Abstract
Importance of the field: Taxanes are agents for the treatment of breast cancer. Paclitaxel is hydrophobic, and available formulations require polyoxyethylated castor oil, Cremphor EL® (CrEL) and an ethanol vehicle to allow parental administration. Nanoparticle albumin-bound paclitaxel (nab-P) is a CrEL-free formulation of paclitaxel. The human albumin-stabilized paclitaxel particles have a size of approximately 130 nm, which allows intravenous infusion without capillary blockage.
Areas covered in this review: Efficacy and safety of nab-P in breast cancer has been compared with paclitaxel and docetaxel in large Phase III and II trials. Additionally, the efficacy and safety of nab-P have been investigated in other single-arm clinical trials, in early and advanced disease.
What the reader will gain: Preclinical and clinical development of the drug across all clinical trials published so far, the approved clinical indications, the benefits of this taxane formulation and a look into the future with emphasis on the application in specific subtypes of breast cancer.
Take home message: nab-P has been approved for the treatment of metastatic breast cancer in patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline-containing therapy is not indicated and represents one of most authoritative and sophisticated applications of nanotechnology in cancer treatment so far.
1. Overview of current breast cancer treatment approaches: chemotherapy
Taxanes are the cornerstone of treatment in both early and advanced breast cancer, as are anthracyclines. A recent Cochrane meta-analysis including 12 studies and more than 21,000 patients evaluated the role of taxanes in the adjuvant treatment of operable breast cancer (stage I – III) Citation[1]. The results showed a statistically significant overall survival (HR = 0.81, p < 0.00001) and disease-free survival (DFS) (HR = 0.81, p < 0.00001) for the taxane-containing regimens compared with the non-taxane regimens. This meta-analysis did not identify any subgroups of patients within the evaluated studies in which a taxane-containing regimen would be more efficacious. According to a meta-analysis of Piccart-Gebhart and colleagues Citation[2], the use of a taxane is beneficial in the metastatic setting. In particular taxane-based combinations were significantly better than anthracycline-based combinations in terms of response rates (RRs) and progression-free-survival (PFS), but not in terms of overall survival. Most patients have been exposed to both an anthracycline (e.g., doxorubicin) and a taxane (docetaxel or paclitaxel) in the adjuvant setting. Treatment of breast cancer with a taxane in the metastatic setting after treatment in the adjuvant setting may be difficult because of residual toxicity. Although taxanes are not cardiotoxic, they can produce peripheral neuropathy, severe neutropoenia, hypersensitivity reactions and edema and create the need for steroid premedication, which makes further administration problematic. Substitution of one taxane for the other is possible, depending on the nature of the chronic toxicity. If the tumor has recurred quickly after administration of adjuvant chemotherapy containing a taxane, managing the disease with a similar anti-microtubule agent such as ixabepilone can be effective Citation[3].
Paclitaxel is highly hydrophobic and commercially available formulations require polyoxyethylated castor oil, Cremphor EL® (CrEL, BASF Corporation, Germany), and an ethanol vehicle to allow parental administration. The amount of CrEL required for paclitaxel is significantly higher than for other drugs containing CrEL, with each millilitre of sterile solution containing 527 mg of purified CrEL Citation[4]. CrEL is considered to be responsible for the hypersensitivity reactions seen in patients during paclitaxel therapy. In vitro, CrEL caused axonal swelling, demyelination and axonal degeneration, and, thus, it may also contribute to the development of neuropathy in patients receiving paclitaxel Citation[5]. The use of CrEL necessitates premedication with antihistamines and corticosteroids to prevent hypersensitivity reactions and, despite these premedications, approximately 40% of all patients will have minor reactions (e.g., flushing and rash) and 3% will have life-threatening reactions Citation[6]. CrEL also causes leaching of the plasticizers from polyvinyl chloride (PVC) bags and infusions sets; thus paclitaxel must be infused via the use of special non-PVC infusion systems and in-line filtration Citation[7-9].
An important consideration in the reintroduction of any anticancer therapy after initial drug treatment is the disease-free interval (DFI): the time elapsed between the end of a cytotoxic treatment and the time the progression of the disease occurs. The DFI is considered to be an indicator of the likelihood of drug resistance and responsiveness of the tumor to retreatment. At present, in anthracycline-resistant disease or following adjuvant cyclophosphamide, methotrexate and fluorouracil, taxane-based combinations or taxane monotherapy are considered the standard of care. In general, retreatment after a taxane therapy with single-agent taxanes, albeit a different one, may not result in a significant clinical benefit. On the other hand, some patients might benefit from a re-challenge with a drug already used at an earlier stage of the disease. With the increased use of anthracyclines and taxanes in early breast cancer, drug resistance is a limiting factor in metastatic breast cancer therapy. In taxane-pretreated patients, other chemotherapy agents (e.g., ixabepilone, capecitabine, vinorelbine and gemcitabine) usually are considered as second-line treatment options for the treatment of advanced disease. In this context, after a first-line failure, nab-P (an alternative taxane formulation) might find its own space especially in women with a prolonged DFI after a previous treatment with a taxane (namely ‘re-challenge’) either in the adjuvant or advanced setting.
2. Introduction to nab-P
Nanoparticle albumin-bound paclitaxel (nab-P; Abraxane®; Abraxis BioScience, Bridgewater, NJ, USA) () is CrEL-free formulation of paclitaxel with activity in patients with breast cancer, both in advanced disease (pre-treated patients) and in the (neo)adjuvant setting (chemo-naive patients), and has demonstrated greater activity and a better safety profile compared with standard solvent-based taxanes, paclitaxel and docetaxel Citation[10,11]. nab-P was approved by the FDA in January 2005 and by the EMEA in 2008 for the treatment of metastatic breast cancer based on a Phase III superiority trial, in which nab-P showed superior efficacy compared with paclitaxel (Taxol®; BristolMyersSquibb). The present review summarizes the existing publications about nab-P in early and advanced breast cancer.
Box 1. Drug summary.
3. Chemistry
The active principle in nab-P is paclitaxel, a natural drug with anti-tumor activity. Paclitaxel derives from Taxus media Citation[4]. Its chemical name is 5β,20-Epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine. Paclitaxel's structural formula is shown in .
Paclitaxel's empirical formula C47H51NO14 and its molecular weight is 853.91. It is highly lipophilic, insoluble in water and melts at approximately 216 – 217°C.
4. Pharmacodynamics of nab-P
nab-P is an anti-microtubule agent. It promotes the assembly of microtubules from tubulin dimers and stabilizes microtubules avoiding depolymerization. This results in the inhibition of the normal dynamic reorganization of the microtubule network that is crucial for cellular functions.
Desai et al. Citation[12] compared nab-P versus paclitaxel in animal models and found (at an equitoxic dose) that the nab-P-treated group had more complete regressions, longer time to recurrence, longer doubling time and prolonged survival. Trans-endothelial transportation of albumin may have contributed to the difference in intratumoral paclitaxel concentration. Albumin is thought to facilitate endothelial transcytosis of unbound and albumin-bound plasma constituents into the extravascular space. This process is initiated by the binding of albumin to a cell surface receptor, 60-kDa glycoprotein (gp60) receptor (albondin), which in turn results in the activation of an intracellular protein (caveolin-1). Subsequently, caveolin-1 activation leads to invagination of the cell membrane to form transcytotic vesicles called caveolae, which transport albumin (and nab-P) across the endothelial cell to the interstitial space Citation[13]. The albumin-binding matricellular protein SPARC (secreted protein, acidic and rich in cysteine), which is often over-expressed in many tumors, may also contribute to the higher intratumoral concentration of nab-P. SPARC has been associated with a poor prognosis in multiple tumors, including squamous cell cancer of the head and neck, Non-small cell lung carcinoma and breast cancer Citation[14-16]. The amount of SPARC over-expression and the role SPARC may have in the increased intratumoral uptake or sequestration of nab-P is an area of active investigation in various malignancies. In nude mice bearing six different human tumor xenografts treated with nab-P or docetaxel, the relative efficacy of nab-P was significantly higher compared with docetaxel in HER2-negative tumors and in HER2-positive tumors with high levels of SPARC. SPARC expression may be a useful biomarker in determining anti-tumor effectiveness for nab-P. Evidence revealed SPARC immunohistochemistry as a biomarker test for nab-P activity through data from three different clinical trials: i) CA-040, a Phase I/II metastatic pancreatic cancer trial of gemcitabine plus nab-P; ii) N057E, a Phase II unresectable stage IV melanoma trial of carboplatin and nab-P; and iii) BRE73, a Phase II neoadjuvant breast cancer trial of gemcitabine, epirubicin and nab-P Citation[17-19]. These preliminary data from three different clinical trials including melanoma, pancreatic and neoadjuvant breast cancer are supportive of the hypothesis that SPARC may be a predictive biomarker of response to nab-P.
