Abstract
Importance of the field: Current therapeutic options for advanced non-small-cell lung cancer (NSCLC) yield relatively modest improvements in survival leading to an ongoing search for new active treatment agents. In the past decade, pemetrexed has had an increasingly established role in the treatment of advanced NSCLC in both first- and second-line settings.
Areas covered in this review: Currently available published data on mechanism of action, pharmacokinetics, safety and efficacy of pemetrexed in the treatment of advanced NSCLC are described. Peer-reviewed publications on the development of pemetrexed and its clinical use in NSCLC were reviewed (1995 – 2009).
What the reader will gain: Pemetrexed is a multitargeted antifolate cytotoxic agent. Key Phase II and Phase III trials are described that have shown pemetrexed's efficacy in both the first- and second-line treatment of advanced NSCLC. The efficacy of pemetrexed seems to vary between squamous and nonsquamous histologies. Possible reasons for this are explored. Additionally, the potential role of pemetrexed in maintenance therapy is discussed.
Take home message: Pemetrexed is an effective treatment for advanced NSCLC, with an overall favorable toxicity profile. There is growing evidence that, in patients treated with pemetrexed, nonsquamous tumors have improved outcomes compared to squamous cell tumors. Pemetrexed may also have a role in maintenance therapy for NSCLC.
1. Introduction
Lung cancer is the leading cause of cancer death globally and in the USA Citation[1]. In the USA, there will be an estimated 219,000 new cases of lung cancer and 159,000 lung cancer deaths in 2009 Citation[2]. The overall 5-year survival rate for lung cancer is 15% Citation[3]. Non-small-cell lung cancer (NSCLC) comprises more than 80% of lung cancer Citation[4]. For the ∼ 50% of patients who present with metastatic disease, or stage III disease that is not amenable to curative therapy, palliative chemotherapy is the mainstay of treatment. A meta-analysis of 16 randomized trials demonstrated survival improvements of 6 – 10 weeks in those who receive chemotherapy compared with supportive care Citation[5]. Current guidelines recommend first-line therapy with two-agent, platinum-based cytotoxic therapy in patients with good performance status Citation[6], based on studies that have shown similar efficacy amongst varying regimens with general overall response rates of 17 – 32%, median time to progression of 3 – 5 months and median survival of 8 – 11 months Citation[7-9]. While chemotherapy has also been shown to effect a survival and quality of life advantage in the second line, the gains are similarly modest Citation[10].
An increased understanding of the molecular basis of lung cancer holds great promise for the advent of ‘personalized’ management for individual patients based on their specific tumor characteristics. For example, tumor expression of genes such as RRM1, the gene that encodes the regulatory subunit of ribonucleotide reductase, and the excision repair cross-complementation group 1 gene (ERCC1), have predictive importance, and molecular markers such as epidermal growth factor receptor (EGFR) are now treatment targets Citation[11,12]. Given these discoveries and the lack of highly effective treatments so far, multiple new agents are being tested in the treatment of NSCLC in both first- and second-line settings. Pemetrexed (), a relatively new treatment with a particular role in the treatment of nonsquamous tumors, is the subject of this review.
Box 1. Drug summary.
2. Introduction to pemetrexed
2.1 Mechanism of action
Folate is an essential element for cell division by serving as a coenzyme in multiple metabolic pathways that lead to synthesis of DNA. These paths accept or donate one-carbon units in what is collectively referred to as ‘one-carbon metabolism’ Citation[13]. Reactions that require folate are essential for purine and pyrimidine base synthesis, upon which cancer cells are dependent for rapid proliferation Citation[14]. The use of antifolates in cancer treatment traces back decades to the development and use of the folate analogs aminopterin and, subsequently, methotrexate Citation[15,16].
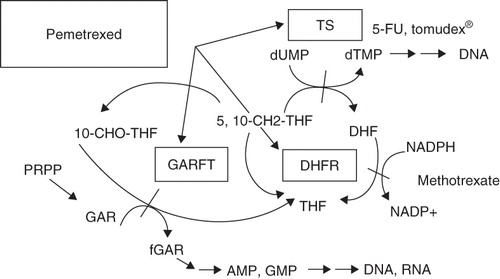
More recently in the 1990s, newer antifolates were developed Citation[17]. Pemetrexed is a folate analog that competes with reduced folate for binding sites, thereby significantly disrupting the activity of multiple folate-requiring enzymes: dihydrofolate reductase (DHFR), glycinamide ribonucleotide formyltansferase (GARFT) and thymidylate synthase (TS) Citation[18-20]. DHFR, which is the primary target of methotrexate, is required for the synthesis of both purines and pyrimidines. Thymidylate synthase, which is also the target of the antimetabolite chemotherapeutic agent 5-fluorouracil, catalyses the transformation of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), which then allows for thymidine synthesis. Thymidylate synthase seems to be the primary target of pemetrexed. Glycinamide ribonucleotide formyltansferase is required for synthesis of purines and was the original target of the investigators developing the newer antifolates (see ). Additionally, recent cell culture experiments have indicated that another folate-dependent enzyme in purine synthesis, aminoimidazolecarboxamide ribonucleotide formyltransferase (AICART), is a secondary target for pemetrexed. In tumor cells where AICART is inhibited, there is accumulation of substrates that cause subsequent inhibition of the mammalian target of rapamycin (mTOR), thereby impeding protein synthesis and cell growth Citation[21]. The varying enzymatic profiles of different tumor lines may have implications for clinical efficacy differences that have been seen based on histology, as will be discussed later.
Pemetrexed is taken into the cell by the reduced folate carrier and then undergoes polyglutamation by folypolyglutamate synthase (FPGS). In in vitro studies, compared with the parent drug, the polyglutamated form of pemetrexed had a 100-fold and 140-fold increase in its ability to inhibit thymidylate synthase and GARFT, respectively Citation[19]. The polyglutamation contributes to increased retention of the drug in cells allowing for prolonged suppression of the target enzymes Citation[22].
2.2 Pharmacokinetics
Initial, single-agent, Phase I dose-escalation studies of pemetrexed determined that a dose of 600 mg/m2 administered over 10 min every 21 days was optimal for Phase II studies. Neutropenia, thrombocytopenia and cumulative fatigue were the dose-limiting toxicities in the study by Rinaldi et al. Citation[23-25]. Neutropenia was a dose-limiting toxicity in the two earlier trials as well. Patients who had a creatinine clearance of < 45 ml/min using the modified Cockcroft and Gault formula were excluded Citation[22]. At the 600-mg/m2 dose, the population pharmacokinetics had a harmonic mean half-life of 3.08 h, an area under the curve (AUC) of 266 μg/h/ml, a mean clearance of 40 ml/min/m2 and a volume of distribution of 7.0 l/m2. The primary route of elimination of the drug was renal excretion with 78% of the drug excreted unchanged in the urine at 24 h.
