Abstract
Importance of the field: Warfarin is the only oral anticoagulant recommended for the prevention of ischemic stroke in atrial fibrillation. A newer and safer anticoagulant is needed because of increased hemorrhagic risks with warfarin, difficult-to-maintain therapeutic levels, and higher drug to drug and food interactions.
Areas covered in this review: Dabigatran etexilate is a new, effective, reversible, rapid-acting, oral direct inhibitor of thrombin. This review focuses on the results of major Phase II and III trials conducted to evaluate the use of dabigatran in prevention of stroke in atrial fibrillation.
What the reader will gain: The objective of this paper is to discuss the use of dabigatran for prevention of stroke in patients with atrial fibrillation and to review its major advantages and disadvantages over warfarin.
Take home message: After the recent publication of Phase III trial RE-LY (randomized evaluation of long-term anticoagulation therapy), the use of dabigatran in atrial fibrillation is more clearly defined. A higher dose of dabigatran may be beneficial in patients who have recurrent ischemic events, despite therapeutic levels of warfarin. A lower dose is potentially safer than warfarin because of fewer hemorrhagic complications. Disadvantages include twice-daily dosing, dyspepsia and higher cost.
1. Introduction
Atrial fibrillation affects more than 2.2 million patients in USA and is associated with a fivefold increased risk of ischemic strokes, which are typically more severe and disabling Citation[1-4]. Approximately 70% of individuals with atrial fibrillation are between 65 and 85 years of age Citation[5]. Projected data from the cross-sectional Anticoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study of 17,974 adults indicated that the number of Americans with atrial fibrillation will increase to more than 5.6 million (2.5-fold), during the next 50 years Citation[6]. Of these, 76% of patients with atrial fibrillation have a moderate to high risk of embolization Citation[7]. Warfarin is the only oral anticoagulant recommended at present for the prevention of ischemic stroke in this high-risk population Citation[8]. However, the effect of warfarin can be altered with changes in diet, liver function and drug interactions involving the P450-cytochromes. Warfarin has a very narrow therapeutic index. A subtherapeutic international normalized ratio (INR) (1.5 – 1.9) reduces its preventive efficacy by a factor of 2 – 3.6 in atrial fibrillation patients compared with the recommended INR values (2.0 – 3.0) Citation[9]. Risk of intracranial hemorrhage with therapeutic INR is small (0.3 – 0.5% per 100 patient-years), but it increases to 2.7% at INR values of 4 – 4.5 and to 9.4% when INR exceeds 4.5 Citation[10]. Approximately 11% of patients receiving long-term warfarin have INR > 5 Citation[11].These limitations of warfarin highlight the need for new oral anticoagulants with predictable pharmacokinetics and pharmacodynamics, minimal food and drug interactions, fixed-dose administration, wider therapeutic window, and requirement of less intense monitoring.
2. Overview of the market
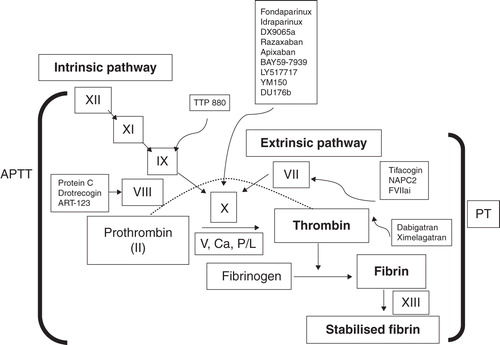
There are many targets for new anticoagulants in the coagulation pathway (). Fondaparinux and idraparinux inhibit Factor Xa (FXa) indirectly requiring antithrombin as a cofactor. A Phase III, open-label atrial fibrillation trial, AMADEUS, evaluating the efficacy of idraparinux versus warfarin ended prematurely owing to a higher rate of adverse events with idraparinux, despite a trend towards greater efficacy (18 cases of thromboembolism with idraparinux and 27 cases with vitamin K antagonists; 0.9 vs 1.3 per 100 patient-years; HR = 0.71, p = 0.007) Citation[12]. In contrast to indirect inhibitors of FXa, there are also many orally active compounds in clinical development for stroke prevention in atrial fibrillation that directly bind to the active site of FXa and block the interaction with its substrates () Citation[13,14]. In the final step of the coagulation pathway, thrombin converts fibrinogen to fibrin. Ximelagatran and dabigatran are oral, direct thrombin inhibitors that prevent fibrin formation, thrombin-mediated activation of Factors V, VIII, XI and XIII and thrombin-induced platelet-aggregation. Ximelagatran demonstrated favorable efficacy for prevention of stroke in atrial fibrillation in two Phase III trials before it was withdrawn from market because of hepatotoxicity Citation[14,15].