Thus, the lack of CrEL-micelle sequestration of paclitaxel and/or the albumin-mediated transport may explain the higher intratumoral accumulation of nab-P compared with paclitaxel. In addition, the endothelial cell binding seen for nab-P could also lead to increased anti-angiogenic activity, resulting in better tumor response Citation[3,20]. In an animal model of ortotopic implants of human breast cancer, combined bevacizumab and nab-P treatment synergistically inhibited tumor growth and metastasis in well-established tumors. This therapy also decreased the incidence of lymphatic and pulmonary metastases by 60 and 100%, respectively Citation[17]. The significant increase in the cure of tumor-bearing mice in the nab-P/bevacizumab-combined group compared with mice treated with monotherapy provides strong motivation for implementing such a strategy in clinical studies Citation[20].
5. Pharmacokinetics and metabolism of nab-P
nab-P is prepared by high-pressure homogenization of paclitaxel in the presence of serum albumin, resulting in a nanoparticle colloidal suspension. The albumin concentration in nab-P is 3 – 4%, which is similar to the albumin concentration in the blood Citation[4]. The human albumin-stabilized paclitaxel particles have an average size of ∼ 130 nm. Injected into the circulation, the 130 nm albumin-paclitaxel particles quickly dissolve into smaller endogenous albumin-sized (∼ 10 nm) complexes, which allows intravenous infusion without the risk of capillary blockage. nab-P can be reconstituted in normal saline at concentrations of 2 – 10 mg/ml compared with 0.3 – 1.2 mg/ml for paclitaxel; therefore, the volume and infusion time are reduced. The nab-P formulation provides several practical advantages over paclitaxel: pre-medications for hypersensitivity reactions are not required, the infusion time is shorter (30 min for nab-P vs 3 h for paclitaxel) and conventional infusion equipment may safely be used as there is no danger of leaching plasticizers from infusion bags or tubing Citation[21-23].
With the development of nab-P and other new taxane formulations, the potential influence of CrEL on efficacy has been an area of active investigation. In albumin-bound, nanoparticle form, paclitaxel is readily bioavailable once infused into the blood circulation. nab-P accumulates in tumors via transcytosis, processes that have shown to be inhibited by CrEL, Tween (polysorbate) 80 and other solvents Citation[21]. Following administration of paclitaxel 20 mg/kg formulated as nab-P or paclitaxel in an MX-1 animal model, the intratumor paclitaxel concentrations were 33% higher for nab-P Citation[21]. Paclitaxel intratumor concentrations were higher even at the first time point measured (5 min) demonstrating the rapid bioavailablity paclitaxel in the albumin-particle-based formulation. Comparative pharmacokinetic data were obtained from 24 patients who were randomly assigned to receive nab-P or paclitaxel at the same dose and schedules that were used in the Phase III randomized trial Citation[21]. These data demonstrated a higher plasma clearance and volume of distribution for paclitaxel administered as nab-P compared with paclitaxel, consistent with the preclinical data and the known paclitaxel sequestration effects of CrEL Citation[21]. Consistent with the metabolic data obtained in a pharmacokinetic substudy of a Phase III trial, the elimination half-lifes were similar for both formulations Citation[21]. Differences in the maximum concentrations and times to maximum concentration were due to differences in the doses administered (260 vs 175 mg/m2) and the infusion duration's (30 min vs 3 h). In vitro studies with human liver microsomes and tissue slices showed that paclitaxel was metabolized primarily to 6α-hydroxypaclitaxel by CYP2C8; and to two minor metabolites, 3′-p-hydroxypaclitaxel and 6α, 3′-p-dihydroxypaclitaxel, by CYP3A4. In vitro, the metabolism of paclitaxel to 6α-hydroxypaclitaxel was inhibited by a number of agents, such as ketoconazole, verapamil, diazepam, quinidine, dexamethasone, cyclosporin, teniposide, etoposide and vincristine, but the concentrations used exceeded those found in vivo following normal therapeutic doses. Testosterone, 17α-ethinyl estradiol, retinoic acid and quercetin, a specific inhibitor of CYP2C8, also inhibited the formation of 6α-hydroxypaclitaxel in vitro. The pharmacokinetics of paclitaxel may also be altered in vivo as a result of interactions with compounds that are substrates, inducers or inhibitors of CYP2C8 and/or CYP3A4. The effect of renal or hepatic dysfunction on the disposition of nab-P has not been investigated in a large population. Possible interactions of paclitaxel with concomitantly administered medications have not been formally investigated.
6. Clinical efficacy
6.1 Advanced disease
nab-P development started with the seminar paper of Gradishar and colleagues in 2005 that established superiority over paclitaxel. The comparative study with docetaxel followed which indicated a more effective outcome than the solvent-free formulation. Several Phase II studies have been published and are reviewed here with regard to activity and toxicity of combinations in particular, with emphasis on the association with target therapies (see and ). As of today, the role of nab-P is in the relapsed setting after taxane failure, a place where various anti-cancer agents are used but which have not demonstrated any clear superiority over another. The choice then depends upon several factors: the characteristics and the choice of patient, physician preference, cost, effectiveness and, not least, the toxicity of the treatment.
6.1.1 Phase III studies
A multinational Phase III clinical trial compared the activity and toxicity of nab-P with paclitaxel in 454 patients with metastatic breast cancer Citation[24]. Patients were randomized to nab-P (260 mg/m2) or paclitaxel (175 mg/m2 over 3 h) every 3 weeks. Patients receiving nab-P had a statistically significant higher response rate (33 vs 19%; p = 0.001) and time to disease progression (TTP; 23 vs 16.9 weeks; p = 0.006). A statistically significant higher activity was seen across different subgroups (e.g., age < 65 years, visceral dominant metastases, previous adjuvant and/or metastatic anthracycline therapy, previous anthracycline therapy only in a metastatic setting, first-line metastatic patients or at least second-line metastatic patients) on subset analysis Citation[25]. There was no difference in median survival for all patients (65 vs 55.7 weeks; p = 0.374); however, for patients receiving second-line or greater therapy, the median survival was statistically significantly longer on the nab-P arm (56.4 vs 46.7; p = 0.024; see for first line and for second or greater lines).
Figure 1. Patient survival over time (A) and patient survival over time in patients who received second-line or greater therapy (B).
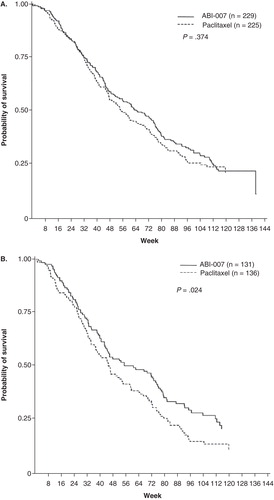
This trial also confirmed the results of the preclinical studies, that the use of the nab-P formulation could result in clinically relevant improvements in the toxicity and the efficacy of paclitaxel therapy. In particular the incidence of any grade 3 and 4 neutropenia was significantly reduced (p < 0.001) in patients receiving nab-P compared with those receiving paclitaxel, despite a 50% increase in paclitaxel dose. Also, the median time-to-improvement for grade 3 peripheral neurotoxicity was 22 days for nab-P and 79 days for paclitaxel. Updated results of the outcome confirmed that patients treated with nab-P had a ∼ 30% reduction in the risk of disease progression compared with paclitaxel, regardless of previous anthracycline exposure. Additionally, nab-P prolonged survival in patients with metastatic breast cancer that was resistant to anthracycline after treatment in the adjuvant or metastatic setting Citation[26].