Importantly, the earliest trials were done without supplementation of folate or vitamin B12. As will be discussed in more detail below, subsequent Phase II trials demonstrated that vitamin supplementation reduced the myelosuppression of pemetrexed and therefore supplementation became standard. Subsequently, additional Phase I trials were done with vitamin supplementation. These studies did not show a significant change in the pharmacokinetic parameters from the Rinaldi study but did establish higher maximum tolerated doses Citation[26,27]. However, as will be discussed further in the next section, subsequent studies at higher doses did not yield improved outcomes.
2.3 Safety and tolerability
The importance of vitamin B12 and folate supplementation emerged after early studies of pemetrexed were marked by significant grade 3 or 4 myelosuppression Citation[28-32]. To evaluate the etiology of this toxicity profile, a multivariate analysis was conducted on 246 patients treated with pemetrexed between 1995 and 1999 Citation[33]. Based on a hypothesis generated by studies in lometrexol that had shown a connection between folate intake and toxicity, the authors demonstrated that pretreatment plasma homocysteine and methylmalonic acid concentrations significantly predicted severe hematological toxicity as well as infection, mucositis, diarrhea and death in patients taking pemetrexed. Thus, as noted above, since December 1999, folic acid and vitamin B12 supplementation have been included in all pemetrexed trials. Two trials evaluating pemetrexed in malignant pleural mesothelioma that incorporated supplementation midway confirmed the significant reduction in toxicity in the patients who received vitamins compared with those who did not receive vitamins, without diminished efficacy Citation[34,35].
Given that nonsteroidal anti-inflammatory drugs (NSAIDs) compete with the antifolate methotrexate, trials of pemetrexed often excluded patients requiring chronic use of aspirin or NSAIDs. Therefore Sweeney et al. did a drug-interaction study, demonstrating that pemetrexed at 500 mg/m2 when given with vitamin supplementation did not require dosage adjustment when used with moderate doses of aspirin (1.3 g/day) in patients with creatinine clearance of ≥ 60 ml/min or with moderate doses of ibuprofen (400 mg every 6 h) in patients with creatinine clearance ≥ 80 ml/min Citation[36]. The authors cautioned against using NSAIDs and pemetrexed concurrently in patients with renal impairment.
A more recent Phase I trial was performed with vitamin supplementation to determine the safety and pharmacokinetics in patients with normal or impaired renal function Citation[37]. The study showed substantial reduction in drug clearance for patients with diminished renal function; however, the increase in systemic exposure was not associated with an increase in drug-related dose-limiting toxicities for vitamin-supplemented patients with glomerular filtration rates (GFR) of ≥ 40 ml/min receiving the 500-mg/m2 dose, though the authors note that calculated creatinine clearance may not exactly correlate with GFR Citation[37].
Rash was also frequently reported in early trials, but improved significantly with routine administration of prophylactic corticosteroid. There has been a case report of pemetrexed associated typhlitis Citation[38].
3. Clinical efficacy and trial data
3.1 Pemetrexed as second-line therapy
Based on the promising activity of pemetrexed, albeit without vitamin supplementation, initial Phase II trials were conducted at a dose of 600 mg/m2. However, owing to excess toxicity in early Phase II trials in both NSCLC and colorectal cancer, the starting dose was reduced to 500 mg/m2 Citation[28,39]. Smit et al. conducted a Phase II trial of pemetrexed 500 mg/m2 in 81 pretreated patients without vitamin supplementation broken into cohorts based on whether or not previous treatment had contained platinum Citation[32]. The overall response rate was 8.9% with a median time to progression (TTP) of 2 months. The response rate was 4.5% in patients who had received previous platinum versus 14.1% in patients who had received non-platinum regimens, but this was not statistically significant. These results led to a Phase III trial by Hanna et al. in previously treated patients Citation[40]. Patients were randomized to receive pemetrexed 500 mg/m2 (n = 283) or docetaxel 75 mg/m2 (n = 288) every 3 weeks Citation[40]. The response rates were similar between the pemetrexed and docetaxel groups at 9.1 and 8.8% respectively. The median progression-free survival (PFS) and 1-year survival were identical for both groups at 2.9 months and 29.7%. Overall survival, the primary end point, was similar for the two arms: 8.3 and 7.9 months. Noninferiority of pemetrexed could not be established based on the prespecified upper bound of the 95% confidence interval to be less than 1.11 for the hazard ratio (HR = 0.99; 95% CI 0.82 – 1.2; noninferiority: p = 0.226). However, pemetrexed met another prespecified efficacy requirement. Using the percentage retention method, it retained the survival benefit of docetaxel when the latter was compared with best supportive care in a different trial Citation[41]. While there was no significant difference between the two arms in reported changes in quality of life, the toxicity analysis demonstrated significantly fewer adverse effects with pemetrexed compared with docetaxel, including grade 3 or 4 neutropenia (5.3 vs 40.2%), febrile neutropenia (1.9 vs 12.7%), hospitalizations for neutropenic fever (1.5 vs 13.4%) and use of granulocyte colony-stimulating factor support (2.6 vs 19.2%). This trial led to the approval of pemetrexed as monotherapy in the second-line setting.
Weiss et al. retrospectively examined whether the elderly patients (age ≥ 70 years) who comprised 15% of the above study population differed in outcomes compared with younger patients Citation[42]. There were no significant differences in efficacy (response rate, TTP, overall survival) or toxicity between elderly and younger patients. Both elderly and younger patients experienced a more tolerable toxicity profile with pemetrexed compared with docetaxel. Postregistration studies have shown similar degrees of efficacy and tolerability. Bearz et al. reported a response rate of 11.2%, a median PFS of 3.0 months, and a median overall survival of 12 months in 160 advanced NSCLC patients treated in Italy Citation[43]. Toxicity was mild with less than 2% of patients experiencing any grade 3 or 4 toxicity. Lee et al. performed a study in a Korean population (n = 81) and reported an overall response rate of 5.1%, median time to progression of 3.1 months and an overall survival of 7.8 months Citation[44].