A similar randomized study was performed in a Chinese population (n = 210) Citation[27]. In this open-label, multicentre study, 210 patients with metastatic breast cancer were assigned to either paclitaxel 175 mg/m2 every 3 weeks or nab-P 260 mg/m2 every 3 weeks for one to six cycles. Compared with paclitaxel, treatment with nab-P was associated with a higher response rate (52 vs 27%) and longer TTP (7.8 vs 5.7 months) without increased toxicity.
As first-line therapy for metastatic breast cancer, nab-P significantly improved PFS compared with docetaxel Citation[28]. The study included 302 patients who were randomized to receive nab-P, 300 mg/m2 every 3 weeks, 100 mg/m2 weekly or 150 mg/m2 weekly, or docetaxel 100 mg/m2 every 3 weeks. nab-P at a dose of 150 mg/m2 weekly achieved longer PFS than docetaxel. The corresponding survival rates were 12.9 and 7.5 months and, by investigator assessment, 14.6 and 7.8 months. On independent radiologist review, the weekly doses of nab-P achieved an overall response rate of 47%, while docetaxel had a response rate of 35%. Although this difference was not statistically significant, the investigator assessment showed a significant trend. Treatment with nab-P every 3 weeks was no better than docetaxel with regard to PFS or overall response rate. Grade 3 or 4 neutropoenia and fatigue were less common in the nab-P groups than in the docetaxel group. Peripheral neurotoxicity, by contrast, occurred with similar frequency and severity across all of the groups.
6.1.2 Phase II studies
A Phase II clinical trial that investigated the activity and toxicity of nab-P 300 mg/m2 every 3 weeks was performed in 63 patients with metastatic breast cancer Citation[29]. The overall response rate was 64% for patients receiving nab-P as first-line therapy. For patients receiving second line and greater therapy the response rate was 21%. The median TTP was 26.6 weeks and the median survival was 63.6 weeks. A second Phase II clinical trial Citation[30] investigated the activity of nab-P 100 mg/m2 (n = 106) or 125 mg/m2 (n = 75) given on days 1, 8 and 15 every 28 days in patients with taxane-refractory metastatic breast cancer. Response rates were 14 and 16% for the 100-mg/m2 and 125-mg/m2 cohorts, respectively; an additional 12 and 21% of patients, respectively, had SD ≥ 4 months. Median PFS were 3 and 3.5 months and 100 mg/m2 at 125 mg/m2 respectively; median survival times were 9.2 months and 9.1 months. nab-P 100 mg/m2 weekly demonstrated the same anti-tumor activity as the 125 mg/m2 weekly schedule and a more favorable safety profile in patients with metastatic breast cancer that had progressed after previous taxane therapy.
A Phase II study of weekly nab-P was presented at the 2008 American Society of Clinical Oncology Meeting. Patients with locally advanced or metastatic breast cancer received nab-P (125 mg/m2) weekly for the first 3 weeks of each 28-day cycle. HER2-positive patients received trastuzumab. The overall response rate was 42.6% (64.7% in the HER2-positive and 31% in HER2-negative subset, respectively). Median TTP was 12.4 months and median survival was 25 months. These results (in particular in the HER2-positive setting) confirmed the above-mentioned studies Citation[19].
Weekly nab-P and gemcitabine combination was studied in an open-label, one-stage, Phase II trial in patients with untreated metastatic breast cancer Citation[31]. nab-P (125 mg/m2) and gemcitabine (1000 mg/m2) were administered on days 1 and 8 of a 21-day cycle until disease progression. A total of fifty patients were enrolled. Four (8%) and twenty-one (42%) patients, respectively, obtained complete response (CR) and partial response (PR) according to RECIST (response evaluation criteria in solid tumors) criteria. Median duration of response was 6.9 months, median PFS 7.9 months and median overall survival was not reached.
Combination of nab-P and capecitabine was also tested Citation[32]. This Phase II, multi-centre, open-label study used nab-P (125 mg/m2 days 1 and 8) and capecitabine (825 mg/m2 p.o. b.i.d. days 1 – 14) on a 3-weekly cycle. Of 38 patients available for analysis of response (out of 43 available for analysis), the overall response rate was 47.5%: PR 39.5% and CR 8%.
In a multicenter, open-label study in the US Oncology Research Network, HER2-negative patients with metastatic breast cancer, receiving first-line chemotherapy were given weekly nab-P 125 mg/m2 on days 1, 8 and 15, and bevacizumab 10 mg/kg on days 1 and 15 of a 28-day cycle Citation[33]. Of the 49 patients enrolled, 27 were evaluable for response. The confirmed overall response rate was 30%. Stable disease > 4 months was 22% (6/27). The median PFS was 9.2 months. Results of this preliminary analysis show that nab-P in combination with bevacizumab has promising PFS and an acceptable safety profile with no unexpected toxicities.
In another Phase II study, patients (untreated HER2-negative metastatic breast cancer or metastases diagnosed ≥ 6 months after primary systemic treatment) received gemcitabine 1500 mg/m2, nab-P 150 mg/m2 and bevacizumab 10 mg/kg on days 1 and 15 of 28-day cycles. Out of 15 patients, ten patients (91%) achieved PR, and one patient (9%) had progression of disease (PD) Citation[34].
Conlin and colleagues investigated three doses of nab-P with bevacizumab Citation[35]. This randomized, Phase II trial compares nab-P at 260 mg/m2 every 3 weeks (arm A) versus 260 mg/m2 every 2 weeks with filgrastim (arm B) versus 130 mg/m2 weekly, all with bevacizumab (15 mg/kg every 3 weeks in arm A, 10 mg/kg every 2 weeks in arms B and C), as first-line therapy for patients with HER2-negative metastatic breast cancer. No significant differences in confirmed CR and PR rates were noted (A: 42%, B: 42%, C: 41%). TTP was longer in arm C (9.2 months) versus both arms B (6.4 months) and A (7.7 months; p = 0.028). As per protocol-specified planned design, arm B was closed early because of an unacceptable safety profile with significantly more grade ≥ 2 fatigue (B: 57%, A: 39%, C: 39%; p = 0.048) and bone pain (B: 19%, A: 10%, C: 4%; p = 0.024). Weekly nab-P with bevacizumab (arm C) resulted in a significantly longer TTP and seems to have the highest therapeutic index; however, sensory neuropathy is limiting, suggesting that a ‘3 week on/1 week off’ schedule could be preferable and should be studied comparatively.
6.1.3 Phase I monotherapy studies
A Phase I trial of nab-P every 3 weeks was performed in patients with metastatic melanoma and breast cancer Citation[10]. The doses investigated were 135, 200, 300 and 375 mg/m2, and the maximum tolerated dose (MTD) was 300 mg/m2. The dose-limiting toxicities (DLTs) were sensory neuropathy (n = 3), stomatitis (n = 2) and superficial keratopathy (n = 2).
A Phase I trial of nab-P on a weekly schedule of days 1, 8 and 15 every 28 days in 39 patients with multiple different types of tumor was done Citation[21]. Doses of 80, 100, 125, 150, 175 and 200 mg/m2 were investigated in both lightly pre-treated and heavily pre-treated patients (defined as one or more of the following: at least 6 cycles of alkylating agent, more than 2 cycles of carboplatin or mitomycin, irradiation to > 25% of the bone marrow, treatment with nitrosoureas, high-dose therapy requiring bone marrow or stem cell support and greater than 1 cycle of investigational agent known to cause a cumulative toxicity). This trial found the MTD was 100 mg/m2 for heavily pre-treated and 150 mg/m2 for lightly pre-treated patients. The DLTs were sensory neurotoxicity and grade 4 neutropoenia.
6.1.4 Combinations with target therapies in HER2-positive disease
Another strategy is to combine nab-P with biologic agents, in particular in HER2-positive disease. A combination with carboplatin, nab-P and trastuzumab has been chosen for a Phase II study. An initial cohort of three patients received nab-P at 75 mg/m2 followed by carboplatin (AUC = 2 weekly on days 1, 8, and 15 every 28 days) + trastuzumab (4 mg/kg × 1, then 2 mg/kg on all weeks) for one cycle. This was well tolerated; thus nab-P was escalated to 100 mg/m2 for all subsequent cycles and patients. At the time of presentation, 30 patients were treated and 12 were on study Citation[36]. Out of 26 patients valuable for response, 46% had a confirmed CR or PR. The median TTP was 16 months (95% CI 7 – 28). This study confirms the comparable synergy between conventional taxane/trastuzumab doublet and nab-P/trastuzumab combinations.