After the advent of standard vitamin supplementation, Phase I trials indicated that doses greater than 500 mg/m2 could be tolerated Citation[26]. Ohe et al. carried out a randomized Phase II comparing doses of 500 and 1000 mg/m2 in 225 previously treated Japanese patients Citation[45]. Comparing the 500-mg/m2 arm with 1000-mg/m2, the response rate was 18.5 versus 14.8% and the median survival time was 16.0 versus 12.6 months respectively. The safety profile of the 500-mg/m2 arm showed generally milder side effects. That 500 mg/m2 should remain the standard dose of pemetrexed was supported by a subsequent Phase III trial published in 2008 comparing 500 mg/m2 with 900 mg/m2 involving 588 patients Citation[46]. Accrual was terminated early after an interim analysis indicated a low probability of improved survival and a numerically increased toxicity in the higher dose arm.
Recently, a Phase II trial exploring the role of combination chemotherapy with pemetrexed in the second-line setting following first-line platinum therapy was published Citation[47]. Two hundred and forty patients were randomized to receive either pemetrexed 500 mg/m2 or pemetrexed plus carboplatin with an AUC of 5. The primary end point was PFS. Median PFS was significantly longer in the combination arm: 4.2 months compared with 2.8 months for pemetrexed alone. There was slightly more grade 3 and 4 hematological toxicity for the combination arm compared with pemetrexed alone with differences in neutropenia (21 vs 7%) and thrombocytopenia (15 vs 2%) being statistically significant. Given an increasing interest in the role of histology in predicting response to pemetrexed (which will be discussed further below), this study also sought to explore genetic polymorphisms that might correlate with outcome in patients treated with pemetrexed.
Notable trials of pemetrexed in the second line are summarized in .
Table 1. Notable trials of pemetrexed in second-line treatment of advanced NSCLC.
3.2 Pemetrexed as first-line therapy
Two studies, done without vitamin supplementation, established the role of pemetrexed as an active single agent in the first-line treatment of NSCLC. Rusthoven et al. enrolled 33 total patients and used the 500-mg/m2 dose following treatment of the first three patients at 600 mg/m2 Citation[28]. There was a 23.3% overall response rate with a median TTP of 3.8 months and median survival of 9.2 months. Thirty-nine per cent of patients had grade 3 or 4 neutropenia, 12% had febrile neutropenia, 39% had grade 3 rash, and 27% had grade 3 lethargy. A retrospective analysis demonstrated that prophylactic steroids reduced the frequency of rash (47.5% in patients without prophylaxis vs 12% of patients who received steroids). Another single-agent study used 600 mg/m2 in 59 patients and showed a 15.8% response rate, median TTP of 4.4 months, median overall survival of 7.2 months and a 1-year survival rate of 32% Citation[31]. Significant toxicities included grade 3 or 4 neutropenia (42% of patients), and reversible grade 3 or 4 liver enzymes elevations (24% of patients). Grade 3 or 4 cutaneous toxicity was seen in 31% of patients but was again noted to improve with administration of steroids in subsequent cycles.
Based on these promising efficacy results, additional studies sought to use pemetrexed in combination with other cytotoxic therapies in the first line. Two Phase II studies, again without vitamin supplementation, combined pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 every 3 weeks. Manegold enrolled 36 patients and showed a response rate of 39% with a median TTP of 6.3 months and median survival time of 10.9 months Citation[29]. Fifty-nine per cent of patients developed grade 3 or 4 neutropenia and 17% developed grade 3 or 4 thrombocytopenia. Shepherd et al. enrolled 31 patients showing a 45% response rate and a median survival of 8.9 months Citation[30]. Grade 3 or 4 neutropenia developed in 37% of patients. (Of note, all subsequent trials discussed in this review incorporated vitamin supplementation.)
Other platinum-based combinations have also been explored to see if the apparent efficacy of combination cisplatin therapy could be achieved with other, potentially less toxic, platinum agents. Zinner et al. administered pemetrexed 500 mg/m2 in combination with carboplatin AUC 6 every 3 weeks to 50 patients. Overall response rate was 24% with a median TTP of 5.4 months and median survival of 13.5 months Citation[48]. Grade 3 or 4 neutropenia developed in 26% of patients. A randomized Phase II trial compared pemetrexed 500 mg/m2 plus oxaliplatin 120 mg/m2 (PemOx) to pemetrexed 500 mg/m2 plus carboplatin AUC of 6 (PemCb), each given every 3 weeks Citation[49]. Forty-one and thirty-nine patients were randomized to each arm respectively. Results were similar between the PemOx and PemCb arms in objective response rate (26.8 vs 31.6%, respectively), median TTP (5.5 vs 5.7 months, respectively) and median overall survival (10.5 months for each arm). Of note, patient compliance with quality-of-life assessment questionnaires was quite high in this study. Approximately 60% of patients in each arm documented either stable or improved symptom metrics. Neutropenia was the most prevalent toxicity with grade 3 or 4 toxicity occurring in 7.3% of PemOx patients and 25.7% of PemCb patients. Neurotoxicity was generally mild with only one patient experiencing more than grade 2 neuropathy in the PemOx group.
Other trials combined pemetrexed with nonplatinum cytotoxic therapy. Stathopoulos did a Phase I/II trial combining pemetrexed with paclitaxel Citation[50]. Based on the Phase I portion of the study, pemetrexed 500 mg/m2 plus paclitaxel 175 mg/m2 was administered to 48 patients every 3 weeks. The overall response rate was 39.6% with a median TTP of 7 months and a median survival of 14 months. Toxicity was notably mild, with 8% of patients experiencing grade 3 or 4 neutropenia and no other significant grade 3 or 4 toxicities. Vinorelbine was combined with pemetrexed in a Phase I/II study by Clarke et al. Citation[51]. Based on the Phase I results, 37 patients were enrolled in the Phase II study and given 500 mg/m2 of pemetrexed on day 1 and 30 mg/m2 of vinorelbine on day 1 and 8 every 3 weeks. The response rate was 38%, median PFS 4.2 months and the overall median survival 7.9 months. Toxicity data were notable for the development of grade 3 or 4 neutropenia in 65% of patients, grade 3 or 4 febrile neutropenia in 11% of patients and grade 3 or 4 fatigue in 27% of patients. The authors noted that, because of the modest overall survival figure, additional Phase III studies would not be pursued with this combination.