Overall studies in advanced disease confirm the higher activity of nab-P single-agent compared with paclitaxel and docetaxel, and the feasibility of combinations with other cytotoxic agents selected for advanced breast cancer (with objective response rates > 40%). Of interest is the association of nab-P with bevacizumab and trastuzumab, respectively, in HER2-negative and -positive disease. In particular, the combination with biological agents seems to be very well tolerated and no life-threatening toxicities were observed.
6.2 Early disease
6.2.1 Neoadjuvant studies
Single-arm, Phase II studies have been done in both operable and locally advanced breast cancer and are reported in the next section. Also, feasibility studies in the adjuvant setting are presented.
In patients with locally advanced or operable breast cancer, neoadjuvant chemotherapy has been demonstrated to increase the chance of breast-conserving surgery when compared with surgery alone Citation[37]. In a meta-analysis, the rate of breast-conserving surgery was significantly higher for patients receiving taxanes, with an absolute difference of 3.4% (p = 0.012) Citation[22]. The rate of pCR was higher for patients receiving taxanes, though not statistically significant. In the sensitivity analysis, patients receiving taxanes as a sequential schedule had a significantly higher probability of achieving pCR, with an absolute difference of 2.4% (p = 0.013) Citation[22].
summarizes studies of nab-P showing anti-tumor activity in the neoadjuvant setting in combination with both chemotherapy and biologic agents, sequentially to anthracycline schedules.
Table 1. Studies with nab-P in the neoadjuvant setting.
A Phase II study, with dose-dense neaoadjuvant adriamicyn plus cyclophsphamide chemotherapy (AC: 60/600 mg/m2) followed by weekly nab-P and carboplatin plus bevacizumab or trastuzumab in HER2-negative or -positive patients (nab-TCB or nab-TCH, respectively) was feasible Citation[23]. Side effects with nab-PCH/PCB included grade 3 arthralgia in two patients and vascular events in one patient. Pathologic CR was documented in 62.5% of HER2-positive tumors after nab-PCH and 30% of HER2-negative tumors after nab-PCB. Among stage II – IV, inflammatory and lobular carcinoma, tumors were reduced to residual ≤ ypT1a in 87.5% HER2-positive breast cancers and in 80% HER2-negative breast cancers. PFS at a median follow-up of 6 months was 100%.
A Phase II study Citation[38] evaluated activity and safety of neoadjuvant nab-P plus gemcitabine and epirubicin and included a correlation with tumor SPARC assessment. Treatment consisted of gemcitabine 2000 mg/m2, epirubicin 50 mg/m2 and nab-P 175 mg/m2 every 14 days for 6 cycles followed by surgery. There were 123 patients enrolled and 112 patients were available for pathologic response analysis. Of those, 82 patients consented to SPARC assessment in their breast tumor. Pathologic CR was obtained in 18% of patients, pathologic PRs in 68%, and pathologic no change (SD; stable disease) in 5%. SPARC immunohistochemical staining was available for analysis in 77 tumor samples, with SPARC positivity noted in 89% of tumors achieving CR at pathologic examination. SPARC-positive tumors showed a trend to improved PFS that was strongly associated with tumoral and not stromal immunohistochemical staining.
In a randomized study with patients (stages II – III HER2-negative breast cancer), neoadjuvant chemotherapy with docetaxel 75 mg/m2, doxorubicin 50 mg/m2, cyclophosphamide 500 mg/m2 with filgrastim support (TAC, arm A) or AC, followed by carboplatin (AUC 2), and nab-P 100 mg/m2 weekly (AC, arm B), or AC plus trastuzumab (arm C) was tested and seemed feasible. Patients with HER2-positive disease received the same treatment to arm B but with 12 weekly doses of trastuzumab given concomitantly with carboplatin and nab-P (arm C). A pCR and/or a minimal residual disease as best pathologic response were observed in 7 and 23% of arm A, 7 and 23% of arm B, and 40 and 0% of arm C. This study confirmed the feasibility of carboplatin and trastuzumab doublet added to nab-P as sequential schedule following anthracycline Citation[39]. This combination unfortunately performed no better than docetaxel/anthracycline combination. Also, nab-P-containing arms (B + C) are not equally comparable with the docetaxel arm (A) because they included two different populations (HER2-negative and -positive).
In a Phase II study, a quadruple combination therapy consisting of carboplatin/nab-P + bevacizumab and trastuzumab was given to patients as neoadjuvant therapy Citation[40]. Patients had cT1 – 4,N0 – 3M0 and HER2-positive breast cancer. nab-P 100 mg/m2 was administered weekly (days 1, 8, and 15 every 28 days) with carboplatin (AUC 6 on day 1 every 28 days) for six cycles, bevacizumab (5 mg/kg weekly for 23 weeks) and trastuzumab (4 mg/kg loading dose followed by 2 mg/kg weekly for 22 doses). Twenty-nine patients have been enrolled and evaluated for safety. Pathologic responses are available for 20 patients and were as follows: pCR in 65% of patients and PR in 35%. Neoadjuvant nab-P/carboplatin/bevacizumab/trastuzumab resulted in a feasible and highly active combination with a high rate of pCR of 65%.
In a Phase II study by the National Surgical Adjuvant Breast and Bowel Project foundation research program, 66 women with locally advanced breast cancer were randomized to receive preoperative nab-P 100 mg/m2 weekly for 12 consecutive weeks followed by: either four cycles of fluorouracil, epirubicyn plus cyclophosphamide (FEC)-100 every 3 weeks (F: 500 mg/m2, E: 100 mg/m2, C: 500 mg/m2) if their cancer was HER2-negative; or four cycles of FEC-75 every 3 weeks (F: 500 mg/m2, E: 75 mg/m2, C: 500 mg/m2) plus weekly trastuzumab if their cancer was HER2-positive Citation[41]. Most frequent adverse events were diarrhoea (grade 2/3: 16/7%), fatigue (grade 2/3: 30/5%) and neurotoxicity (grade 2/3: 9/5%). Pathologic CR in breast was 29% overall and 58% in the HER2-positive subset. The efficacy results of this study were in accordance with the activity of trastuzumab in the neoadjuvant setting.
nab-P has also been investigated in combination with capecitabine. This was a Phase II study, that enrolled 14 patients with breast cancer (stage II – IIIB disease) who received four cycles of neoadjuvant capecitabine (825 mg/m2 p.o. b.i.d. for 14 days) and nab-P (260 mg/m2 i.v.) on day 1 every 21 days Citation[42]. Seven per cent of patients obtained a pCR, 71% a PR, 21% SD, and 7% PD. Out of six ‘triple-negative’ patients, four had PR, one SD and one PD.
6.2.2 Adjuvant studies
The availability of a less toxic and more powerful taxane like nab-P is ideal for use in the adjuvant phase, in which a potentially curative treatment has to be offered to patients with good prognosis, but avoiding risk of serious and cumulative toxicities ().
Table 2. Studies with nab-P in the adjuvant setting.
In a Phase II study, patients received dose-dense anthracycline chemotherapy followed by nab-P and bevacizumab in the adjuvant setting Citation[43]. Patients with resected HER2-negative breast cancer and normal cardiac function were treated. Chemotherapy schedule was AC (60/600 mg/m2) for 4 cycles then nab-P (260 mg/m2) every 2 weeks for 4 cycles with pegfilgrastim on day 2 plus bevacizumab for 1 year (10 mg/kg i.v. every 2 weeks for 8 cycles with chemotherapy, then 15 mg/kg every 3 weeks). More relevant grade 3/4 toxicities were: neutropoenia (grade 4; 6.8%), diarrhoea (grade 3; 2.3%), hypertension (grade 3; 2.3%), neurotoxicity (grade 3; 2.3%), fatigue (grade 3; 2.3%) and mucositis (grade 3; 2.3%). No cardiac dysfunction has been observed with bevacizumab plus AC-nab-P in 44 patients enrolled from date of presentation. In another similar study, adjuvant bevacizumab plus dose-dense doxorubicin/cyclophosphamide (AC) followed by nab-P seemed safe Citation[44]. In the interim analysis of 80 patients, 16 discontinued treatment owing to toxicity: asymptomatic ejection fraction decline (n = 3), hypertension (n = 3), wound healing (n = 3), headache (n = 2), sensory neurotoxicity (n = 2), pain (n = 1), hypersensitivity reaction (n = 1) and pneumonitis (n = 1). Last analysis showed no cardiac concern related to bevacizumab addition.