Given gemcitabine's activity in NSCLC, a series of trials sought to combine it with pemetrexed and to compare it with platinum-based regimens. Monnerat et al. published a Phase II trial using day 1 gemcitabine 1250 mg/m2 and then day 8 gemcitabine followed 90 min later by pemetrexed 500 mg/m2 in 60 patients on a 3-week cycle Citation[52]. (Vitamin supplementation was started midway through this trial.) The overall response rate was 15.5% with a median PFS of 5.0 months. The majority of patients also reported improvement or stability in their Lung Cancer Symptom Scale assessments in anorexia, fatigue, cough, dyspnea, hemoptysis and pain. Grade 3 or 4 neutropenia developed in 62% of patients, with 15% having grade 3 or 4 febrile neutropenia. Grade 3 or 4 fatigue occurred in 23% of patients. Ma et al. conducted a Phase II trial that examined three different schedules of gemcitabine combined with pemetrexed Citation[53]. One hundred and fifty-two patients were randomized to receive day 1 pemetrexed 500 mg/m2 followed by gemcitabine 1250 mg/m2 and then day 8 gemcitabine; or day 1 gemcitabine followed by pemetrexed and day 8 gemcitabine; or day 1 gemcitabine and day 8 pemetrexed followed by gemcitabine. The second arm was closed after an interim analysis showed inferior efficacy. There was a rate of 64% grade 3 or 4 neutropenia with 5% of patients having grade 3 or 4 febrile neutropenia and 8.5% grade 3 or 4 thrombocytopenia in the first arm compared with 69%, 5 and 19%, respectively, in the third arm. The authors concluded that the first schedule demonstrated equivalent efficacy, with a median TTP of 11.4 months, but with a lower toxicity profile compared with the third arm.
Based on pharmacokinetic data that indicated that the 90-min delay between pemetrexed and gemcitabine was not required, two trials sought to determine whether the gemcitabine and pemetrexed could be administered in a more rapid sequence. Treat and colleagues administered gemcitabine 1250 mg/m2 on days 1 and 8, with immediately sequenced pemetrexed 500 mg/m2 on day 1 every 3 weeks to 53 patients Citation[54]. The overall response rate was 30.2%, median TTP 3.3 months and median survival 10.3 months. Grade 3 or 4 neutropenia was seen in 43% of patients; grade 3 or 4 febrile neutropenia occurred in 7.5% of patients; and grade 3 or 4 fatigue occurred in 11% of patients. West and colleagues did a similar study looking at rapid succession dosing Citation[55]. Fifty-four patients were enrolled in a Phase II trial that administered gemcitabine 1250 mg/m2 on days 1 and 8 with pemetrexed immediately following the day 8 gemcitabine dose. The response rate was 13%, median PFS 4.6 months and median overall survival 12.4 months. Grade 3 or 4 neutropenia occurred in 40% of patients; grade 3 or 4 febrile neutropenia occurred in 11% of patients; and grade 3 or 4 thrombocytopenia occurred in 11% of patients. Twenty-one per cent of patients experienced grade 3 or 4 fatigue. The results indicated that the more convenient dosing schedule did not significantly alter efficacy or toxicity. Additional studies looked at different schedule combinations of pemetrexed and gemcitabine, though no specific schedule or frequency of doses emerged as clearly superior Citation[56-58].
Two large Phase III trials studying pemetrexed in the first-line setting have been published. Scagliotti et al. conducted a noninferiority study randomizing 1725 patients to compare day 1 cisplatin 75 mg/m2 plus gemcitabine 1250 mg/m2 on days 1 and 8 with a regimen of day 1 cisplatin 75 mg/m2 plus pemetrexed 500 mg/m2, all given every 3 weeks for up to six cycles Citation[59]. The primary end point was overall survival. Overall survival for patients in the cisplatin/pemetrexed group was noninferior compared with cisplatin/gemcitabine, with a median overall survival of 10.3 months for each arm (HR = 0.94; 95% CI 0.84 – 1.05). Survival rates for the cisplatin/pemetrexed arm at 12 and 24 months were 43.5 and 18.9% respectively, compared with 41.9 and 14.0% for the cisplatin/gemcitabine arm. Progression-free survival in the cisplatin/pemetrexed arm was also noninferior compared with the cisplatin/gemcitabine arm, with a time of 4.8 months and 5.1 months respectively. Grade 3 or 4 drug-related toxicities were significantly lower for cisplatin/pemetrexed compared with cisplatin/gemcitabine (neutropenia: 15 vs 27%; thrombocytopenia: 4 vs 13%; p ≤ 0.001). Grade 3 or 4 nausea was significantly more common in the cisplatin/pemetrexed arm compared with cisplatin/gemcitabine (7.2 vs 3.9%; p = 0.004). Of note, this trial included a prespecified analysis of survival by histological subtype, the first such prospective analysis. The effect on survival of cisplatin/pemetrexed relative to cisplatin/gemcitabine was significantly different according to nonsquamous versus squamous histology, as will be discussed further in the next section. Factors that had a statistically significant prognostic impact on survival independent of treatment included: sex, race, performance status, disease stage and histology. The authors noted in their conclusions that the modest improvement in overall survival seen in this study compared with previous studies with platinum may reflect improvements in NSCLC staging, a relatively higher proportion of stage IIIB patients and the exclusion of patients with an ECOG performance status of 2.
Recently, Gronberg and colleagues in Norway published a Phase III trial that compared pemetrexed 500 mg/m2 plus carboplatin AUC 5 on day 1 with a regimen of days 1 and 8 gemcitabine 1000 mg/m2 plus carboplatin AUC 5 on day 1, both given every 3 weeks for up to four cycles Citation[60]. The primary end point was health-related quality of life (with a focus on global quality of life, nausea/vomiting, fatigue and dyspnea), with secondary end points of overall survival, safety and tolerability. Four hundred and forty-six patients were randomized. Of note, 22% of patients in the pemetrexed/carboplatin arm and 23% of patients in the gemcitabine/carboplatin arm had an ECOG performance status of 2. There was a high rate (87%) of completion of the quality-of-life questionnaires for this study. There was no significant difference found between the two arms in quality-of-life end points. There was no difference in median overall survival between the pemetrexed/carboplatin group versus the gemcitabine/carboplatin (7.0 vs 7.3 months, respectively) or in 1-year survival rate (34 vs 31%, respectively). Patients in the gemcitabine/carboplatin arm, compared with the pemetrexed/carboplatin arm, had significantly more grade 3 or 4 neutropenia (51 vs 40%; p = 0.024), grade 3 or 4 thrombocytopenia (56 vs 24%; p < 0.001), need for transfusions of red blood cells (43 vs 29%; p = 0.003) and platelets (9 vs 3%; p = 0.007). There was no significant difference in the frequency of any single grade 3 or 4 nonhematologic toxicity, but more patients on the gemcitabine/carboplatin arm had one or more grade 3 or 4 toxicities (28 vs 19% for pemetrexed/carboplatin; p = 0.037).
Notable trials of pemetrexed in the first line are summarized in and .
Table 2. Phase III trials of pemetrexed in the first-line treatment of NSCLC.
Table 3. Notable Phase II trials of pemetrexed in the first line.
4. Impact of histology
An intriguing aspect in the development of pemetrexed has been the role of histology in predicting efficacy. Historically, different histologies (adenocarcinoma, large cell carcinoma, squamous carcinoma) in NSCLC have not been considered predictive of response to treatment, though studies were seldom designed in a manner to allow subset analyses of this nature Citation[61,62]. However, retrospective and subgroup analyses of pemetrexed trials suggested a differing response amongst patients with squamous versus nonsquamous histology. In their Phase II trial examining different doses of pemetrexed in the second line, Ohe et al. reported that the median survival time of patients with nonsquamous carcinoma was significantly longer compared with squamous cell carcinoma (16.0 vs 9.3 months; p = 0.0026) Citation[45]. The trial was not designed for subgroup analyses but the results were provocative. Peterson and colleagues performed a retrospective analysis on the large Phase III trial conducted by Hanna et al., to determine the impact of histology Citation[63]. A treatment-by-histology interaction was statistically significant, demonstrating that patients with nonsquamous histology had higher survival compared with all others on trial. Docetaxel's efficacy, however, did not significantly differ between squamous and nonsquamous groups.
In the recent Phase II study combining gemcitabine and pemetrexed, West et al. noted modestly superior efficacy results for pemetrexed in nonsquamous patients compared with the squamous group with a median PFS of 5.4 versus 4.0 months respectively.
Given the interest in the role of histology, Scagliotti and colleagues designed their large Phase III trial to include a prespecified analysis of overall survival by histology, though the patients were not randomized by histology Citation[59]. In the 847 patients with adenocarcinoma, there was significantly improved survival in the cisplatin/pemetrexed arm compared with the cisplatin/gemcitabine arm (12.6 vs 10.9 months, respectively; HR = 0.84; 95% CI 0.71 – 0.99; p = 0.03). However, in the 473 patients with squamous cell carcinoma the opposite trend was seen, with improved survival in the cisplatin/gemcitabine arm compared with the cisplatin/pemetrexed arm (10.8 vs 9.4 months, respectively; HR = 1.23; 95% CI 1.00 – 1.51; p = 0.05). To assess further the role of histology, Scagliotti et al. conducted a retrospective review of the interaction of histology and efficacy using data from this trial as well as from the earlier Phase III trial by Hanna et al. that looked at pemetrexed in the second line Citation[40,64]. Cox proportional hazard models were used to test for covariate-adjusted treatment-by-histology interactions. Analysis showed that the treatment arms in both studies were well balanced for histology, though the Hanna study had some numerical imbalances in other prognostic factors across histologic subgroups, including gender, stage and performance status. The statistically adjusted results indicated that overall there was significant treatment-by-histology interaction for both overall survival and PFS. In the recent Phase III trial by Gronberg et al., comparing gemcitabine/carboplatin with pemetrexed/carboplatin, a subgroup analysis did not find any association between survival and histology, though this was not a prespecified subgroup analysis Citation[60]. Overall, the results led to a modification in the approval for pemetrexed, whereby its indications stipulate that it should be used in NSCLC of a histological subtype other than squamous cell. Results of selected studies that have analyzed the impact of histology are summarized in .
Table 4. Results from analyses of histology in selected trials of pemetrexed.
One of the proposed mechanisms to explain the varying efficacy by histological subtype is differences in the expression of thymidylate synthase (TS), which is the primary enzymatic target of pemetrexed, in different tumor histologies. While results have been at times inconsistent, higher levels of TS have been shown to predict poor clinical outcome in patients treated with 5-fluorouracil chemotherapy regimens in earlier studies Citation[65]. In NSCLC, tumor cells with higher TS expression have higher proliferative activity Citation[66], which may predict worse clinical outcome Citation[67,68]. A study by Ceppi and colleagues demonstrated significantly higher levels of TS expression in squamous cell carcinoma than in adenocarcinoma Citation[69]. Preclinical data have indicated a reduced activity of pemetrexed in cells with high expression of TS Citation[70]. Given that functional gene polymorphisms of TS have been correlated with outcome in patients receiving methotrexate, Smit et al. assessed pemetrexed drug pathway-associated gene polymorphisms as part of their Phase II study comparing pemetrexed with pemetrexed plus carboplatin in the second line Citation[47]. Blood samples from 127 patients with baseline characteristics similar to the study population were collected. They did not find a correlation between high and low TS expression genotype and tumor histology leading to the authors' recommendation that real-time PCR be used to evaluate for levels of TS in tumor cells. They did find that patients with homozygous mutations for methylenetetrahydrofolate reductase C677T allele had a significant correlation with improved clinical outcome.
5. Role in maintenance therapy
There is no accepted consensus definition as of yet of maintenance therapy in NSCLC. Some authorities suggest that early second-line therapy implies interval progression, whereas maintenance implies continuation of the original regimen Citation[71]. Regardless, maintenance therapy in its most basic form can be thought of as a continuation of therapy beyond a set number of cycles (typically 4 – 6 cycles in the first-line setting for NSCLC) after the initial regimen has achieved a response. The role of maintenance therapy in the treatment of advanced NSCLC has not been clearly defined and there have been few trials to guide the decision of whether or not to continue chemotherapy beyond a specified number of initial cycles Citation[71,72]. A meta-analysis showed that continuing treatment beyond a set number of cycles provided significant improvement in PFS but a modest improvement in overall survival and possible impairments in health-related quality of life Citation[73]. In a recent, double-blind, Phase III trial of pemetrexed in the maintenance setting for advanced lung cancer, 441 patients were randomized 2:1 to either pemetrexed 500 mg/m2 or best supportive care following four cycles of platinum every 3 weeks until disease progression Citation[74]. Patients in the pemetrexed arm had significantly better overall survival (13.4 vs 10.6 months; HR = 0.79, 95% CI 0.65 – 0.95, p = 0.012) and PFS (4.3 vs 2.6 months; HR = 0.50, p < 0.0001). The improvements in efficacy were seen primarily in patients with nonsquamous histology. In terms of drug-related adverse effects, grade 3 or 4 toxicities in general were higher in the pemetrexed compared with the supportive care arm (16 vs 4%, respectively p < 0.0001) and there was significantly more grade 3 or 4 neutropenia (2.9 vs 0%, respectively) and fatigue (5 vs 0.5%, respectively). Based on this recent study, pemetrexed was approved for use in the maintenance setting for patients with NSCLC who have not had disease progression following four cycles of initial platinum-based therapy that did not include pemetrexed.