In a pilot study patients with early-stage breast cancer received adjuvant dose-dense AC followed by dose-dense nab-P (260 mg/m2 every 2 weeks). The treatment was feasible and showed a favorable safety profile Citation[45]. Patients (18 – 70 years old; n = 30) had operable breast cancer (T1 – 3N1 – 2 or node-negative (N0) > 2 cm, or > 1 cm and oestrogen and progesterone-receptor negative). Toxicities were comparable to those reported in the Cancer and Leukemia Breast Group (CALGB) 9741 trial, a Phase III trial comparing standard with dose-dense (every 2 weeks) anthracycline and paclitaxel combinations in an adjuvant setting Citation[46]. Peripheral neurotoxicity was common, but was generally transient, and with most patients having grade 1 or complete resolution of neuropathy after the last cycle Citation[45]. The same anthracycline schedule followed by dose-dense paclitaxel (175 mg/m2) or nab-P (260 mg/m2) plus bevacizumab was reported in 2009 Citation[47]. A 45% higher paclitaxel dose was delivered safely in the nab-P arm compared with the paclitaxel arm. nab-P administered following athracycline therapy was manageable and safe.
An anthracycline-free taxane doublet (docetaxel/cyclophosphamide) demonstrated superior disease-free and overall survival compared with doxorubicin/cyclophosphamide in the treatment of early stage breast cancer with an acceptable safety profile, with grade 3/4 neutropoenia of 61% and no cardiotoxicity Citation[48]. As a consequence, a multicenter Phase II pilot trial of weekly nab-P/cyclophosphamide was conducted to test the safety and tolerability of this combination in early-stage breast cancer. Sixty-three patients were enrolled in 2008; 33 of whom were evaluable for safety with a median of two cycles. Treatment consisted of nab-P (100 mg/m2 on days 1, 8, and 15) with cyclophosphamide (600 mg/m2 on day 1 every 3 weeks) for four cycles. Grade 3/4 neutropoenia was observed in 30% with only one grade 4 event. There were grade 3/4 non-hematologic toxicities and no left ventricular ejection fraction fall in patients receiving trastuzumab Citation[49].
In summary nab-P shows impressive activity in the neoadjuvant setting, according to the clear superiority demonstrated in the advanced setting. The burden of toxicity was low and the combination with biologics seems interesting and to be working well both in HER2-negative and -positive disease. Appealing and fascinating is the synergistic combination with platinum salts and trastuzumab which obtained a high rate of complete response. So far no clear superiority has been demonstrated in pCR or prolonged outcome with nab-P as neoadjuvant therapy despite this treatment being manageable in terms of toxicity. In fact, no Phase III randomized studies comparing nab-P with the standard taxane formulations have been conducted. This will be the target of future neoadjuvant trials.
There is a potential place for nab-P in the adjuvant setting where manageable toxicities and a potential improvement in dose delivery are observed in Phase II studies, although up to today a greater efficacy has not yet been demonstrated in randomized studies.
7. Safety and tolerability
Unless otherwise noted, the following discussion refers to the primary safety database of 229 patients with metastatic breast cancer treated with single-agent nab-P in the randomized, controlled trial. The frequency and severity of important adverse events for the study are presented in . In some instances, rare severe events observed with paclitaxel injection may be expected to occur with nab-P.
Table 3. Studies with nab-P in monochemotherapy for metastatic disease.
Table 4. Studies with nab-P in combination with other agents for metastatic disease.
7.1 Hematologic
Neutropoenia, the most important hematologic toxicity, was dose dependent and reversible. Among patients with metastatic breast cancer in the randomized trial, neutrophil counts declined below 500 cells/mm3 (grade 4) in 9% of the patients treated with a dose of 260 mg/m2 compared with 22% in patients receiving paclitaxel injection at a dose of 175 mg/m2. Febrile neutropoenia was reported in 2% of patients in the nab-P arm and 1% of patients in the paclitaxel arm. Thrombocytopenia was uncommon. Anemia of any grade (Hb < 11 g/dl) was observed in 33% of patients treated with nab-P in the randomized trial and was severe (Hb < 8 g/dl) in 1% of the cases. Among all patients with normal baseline hemoglobin, 31% became anemic on study and 1% had severe anemia.
7.2 Hypersensitivity reactions
In the randomized, controlled metastatic breast cancer study, grade 1 or 2 hypersensitivity reactions occurred on the day of nab-P administration and consisted of dyspnoea (1%) and flushing, hypotension, chest pain and arrhythmia (all < 1%). The use of nab-P in patients previously exhibiting hypersensitivity to paclitaxel injection or human albumin has not been studied.
During postmarketing surveillance, rare occurrences of severe hypersensitivity reactions have been reported with nab-P. Unfortunately the use of nab-P in patients previously exhibiting hypersensitivity to paclitaxel injection or human albumin has not been studied. Patients who experience a severe hypersensitivity reaction to nab-P should not be re-challenged with the drug.
7.3 Cardiovascular
Hypotension, during the 30-min infusion, occurred in 5% of patients in the randomized metastatic breast cancer trial. Bradycardia, during the 30-min infusion, occurred in < 1% of patients. These vital sign changes most often caused no symptoms and required neither specific therapy nor treatment discontinuation. Severe cardiovascular events possibly related to single-agent nab-P occurred in ∼ 3% of patients in the randomized trial. These events included chest pain, cardiac arrest, supraventricular tachycardia, edema, thrombosis, pulmonary thromboembolism, pulmonary emboli and hypertension. Cases of cerebrovascular attacks (strokes) and transient ischemic attacks have been reported rarely. Electrocardiogram (ECG) abnormalities were common among patients at baseline. Among patients with a normal ECG before study entry, 35% developed an abnormal tracing while in the study. The most frequently reported ECG modifications were non-specific repolarization abnormalities, sinus bradycardia and sinus tachycardia.
7.4 Respiratory
Reports of dyspnoea (12%) and cough (6%) were reported after treatment with nab-P in the randomized trial. Rare reports (< 1%) of pneumothorax were reported after treatment with nab-P. Rare reports of interstitial pneumonia, lung fibrosis and pulmonary embolism have been received as part of the continuing surveillance of paclitaxel injection safety and may occur following nab-P treatment. Rare reports of radiation pneumonitis have been received in patients receiving concurrent radiotherapy. There is no experience with the use of nab-P with concurrent radiotherapy.
7.5 Neurologic
The frequency and severity of neurologic manifestations were influenced by previous and/or concomitant therapy with neurotoxic agents. In general, the frequency and severity of neurologic manifestations were doses dependent in patients receiving single-agent nab-P. In the randomized trial, sensory neuropathy was observed in 71% of patients (10% severe) in the nab-P arm and in 56% of patients (2% severe) in the paclitaxel injection arm. The frequency of sensory neuropathy increased with cumulative doses. Sensory neuropathy was the cause of nab-P discontinuation in 7/229 (3%) patients in the randomized trial. In the randomized comparative study, 24 patients (10%) treated with nab-P developed grade 3 peripheral neuropathy; of these patients, 14 had documented improvement after a median of 22 days; 10 patients resumed treatment at a reduced dose of nab-P and 2 discontinued because of peripheral neuropathy. Of the 10 patients without documented improvement, 4 discontinued the study because of peripheral neuropathy. No incidences of grade 4 sensory neuropathies were reported in the clinical trial. Only one incident of motor neuropathy (grade 2) was observed in either arm of the controlled trial. Cranial nerve palsies have been reported during postmarketing surveillance of nab-P. Reports of autonomic neuropathy resulting in paralytic ileus have been received as part of the continuing surveillance of paclitaxel injection safety. Ocular/visual disturbances occurred in 13% of all patients (n = 366) treated with nab-P in single-arm and randomized trials and 1% were severe. The severe cases (keratitis and blurred vision) were reported in patients in a single-arm study who received higher doses than those recommended (300 or 375 mg/m2). These effects have generally been reversible. However, rare literature reports of abnormal visual-evoked potentials in patients treated with paclitaxel have indicated persistent optic nerve damage.