6. Regulatory affairs
At present, pemetrexed is approved in the USA and Europe for the following indications in NSCLC Citation[75,76]:
In combination with cisplatin in the first-line treatment of locally advanced or metastatic NSCLC other than predominantly squamous histology
As monotherapy for second-line treatment in patients with locally advanced or metastatic NSCLC other than predominantly squamous histology.
Additionally, in the USA pemetrexed is approved for the indication Citation[75]:
As maintenance treatment in patients with NSCLC who have not had disease progression after four cycles of platinum-based first-line chemotherapy.
7. Conclusions
Pemetrexed is an efficacious agent in the treatment of advanced NSCLC. Trials have shown its activity in both first- and second-line settings with an overall favorable toxicity profile. Additionally, there is evidence that patients with nonsquamous tumors have improved outcomes with pemetrexed compared with their counterparts with squamous cell cancer.
8. Expert opinion
The treatment options for patients with metastatic lung cancer have increased over the last decade. Various combination regimens have resulted in improved outcomes, with median survival of 1 year and a substantial minority of patients living 2 years and longer. Given the efficacy and tolerability of pemetrexed in the treatment of advanced NSCLC, current and upcoming studies seek to combine it with newer targeted and biological agents. A Phase II trial using pemetrexed plus bevacizumab every 3 weeks in the second line had PFS of 4.0 months and overall survival of 8.6 months Citation[77]. Another Phase II trial in the first-line treatment was recently published that combined pemetrexed, carboplatin and bevacizumab for six cycles followed by pemetrexed and bevacizumab until disease progression Citation[78]. There was a 55% response rate with a PFS of 7.8 months and a median survival of 14.1 months with tolerable toxicity. Other Phase II and III trials using pemetrexed and bevacizumab are now underway Citation[79,80], including the Phase III PointBreak study, which will compare pemetrexed/carboplatin/bevacizumab followed by pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by bevacizumab, with overall survival as the primary outcome Citation[81]. The first-line use of carboplatin/pemetrexed/bevacizumab seems to be increasing, but we should exercise caution and await the results of these comparative studies.
Erlotinib, docetaxel and pemetrexed are approved agents by the FDA for use in previously treated patients with advanced NSCLC. At present, multiple Phase III trials are underway comparing erlotinib and pemetrexed in pretreated advanced NSCLC patients Citation[82,83]. Another Phase II trial is comparing erlotinib versus pemetrexed plus erlotinib in the second line Citation[84].
Studies discussed in this review suggest that histology plays a key role in predicting the response to pemetrexed and that it is particularly effective in nonsquamous tumors. The strongest comparison is with gemcitabine. The histology data for docetaxel presented above are based on retrospective subset analyses and no such information is available for paclitaxel, vinorelbine or erlotinib. Therefore, in practical terms, we know that pemetrexed should not be used in patients with squamous cell lung cancer, but we still do not have adequate means to select from among the other treatment options. While there are hypotheses trying to explain the histology findings, it seems likely that a better understanding of the unique molecular underpinnings of a given tumor will be ultimately more important than the relatively crude histopathologic assessment. The promise of individually ‘tailored’ therapy is great, though it is not fully realized as yet.
The use of pemetrexed in a maintenance setting is also intriguing, though more studies are needed to establish more clearly this new treatment paradigm. One concern regarding the results of the study by Ciuleanu et al. is whether the finding of increased overall survival with maintenance therapy represents a true impact of the ongoing therapy as opposed to reflecting that patients with immediate access to effective therapy tend to do better than patients who have a delay and are therefore less likely ever to receive any additional therapy Citation[71,85]. In the arm randomized to pemetrexed maintenance, 98% received second-line therapy with pemetrexed and 51% received poststudy treatment; whereas in the placebo arm only 67% of patients received poststudy treatment and only 18% received pemetrexed. In a trial of immediate versus delayed docetaxel following first-line chemotherapy, there was improved PFS, but not overall survival. Only two-thirds of the patients on the delayed arm received docetaxel. When the study results were analyzed based on the subsets of patients who actually received docetaxel, the overall survival was identical Citation[86]. Thus, it may be more important to monitor patients closely so that second-line treatment is initiated before clinical deterioration rather than rigidly apply the maintenance approach. Accordingly, multiple studies are now recruiting patients to explore the role of maintenance in greater depth, including the aforementioned PointBreak trial Citation[81]. A randomized, double-blind, placebo-controlled, Phase III trial is looking at PFS in patients with advanced nonsquamous NSCLC treated with pemetrexed plus cisplatin followed by either pemetrexed maintenance or best supportive care Citation[87]. Another Phase III trial seeks to compare pemetrexed plus carboplatin followed by maintenance pemetrexed versus paclitaxel plus carboplatin plus bevacizumab followed by maintenance bevacizumab in advanced nonsquamous tumors Citation[88]. Selected ongoing trials of pemetrexed are described in .
Table 5. Selected ongoing trials involving pemetrexed in advanced NSCLC.
Despite the grim prognosis for advanced NSCLC, incremental improvements in outcomes are being realized with new cytotoxic and biologic/targeted therapies. Given its good efficacy, and relatively mild toxicity profile, pemetrexed seems to be an important therapeutic option in the first- and second-line setting of advanced NSCLC and potentially in the maintenance setting as well. Many studies are now underway to define better its role in the treatment of NSCLC. Additional assessments of its efficacy, particularly in poorer performance-status patients, but also of its impact on quality of life and its cost effectiveness, will be helpful in allowing a clinician to choose most appropriately from the growing number of treatment options available.
Declaration of interest
KH Dragnev has received speaking and consulting fees from Eli Lilly. JR Rigas and AD Fuld declare no conflicts of interest.
Notes
Bibliography
- Garcia M, Jemal A, Ward E, Global Cancer Facts & Figures 2007. Available from: http://www.cancer.org/downloads/STT/Global_Facts_and_Figures_2007_rev2.pdf [Last accessed March 2010]
- Jemal A, Siegel R, Ward E, Cancer Statistics, 2009. CA Cancer J Clin 2009;59:225-49
- Horner M, Ries L, Krapcho M, Neyman N, editors. SEER Cancer Statistics Review, 1975-2006. Available from: http://seer.cancer.gov/csr/1975_2006/ [Last accessed 7 November 2009]
- Yang P, Allen MS, Aubry MC, Clinical features of 5,628 primary lung cancer patients. Chest 2005;128:452-62
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25
- Azzoli CG, Baker S Jr, Temin S, American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage i.v. non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66
- Schiller JH, Harrington D, Belani CP, Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8
- Scagliotti GV, De Marinis F, Rinaldi M, Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285-91
- Fossella F, Pereira JR, von Pawel J, Randomized, multinational, Phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. Jf Clin Oncol 2003;21:3016-24
- Noble J, Ellis PM, Mackay JA, Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2006;1:1042-58
- Zheng Z, Chen T, Li X, DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 2007;356:800-8
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80
- Bailey LB, Gregory JF III. Folate metabolism and requirements. J Nutr 1999;129:779-82
- Saif MW, Chu E. Antimetabolites. In: DeVita VT Jr, Rosenberg AE, Lawrence TS, editors. Cancer: Principles & Practice of Oncology, 8th edition. Philadelphia: Lippincott, Williams & Wilkins, 2008. p. 427-36
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 2007;6:404-17
- Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948;238:787-93
- Calvert AH, Walling JM. Clinical studies with MTA. Brit J Cancer 1998;78(Suppl 3):35-40
- Shih C, Chen VJ, Gossett LS, LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res 1997;57:1116-23
- Mendelsohn LG, Shih C, Chen VJ, Enzyme inhibition, polyglutamation, and the effect of LY231514 (MTA) on purine biosynthesis. Semin Oncol 1999;26:42-7
- Schultz RM, Patel VF, Worzalla JF, Shih C. Role of thymidylate synthase in the antitumor activity of the multitargeted antifolate, LY231514. Anticancer Res 1999;19:437-43
- Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res 2009;69:5467-74
- Hanauske AR, Chen V, Paoletti P, Niyikiza C. Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 2001;6:363-73
- Rinaldi DA, Burris HA, Dorr FA, Initial Phase I evaluation of the novel thymidylate synthase inhibitor, LY231514, using the modified continual reassessment method for dose escalation. J Clin Oncol 1995;13:2842-50
- McDonald AC, Vasey PA, Adams L, A Phase I and pharmacokinetic study of LY231514, the multitargeted antifolate. Clin Cancer Res 1998;4:605-10
- Rinaldi DA, Kuhn JG, Burris HA, A Phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 1999;44:372-80
- Nakagawa K, Kudoh S, Matsui K, A Phase I study of pemetrexed (LY231514) supplemented with folate and vitamin B12 in Japanese patients with solid tumours. Brit J Cancer 2006;95:677-82
- Takimoto CH, Hammond-Thelin LA, Latz JE, Phase I and pharmacokinetic study of pemetrexed with high-dose folic acid supplementation or multivitamin supplementation in patients with locally advanced or metastatic cancer. Clin Cancer Res 2007;13:2675-83
- Rusthoven JJ, Eisenhauer E, Butts C, Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small-cell lung cancer: a Phase II study. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1999;17:1194-9
- Manegold C, Gatzemeier U, von Pawel J, Front-line treatment of advanced non-small-cell lung cancer with MTA (LY231514, pemetrexed disodium, ALIMTA) and cisplatin: a multicenter Phase II trial. Ann Oncol 2000;11:435-40
- Shepherd FA, Dancey J, Arnold A, Phase II study of pemetrexed disodium, a multitargeted antifolate, and cisplatin as first-line therapy in patients with advanced nonsmall cell lung carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group. Cancer 2001;92:595-600
- Clarke SJ, Abratt R, Goedhals L, Phase II trial of pemetrexed disodium (ALIMTA, LY231514) in chemotherapy-naive patients with advanced non-small-cell lung cancer. Ann Oncol 2002;13:737-41
- Smit EF, Mattson K, von Pawel J, ALIMTA (pemetrexed disodium) as second-line treatment of non-small-cell lung cancer: a Phase II study. Ann Oncol 2003;14:455-60
- Niyikiza C, Baker SD, Seitz DE, Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 2002;1:545-52
- Scagliotti GV, Shin D-M, Kindler HL, Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 2003;21:1556-61
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44
- Sweeney CJ, Takimoto CH, Latz JE, Two drug interaction studies evaluating the pharmacokinetics and toxicity of pemetrexed when coadministered with aspirin or ibuprofen in patients with advanced cancer. Clin Cancer Res 2006;12:536-42
- Mita AC, Sweeney CJ, Baker SD, Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol 2006;24:552-62
- Shvartsbeyn M, Edelman MJ. Pemetrexed-induced typhlitis in non-small cell lung cancer. J Thorac Oncol 2008;3:1188-90
- Cripps C, Burnell M, Jolivet J, Phase II study of first-line LY231514 (multi-targeted antifolate) in patients with locally advanced or metastatic colorectal cancer: an NCIC Clinical Trials Group study. Ann Oncol 1999;10:1175-9
- Hanna N, Shepherd FA, Fossella FV, Randomized Phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97
- Shepherd FA, Dancey J, Ramlau R, Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103
- Weiss GJ, Langer C, Rosell R, Elderly patients benefit from second-line cytotoxic chemotherapy: a subset analysis of a randomized Phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2006;24:4405-11
- Bearz A, Garassino I, Cavina R, Pemetrexed single agent in previously treated non-small cell lung cancer: a multi-institutional observational study. Lung Cancer 2008;60:240-5
- Lee H-Y, Ahn M-J, Park YH, Adenocarcinoma has an excellent outcome with pemetrexed treatment in Korean patients: a prospective, multicenter trial. Lung Cancer 2009;66:338-43
- Ohe Y, Ichinose Y, Nakagawa K, Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res 2008;14:4206-12
- Cullen MH, Zatloukal P, Sorenson S, A randomized Phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol 2008;19:939-45
- Smit EF, Burgers SA, Biesma B, Randomized Phase II and pharmacogenetic study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced non-small-cell lung cancer. J Clin Oncol 2009;27:2038-45
- Zinner RG, Fossella FV, Gladish GW, Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer 2005;104:2449-56
- Scagliotti GV, Kortsik C, Dark GG, Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, Phase II trial. Clin Cancer Res 2005;11:690-6
- Stathopoulos GP, Dimitroulis J, Toubis M, Pemetrexed combined with paclitaxel in patients with advanced or metastatic non-small-cell lung cancer: a Phase I-II trial. Lung Cancer 2007;57:66-71