7.6 Arthralgia/myalgia
Forty-four per cent of patients treated in the randomized trial experienced arthralgia/myalgia; 8% experienced severe symptoms. The symptoms were usually transient, occurred 2 or 3 days after nab-P administration and resolved within a few days.
7.7 Hepatic
Among patients with normal baseline liver function treated with nab-P in the randomized trial, 7, 36 and 39% had elevations in bilirubin, alkaline phosphotase and transaminases, respectively. Grade 3 or 4 elevations in gamma glutamil transferase were reported for 14% of patients treated with nab-P and 10% of patients treated with paclitaxel injection in the randomized trial.
7.8 Renal
Overall, 11% of patients experienced creatinine elevation, 1% severe. No discontinuations, dose reductions or dose delays were caused by renal toxicities.
7.9 Gastrointestinal
Nausea/vomiting, diarrhoea and mucositis were reported by 33, 27 and 7%, respectively, of nab-P treated patients in the randomized trial.
Rare reports of intestinal obstruction, intestinal perforation, pancreatitis and ischemic colitis have been received as part of the continuing surveillance of paclitaxel injection safety and may occur following nab-P treatment. Rare reports of neutropenic enterocolitis (typhlitis), despite the coadministration of granulocyte colony-stimulating factor, were observed in patients treated with paclitaxel injection alone and in combination with other chemotherapeutic agents.
7.10 Injection-site reaction
Injection-site reactions have occurred infrequently with nab-P and were mild in the randomized clinical trial. Recurrence of skin reactions at a site of previous extravasation following administration of paclitaxel injection at a different site (i.e., ‘recall’) has been reported rarely.
Rare reports of more severe events such as phlebitis, cellulitis, induration, skin exfoliation, necrosis and fibrosis have been received as part of the continuing surveillance of paclitaxel injection safety. In some cases the onset of the injection site reaction in paclitaxel-injected patients either occurred during a prolonged infusion or was delayed by 1 week to 10 days.
Given the possibility of extravasation, it is advisable to closely monitor the infusion site for possible infiltration during drug administration.
7.11 Asthenia
Asthenia was reported in 47% of patients (8% severe) treated with nab-P in the randomized trial. Asthenia included reports of asthenia, fatigue, weakness, lethargy and malaise.
7.12 Other clinical events
Rare cases of cardiac ischemia/infarction and thrombosis/embolism possibly related to nab-P treatment have been reported. Alopecia was observed in almost all of the patients. Nail changes (changes in pigmentation or discoloration of nail bed) were uncommon. Edema (fluid retention) was infrequent (10% of randomized trial patients); no patients had severe edema.
7.13 Accidental exposure
No reports of accidental exposure to nab-P have been received. However, upon inhalation of paclitaxel, dyspnoea, chest pain, burning eyes, sore throat and nausea have been reported. Following topical exposure, events have included tingling, burning and redness.
8. Regulatory affairs
nab-P is FDA approved and indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Previous therapy should have included an anthracycline unless clinically contraindicated.
nab-P monotherapy is indicated in Europe for the treatment of metastatic breast cancer in patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline-containing therapy is not indicated.
9. Conclusion
Application of nanotechnology to medical science has been emerging as a new field of interdisciplinary research among medicine, biology, toxicology, pharmacology, chemistry, material science, engineering and mathematics and is expected to bring a major breakthrough to address unsolved medical issues. Nanotechnology was originally defined as ‘the creation of useful materials, devices, and systems used to manipulate matter that are small scale ranging between 1 and 100 nm’ Citation[59].
nab-P was developed to improve the solubility of paclitaxel. This formulation improves the toxicity profile of conventional paclitaxel therapy formulated with cremophor. These vectors are not specifically targeted against any molecule expressed on the tumor cells or the endothelium and have been classified as ‘first-generation’ vectors.
The extensive preclinical data in fact indicated that the CrEL vehicle used in the standard formulation of paclitaxel might contribute to the toxicity of paclitaxel therapy. The nab technology has allowed the development of a new formulation of paclitaxel, nab-P, which eliminates the need for premedication for hypersensitivity reactions, reduces the infusion time and allows paclitaxel to be administered with standard infusion equipment. Preclinical data have indicated that nab-P has less toxicity and greater anti-tumor activity than paclitaxel. Preclinical studies have also demonstrated that the nab-P formulation results in higher intratumoral levels of paclitaxel through the mechanisms of albumin transportations into malignant cells and, potentially, sequestration of nab-P by SPARC. A Phase III clinical trial in patients with metastatic breast cancer compared nab-P (260 mg/m2) with paclitaxel (175 mg/m2) every 3 weeks, and patients treated with nab-P had a higher response rate and TTP. Patients on the nab-P arm had a statistically significantly lower rate of grade 4 neutropenia (9 vs 22%, respectively; p < 0.001) and a higher rate of grade 3 sensory neuropathy (10 vs 2%, respectively; p < 0.001). nab-P 150 mg/m2 weekly demonstrated significantly longer PFS than standard-dose docetaxel by both independent radiologists (12.9 vs 7.5 months, respectively; p = 0.0065) and an investigator (14.6 vs 7.8 months, respectively; p = 0.012) assessment. Grade 3 or 4 fatigue, neutropenia and febrile neutropenia were less frequent in all nab-P arms. The frequency and grade of peripheral neuropathy were similar in all arms. nab-P is emerging as a more active and less toxic formulation of paclitaxel. Its convenience is linked to the need for no premedication for hypersensitivity reaction and higher velocity of administration.
On 7 January 2005, the FDA approved nab-P for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Previous therapy should have included an anthracycline unless clinically contraindicated. In Europe (EMEA) nab-P monotherapy has been approved for the treatment of metastatic breast cancer in patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline-containing therapy is not indicated.
Given its improved safety profile, potentially enhanced efficacy and comparable economic impact Citation[50], nab-P (weekly or every 3 weeks) can be considered a reasonable alternative to docetaxel as first-line chemotherapy for advanced breast cancer.
At the moment, the question that remains unanswered is whether or not weekly nab-P is equally active to one of the best comparator taxane arms, weekly paclitaxel. Certainly, the administration benefits (no need for premedication, rate and modality of infusion) makes nab-P an attractive comparison arm (in adjuvant phase) with standard weekly paclitaxel, representing the better taxane schedule that may be offered to breast cancer patients. In conclusion nab-P represents one of the most authoritative exponents of nanotechnology in the treatment of breast cancer.
10. Expert opinion
The new avenue of nanotechnology offers potential solutions to the historical challenge that has rendered breast cancer so difficult to contain and eradicate: the extreme biologic diversity of the disease presentation in the patient population and in the genetic profile of any individual disease, the multiple pathways that drive disease progression, the onset of ‘resistance’ to established therapeutic cocktails and last, but not least, the gravity of the side effects to treatment, which result from generally very poor distribution of the injected therapeutic agents in the body. A fundamental requirement for success in the development of new therapeutic strategies is that breast cancer specialists (clinicians, the pharmaceutical and the basic biologic laboratory) and nanotechnologists (engineers, physicists, chemists and mathematicians) maximize their ability to work in close collaboration.
The setting of breast cancer, from our point of view, is a challenge for nanotechnology. Anthracycline and taxanes have revolutionized the treatment and in most cases offered a chance of cure for breast cancer patients. Toxic effects and efficacy, however, could be ameliorated because long-term survivors still suffer today from long-term adverse effects (cardiovascular toxicity, neurological toxicity, etc.) and, despite these advancements, breast cancer morbidity and mortality is unacceptably high. Breast cancer is the field of medicine with the greatest presence of nanotechnological therapeutic agents in the clinic. A pegylated form of liposomally encapsulated doxorubicin is routinely used for treatment against metastatic disease, and nab-P was approved for locally recurrent and metastatic breast cancer in 2005. These drugs have yielded substantial clinical benefit, and are steadily gathering greater beneficial impact.