- Clarke SJ, Boyer MJ, Millward M, A Phase I/II study of pemetrexed and vinorelbine in patients with non-small cell lung cancer. Lung Cancer 2005;49:401-12
- Monnerat C, Le Chevalier T, Kelly K, Phase II study of pemetrexed-gemcitabine combination in patients with advanced-stage non-small cell lung cancer. Clin Cancer Res 2004;10:5439-46
- Ma CX, Nair S, Thomas S, Randomized Phase II trial of three schedules of pemetrexed and gemcitabine as front-line therapy for advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5929-37
- Treat J, Bonomi P, McCleod M, Administration of pemetrexed immediately following gemcitabine as front-line therapy in advanced non-small cell lung cancer: a Phase II trial. Lung Cancer 2006;53:77-83
- West HL, Wakelee HA, Perry MC, Gemcitabine and pemetrexed administered in rapid sequence as front-line chemotherapy for advanced non-small-cell lung cancer: a Phase II clinical trial. Ann Oncol 2009;20:850-6
- Gridelli C, Kaukel E, Gregorc V, Single-agent pemetrexed or sequential pemetrexed/gemcitabine as front-line treatment of advanced non-small cell lung cancer in elderly patients or patients ineligible for platinum-based chemotherapy: a multicenter, randomized, Phase II trial. J Thorac Oncol 2007;2:221-9
- Dudek AZ, Larson T, McCleod MJ, Phase I/2 dose escalating study of twice-monthly pemetrexed and gemcitabine in patients with advanced cancer and non-small cell lung cancer. J Thorac Oncol 2008;3:394-9
- Blakely LJ, Schwartzberg L, Keaton M, A Phase II trial of pemetrexed and gemcitabine as first line therapy for poor performance status and/or elderly patients with stage IIIB/IV non-small cell lung cancer. Lung Cancer 2009;66:97-102
- Scagliotti GV, Parikh P, von Pawel J, Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51
- Gronberg BH, Bremnes RM, Flotten O, Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:3217-24
- Tiseo M, Bartolotti M, Gelsomino F, Ardizzoni A. First-line treatment in advanced non-small-cell lung cancer: the emerging role of the histologic subtype. Expert Rev Anticancer 2009;9:425-35
- Selvaggi G, Scagliotti GV. Histologic subtype in NSCLC: does it matter? Oncology 2009;23:1133-40
- Peterson P, Park K, Fossella F, Is pemetrexed more effective in patients with non-squamous histology? A retrospective analysis of a Phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC). EJC Suppl 2007;5:363-4
- Scagliotti G, Hanna N, Fossella F, The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 2009;14:253-63
- Lurje G, Manegold PC, Ning Y, Thymidylate synthase gene variations: predictive and prognostic markers. Mol Cancer Ther 2009;8:1000-7
- Nakagawa T, Otake Y, Yanagihara K, Expression of thymidylate synthase is correlated with proliferative activity in non-small cell lung cancer (NSCLC). Lung Cancer 2004;43:145-9
- Nakagawa T, Tanaka F, Otake Y, Prognostic value of thymidylate synthase expression in patients with p-stage I adenocarcinoma of the lung. Lung Cancer 2002;35:165-70
- Shintani Y, Ohta M, Hirabayashi H, New prognostic indicator for non-small-cell lung cancer, quantitation of thymidylate synthase by real-time reverse transcription polymerase chain reaction. Int J Cancer 2003;104:790-5
- Ceppi P, Volante M, Saviozzi S, Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589-96
- Giovannetti E, Mey V, Nannizzi S, Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol 2005;68:110-18
- Schiller JH, Ramalingam SS. Duration of chemotherapy for metastatic non-small-cell lung cancer: more may be better after all. J Clin Oncol 2009;27:3265-7
- Grossi F, Aita M, Follador A, Sequential, alternating, and maintenance/consolidation chemotherapy in advanced non-small cell lung cancer: a review of the literature. Oncologist 2007;12:451-64
- Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol 2009;27:3277-83
- Ciuleanu T, Brodowicz T, Zielinski C, Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, Phase III study. Lancet 2009;374:1432-40
- US Food and Drug Adminstration Center for Drug Evaluation and Research. Alimta. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021462s018s021s022lbl.pdf [Last accessed November 2009]
- European Medicines Agency. Summary of Product Characteristics. Alimta. Available from: http://www.emea.europa.eu/humandocs/Humans/EPAR/alimta/alimta.htm [Last accessed November 2009]
- Adjei AA, Mandrekar SJ, Dy GK, Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG Study N0426. J Clin Oncol 2009.23.6406
- Patel JD, Hensing TA, Rademaker A, Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol 2009;27:3284-9
- A Phase II trial of carboplatin, bevacizumab and pemetrexed in advanced non-small cell lung cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT00614822 [Last accessed January 2010]
- Pemetrexed plus bevacizumab in pretreated, advanced or metastatic non small cell lung cancer (NSCLC). Available from: http://clinicaltrials.gov/ct2/show/NCT00741221 [Last accessed January 2010]
- Patel JD, Bonomi P, Socinski MA, Treatment rationale and study design for the pointbreak study: a randomized, open-label Phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2009;10:252-6
- A study of tarceva (erlotinib) and standard of care chemotherapy in patients with advanced, recurrent, or metastatic non-small cell lung cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT00556322 [Last accessed November 2009]
- Trial of pemetrexed versus erlotinib in pretreated patients with non small cell lung cancer (NSCLC). Available from: http://clinicaltrials.gov/ct2/show/NCT00440414 [Last accessed November 2009]
- A Phase II study of pemetrexed versus pemetrexed plus erlotinib in second-line treatment in patients with nonsquamous NSCLC. Available from: http://clinicaltrials.gov/ct2/show/NCT00447057 [Last accessed January 2010]
- Stinchcombe TE, West HL. Maintenance therapy in non-small-cell lung cancer. Lancet 2009;374:1398-400
- Fidias PM, Dakhil SR, Lyss AP, Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591-8
- A study of induction and maintenance treatment of advanced non-squamous non small cell lung cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT00789373 [Last accessed November 2009]
- Study of patients with advanced non-small cell lung cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT00948675 [Last accessed January 2010]
- Ciuleanu T, Brodowicz T, Zielinski C, Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, Phase III study. Lancet 2009;374:1432-40
- Kubota K, Niho S, Enatsu S, Efficacy differences of pemetrexed by histology in pretreated patients with stage IIIB/IV non-small cell lung cancer: review of results from an open-label randomized phase ii study. J Thorac Oncol 2009;4(12):1530-6
- Trial of poor performance status patients (ToPPS). Available from: http://clinicaltrials.gov/ct2/show/NCT00892710 [Last accessed March 2010]
- Pemetrexed, carboplatin, and bevacizumab as first-line therapy in treating older patients with stage IIIB or stage IV non-small cell lung cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT00798603 [Last accessed March 2010]