Specifically, without the need for CrEL present in the standard paclitaxel formulation, the reasons to use nab-P are at least the following 10 good reasons:
Patients treated with nab-P had a superior TTP and overall survival compared with paclitaxel-treated patients, regardless of previous anthracycline exposure. nab-P 150 mg/m2 weekly demonstrated significantly longer PFS than docetaxel by both independent radiologist assessment (12.9 vs 7.5 months, respectively; p = 0.0065) and investigator assessment (14.6 vs 7.8 months, respectively; p = 0.012) in first-line treatment of metastatic breast cancer.
nab-P permits a higher dose of paclitaxel to be administered with comparable toxicity (260 mg/m2 compared with 175 mg/m2 for paclitaxel).
nab-P increases intratumor paclitaxel concentrations by 33% (data from an equivalent-dose animal model).
nab-P eliminates solvent-related severe hypersensitivity reactions, including anaphylactic reactions and death, permitting administration of paclitaxel over 30 min without premedication.
nab-P eliminates need for specialised intravenous tubing required for CrEL products (to prevent leaching of plasticizers).
nab-P results in more rapid clearance from the plasma and predictable (linear) pharmacokinetics.
nab-P reduces neutropenia; in fact the incidence of any-grade and grade 4 neutropenia was significantly reduced in patients receiving nab-P compared with those receiving CrEL paclitaxel, despite a 50% increase in paclitaxel dose.
In the absence of CrEL, nab-P was administered for a median of six cycles (cf. five cycles with paclitaxel in the Phase III trial) and the neuropathy which occurred with nab-P was transient, although, as expected from the higher dose of paclitaxel delivered, the frequency of peripheral neuropathy was greater with nab-P.
From an economic point of view, when all of the cost components were combined for the entire population of the Phase III trial, patients in the nab-P 100 mg/m2 weekly and 300 mg/m2 every 3 weeks groups had comparable average costs to the conventional docetaxel arm.
There is a strong rationale for combining nab-P with anti-angiogenetic therapy and to use it in SPARC-rich or triple-receptor-negative tumors.
Interesting possible developments with nab-P over the next few years are its comparison with ixabepilone and use in the treatment of triple negative disease. Ixabepilone is a semi-synthetic analogue of epothilone B. Ixabepilone is indicated in combination with capecitabine for the treatment of patients with metastatic or locally advanced breast cancer resistant to treatment with an anthracycline and a taxane, or whose cancer is taxane resistant and for whom further anthracycline therapy is contraindicated. Ixabepilone is also indicated as monotherapy for the treatment of metastatic or locally advanced breast cancer in patients whose tumors are resistant or refractory to anthracyclines, taxanes, and capecitabine Citation[3].
A CALGB, randomized, Phase III trial is studying bevacizumab to see how well it works when given together with paclitaxel, nab-P or ixabepilone in treating patients with locally recurrent, stage IIIB or stage IV breast cancer. The study has been accruing 900 patients with primary objectives to compare the PFS of patients with locally recurrent, stage IIIB or IV breast cancer treated with nab-P versus ixabepilone versus paclitaxel with concomitant bevacizumab.
A challenging paradigm is the triple-receptor-negative breast cancer, an aggressive and poor prognostic breast cancer subtype. It is a particularly chemo-responsive disease, with particular anthracycline and taxane sensitivity. Caveolae are special invaginated microdomains of the plasma membrane found in the majority of mammalian cells and serve as membrane organizing centers. Caveolae are specialized sub-domains of the plasma membrane found in most cell types and particularly abundant in highly differentiated cells such as endothelial cells, adipocytes or muscle cells. Three members of the caveolin family (CAV1, CAV2 and CAV3) have been identified and they play a pivotal role in intracellular trafficking of cellular components and in signal transduction Citation[51]. Despite the controversy about the distribution of CAV1 and CAV2 in normal and invasive breast cancer, recent studies confirmed the preferential expression of both genes and their proteins in normal myoepithelial cells. Furthermore, Savage et al., have recently reported high prevalence of CAV1 and CAV2 expression in basal-like breast carcinomas, and observed CAV1 gene amplification in a small subgroup of basal-like breast cancers. Moreover, these findings support Pinilla et al. who described an association between CAV1 expression and sporadic basal-like breast cancers and familial BRCA1 tumors Citation[52-54]. CAV1 and CAV2 protein expression was also assessed on a tissue microarray containing 880 unselected invasive breast cancer cases, by means of immunohistochemistry. CAV1 and CAV2 expression was observed in 13.4 and 5.9% of all breast cancers, respectively. Their expression was strongly associated with a high histological grade, lack of steroid hormone receptor positivity (ER and PR), and expression of basal markers (basal cytokeratins, P63, P-cadherin). In addition, there was a significant association between CAV1 and CAV2 expression and basal-like phenotype. On univariate analysis only CAV2 had a prognostic impact on breast cancer-specific survival; however, this was not independent from other traditional markers on multivariate analysis Citation[55]. Conversely an absence of stromal CAV-1 was associated with early disease recurrence, advanced tumor stage and lymph node metastasis, resulting in a 3.6-fold reduction in PFS. Interestingly, in lymph node-positive patients, an absence of stromal CAV-1 predicted an 11.5-fold reduction in 5-year PFS Citation[56]. Targeting the tumor vasculature is an extremely promising approach for cancer treatment. Doing so with antibodies to proteins expressed exclusively in the caveolae of tumor endothelia has been the object of active research Citation[57,58]. Such an approach would, in theory, offer the combined advantages of tumor-specific endothelial expression of the target antigen and caveolae-mediated transcytosis of the antibody and its therapeutic load from the blood to the subendothelial space. This intriguing scenario also found the basis of the therapeutic association of nab-P and anti-angiogenic therapy. Unfortunately, in Gradishar et al.'s Phase III study, no subgroup analysis of molecular phenotypes for differential efficacy of the treatment was done Citation[39,43]. An ongoing Phase II study of nab-P, carboplatin and bevacizumab in ‘triple-negative’ (demonstrating no expression for oestrogen, progesterone or HER2 receptors) metastatic breast cancer has been accruing patients. Finally, the next years will probably see more reports of caveolin mutations or ablation in human disease, which will enhance our knowledge of caveolae physiological roles and reinforce the potential of caveolae and caveolin therapeutic targeting.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Notes
Bibliography
- Ferguson T, Wilcken N, Vagg R, Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev 2007;CD004421
- Piccart-Gebhart MJ, Burzykowski T, Buyse M, Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 2008;26:1980-6
- Thomas ES, Gomez HL, Li RK, Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 2007;25:5210-17
- Available from: http://www.abraxane.com/resources/Abraxane_Healthcare_ Professional_Prescribing_Information.pdf
- Windebank AJ, Blexrud MD, de Groen PC. Potential neurotoxicity of the solvent vehicle for cyclosporine. J Pharmacol Exp Ther 1994;268:1051-6
- Irizarry LD, Luu TH, McKoy JM, Cremophor EL-containing paclitaxel-induced anaphylaxis: a call to action. Community Oncol 2009;132-4
- Gelderblom H, Verweij J, Nooter K, The drawbacks and advantages of vehicle selectionfor drug formulation. Eur J Cancer 2001;37:1590-8
- Authier N, Gillet JP, Fialip J, Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res 2001;3:301-6
- Weiss RB, Donehower RC, Wiernik PH, Hypersensitivity reactions from taxol. J Clin Oncol 1990;8:1263-8
- Ibrahim NK, Desai N, Legha S, Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res 2002;8:1038-44
- Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol 2006;17:735-49
- Desai NP, Trieu V, Hwang LY, Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anticancer Drugs 2008;19:899-909
- Desai N, Trieu V, Yao Z, Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006;12:1317-24
- Chin D, Boyle GM, Williams RM, Novel markers for poor prognosis in head and neck cancer. Int J Cancer 2005;113:789-97
- Koukourakis MI, Giatromanolaki A, Brekken RA, Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res 2003;63:5376-80
- Watkins G, Douglas-Jones A, Bryce R, Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids 2005;72:267-72
- Von Hoff D, Ramanathan E, Borad M, SPARC correlation with response to gemcitabine (G) plus nab-paclitaxel (nab-P) in patients with advanced metastatic pancreatic cancer: a phase I/II study [abstract 4525]. J Clin Oncol 2009;27(Suppl):15s
- Markovic SN, Suman VJ, Kottschade LA, A phase II trial of carboplatin (C) and nab-paclitaxel (ABI-007-nab-P) in patients with unresectable stage IV melanoma: final data from N057E [abstract 9055]. J Clin Oncol 2009;27(Suppl):15s
- Mirtsching B, Cosgriff T, Harker G, Single-agent nab-paclitaxel given weekly (3/4) as first-line therapy for metastatic breast cancer (an International Oncology Network study, #I-04-012). J Clin Oncol 2008;26(Suppl): abstract 1118
- Volk LD, Flister MJ, Bivens CM, Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia 2008;10:613-8
- Nyman DW, Campbell KJ, Hersh E, Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol 2005;23:7785-93
- Cuppone F, Bria E, Carlini P, Taxanes as primary chemotherapy for early breast cancer: meta-analysis of randomized trials. Cancer 2008;113:238-46
- Berstein JA, Mehta R. In vivo response-adapted dose-dense (dd) doxorubicin and cyclophosphamide (AC) -> weekly carboplatin and albumin-bound paclitaxel (nab-TC)/trastuzumab (H)/bevacizumab (B) in large and inflammatory breast cancer (BC): a phase II study. ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2007;25(Suppl 18S):11078
- Gradishar WJ, Tjulandin S, Davidson N, Superior efficacy of albumin-bound paclitaxel, ABI-007, compared with polyethylated castor oil-based paclitaxel in women with metastatic breast cancer: results of a Phase III trial. J Clin Oncol 2005;23:1-10
- Davidson N, Tjulandin S, O'shaughnessy J, Overall survival analysis of a randomized phase III trial comparing nab-paclitaxel with solvent-based paclitaxel in patients with metastatic breast cancer previously treated with anthracycline. Eur J Cancer 2008;218(Suppl 6): abstract 569
- Vukelja SJ, O'shaughnessy J, Krasnojon D, Efficacy of Nab-paclitaxel in patients with poor prognostic factors or with anthracycline-resistant metastatic breast cancer (MBC). J Clin Oncol 2008;26(Suppl): abstract 108242
- Guan Z, Feng F, Li QL, Randomized study comparing nab-paclitaxel with solvent-based paclitaxel in Chinese patients (pts) with metastatic breast cancer (MBC). ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2007;25(Suppl 18S):1038
- Gradishar WJ, Krasnojon D, Cheporov S, Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 2009;27:3611-19
- Ibrahim NK, Samuels B, Page R, Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol 2005;23:6019-26
- Blum JL, Savin MA, Edelman G, Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer 2007;7:850-6
- Roy V, LaPlant BR, Gross GG, ; North Central Cancer Treatment Group. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531). Ann Oncol 2009;20:449-53
- Somer BG, Schwartzberg LS, Arena F, Phase II trial of nab-paclitaxel (nanoparticle albumin-bound paclitaxel (ABX)) + capecitabine (XEL) in first-line treatment of metastatic breast cancer (MBC). ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2007;25(Suppl 18S):1053
- Danso MA, Blum JL, Robert NJ, Phase II trial of weekly nab-paclitaxel in combination with bevacizumab as first-line treatment in metastatic breast cancer. J Clin Oncol 2008;26(Suppl): abstract 1075
- Gluck S, Lobo C, Reis I, Phase II study of nab-paclitaxel, bevacizumab, and gemcitabine for first-line therapy of patients with HER2-negative metastatic breast cancer (MBC). J Clin Oncol 2008;26(Suppl): abstract 1089
- Conlin AK, Hudis CA, Bach A, Randomized phase II trial of nanoparticle albumin-bound paclitaxel in three dosing schedules with bevacizumab as first-line therapy for HER2-negative metastatic breast cancer (MBC). J Clin Oncol 2009;27(Suppl 15s); abstract 1006
- Seidman AD, Conlin AK, Bach A, Phase II study of weekly nanoparticle albumin bound (nab)paclitaxel with carboplatin and trastuzumab as 1st-line therapy for HER2-positive metastatic breast cancer (MBC). J Clin Oncol 2008;26(Suppl): abstract 1047
- Rastogi P, Anderson SJ, Bear HD, Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85 Erratum in: J Clin Oncol 2008;26:2793
- Inhorn RC, Daniel B, Daniel D, Correlation of SPARC, ER, PR, and HER2 tumor with progression-free survival from a phase II neoadjuvant trial of gemcitabine, epirubicin, and nab-paclitaxel. J Clin Oncol 2009;27(Suppl 15s): abstract 618
- Paz IB, Lau S, Garberoglio C, Nab-paclitaxel and carboplatin with or without trastuzumab (trast) as part of neoadjuvant chemotherapy (NCT) in patients with stage II – III breast cancer (BC). J Clin Oncol 2008;26(Suppl): abstract 567
- Yardley DA, Raefsky E, Castillo R, Results of a multicenter pilot study of weekly nab-paclitaxel, carboplatin with bevacizumab, and trastuzumab as neoadjuvant therapy in HER2+ locally advanced breast cancer with SPARC correlatives. J Clin Oncol 2009;27(Suppl 15s): abstract 527
- Robidoux A, Buzdar AU, Quinaux E, A phase II neoadjuvant trial of sequential weekly nanoparticle albumin-bound paclitaxel followed by 5-fluorouracil/epirubicin/cyclophosphamide in locally advanced breast cancer. Clin Breast Cancer 2010;10:81-6
- Veerapaneni A, Boisvert M, Choi A, Aggarwal A. Capecitabine and ABI-007 chemotherapy as neoadjuvant treatment of locally advanced breast cancer. J Clin Oncol 2008;26(Suppl): abstract 11535
- Dickler MN, Traina T, Panageas K, Adjuvant (adj) bevacizumab (B) plus dose-dense (dd) doxorubicin/cyclophosphamide (AC) followed by nanoparticle albumin- bound paclitaxel (nab-p) in early stage breast cancer (BC) patients (pts): cardiac safety. ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2007;25(Suppl 18S):567
- McArthur HL, Rugo H, Nulsen B, Cardiac safety of adjuvant bevacizumab (B) plus dose-dense doxorubicin/cyclophosphamide (AC) followed by nanoparticle albumin-bound paclitaxel (nab-P) in patients with early stage breast cancer [abstract 4104]. San Antonio, TX. Breast Cancer Symposium 10 – 15 December; 2008
- Pippen J, O'shaughnessy J, Krekow L, Adriamycin plus cytoxan followed by dose-dense nab-paclitaxel is safe in women with early-stage breast cancer: a pilot study [abstract 0151]. 11th International Conference on Primary Therapy of Early Breast Cancer; 11 – 14 March 2009; St Gallen, Switzerland
- Citron ML, Berry DA, Cirrincione C, Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431-9
- Pippen J, Paul D, Richards D, Dose-dense nab-paclitaxel vs paclitaxel with bevacizumab following adriamycin and cytoxan is safe as adjuvant therapy in patients with early-stage breast cancer [abstract 0152]. 11th International Conference on Primary Therapy of Early Breast Cancer; 11 – 14 March 2009; St Gallen, Switzerland
- Jones S, Holmes FA, O'shaughnessy J, Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol 2009;27:1177-83
- Raefsky E, Inhorn R, Lange M, Preliminary safety results from a multicentre phase II trial of nanoparticle-albumin bound paclitaxel/cyclophsphamide in early breast cancer plus trastuzumab in HER-+ patients (pts). J Clin Oncol 2009;27(Suppl 15s): abstract e11509
- Dranitsaris G, Coleman R, Gradishar W. nab-Paclitaxel weekly or every 3 weeks compared to standard docetaxel as first-line therapy in patients with metastatic breast cancer: an economic analysis of a prospective randomized trial. Breast Cancer Res Treat 2010;119(3):717-24
- Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv 2003;3:445-64
- Pinilla SM, Honrado E, Hardisson D, Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat 2006;99:85-90
- Savage K, Lambros MB, Robertson D, Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res 2007;13:90-101
- Savage K, Leung S, Todd SK, Distribution and significance of caveolin 2 expression in normal breast and invasive breast cancer: an immunofluorescence and immunohistochemical analysis. Breast Cancer Res Treat 2008;110:245-56
- Elsheikh SE, Green AR, Rakha EA, Caveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer 2008;99:327-34
- Witkiewicz AK, Dasgupta A, Sotgia F, An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol 2009;174:2023-34 Epub 2009 May 1
- Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer 2003;3:571-81
- Gradishar WJ, Tjulandin S, Davidson N, Superior efficacy of albumin-bound paclitaxel, ABI-007, compared with polyethylated castor oil-based paclitaxel in women with metastatic breast cancer: results of a Phase III trial. J Clin Oncol 2005;23(31):1-10
- Available from: http://nano.cancer.gov