3. Dabigatran etexilate
Dabigatran (BIBR 953 ZW; ) is a small molecule that specifically and reversibly inhibits thrombin with similar structure to α-NAPAP (N-alpha-(2-naphthylsulfonylglycyl)-4-amidinophenylalanine piperidide), a benzamidine-based thrombin inhibitor Citation[16-18].
Box 1. Drug summary.
3.1 Chemistry
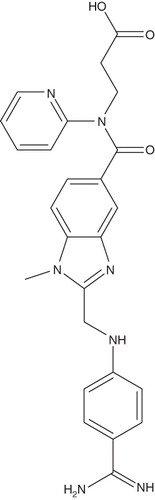
Dabigatran etexilate, Ethyl N-{[2-({[4-((E)-amino{[(hexyloxy) carbonyl] imino} methyl) phenyl] amino} methyl)-1-methyl-1H-benzimidazol-5-yl]carbonyl}-Npyridin-2-yl-β-alaninate methanesulfonate is an orally absorbable prodrug form of dabigatran, formed by addition of a hydrophobic side-chain to the original molecule () Citation[19]. It corresponds to a molecular formula of C35H45N7O8S. The molecular mass is 723.86 for the salt and 627.75 for the free base Citation[20]. Dabigatran etexilate is converted to active form dabigatran by esterase-catalyzed hydrolysis in plasma and in the liver Citation[20].
3.2 Pharmacodynamics
Dabigatran is a synthetic, nonpeptide competitive, rapid-acting and reversible inhibitor of thrombin, the most potent physiological agonist of platelet aggregation Citation[17]. As the binding with thrombin is selective, and reversible, its anticoagulant effects are more predictable than other irreversible thrombin inhibitors like hirudin Citation[21]. In in vitro and ex vivo studies, dabigatran showed high thrombin selectivity (700- to 10,000-fold higher than any other key factors in the coagulation cascade) Citation[22]. Dabigatran showed a potent inhibitory effect on thrombin-induced platelet aggregation; a concentration of 10 nmol/liter achieved 50% maximal inhibition (IC50) Citation[22]. In addition, dabigatran inhibits tissue factor-induced thrombin generation in human platelet-poor plasma in a concentration-dependent manner and decreases endogenous thrombin generation Citation[22]. In platelet-rich plasma from healthy adults, dabigatran had a more potent inhibitory effect on tissue factor-induced platelet aggregation than two FXa inhibitors, rivaroxaban and apixaban Citation[23]. Therefore, dabigatran may be effective in preventing both venous thrombosis and arterial thrombosis, which is more dependent on platelet aggregation Citation[23].
Two double-blind, randomized, placebo-controlled studies were done; the first a single-dose and the second a multiple-dose escalating study Citation[24]. The effects of dabigatran were assessed using coagulation tests including activated partial thromboplastin time (aPTT), prothrombin time (PT), INR, thrombin time (TT) and ecarin clotting time (ECT). Single, orally administered doses (10 – 400 mg) led to rapid, dose-dependent increases in mean INR, TT and ECT, with the maximum anticoagulant effect occurring at the maximum plasma dabigatran concentration(Cmax) indicating that thrombin inhibition by dabigatran is a direct effect linked to the central plasma compartment Citation[24]. Coagulation parameters closely followed drug concentrations when healthy men were given dabigatran 50 – 400 mg three times daily. Maximum effects were achieved within 2 h of administration. Twelve hours after administration, blood coagulation prolongation was reduced to ≈ 50% of its maximum Citation[24]. Similar pharmacodynamic effects were observed in healthy older subjects Citation[25].
3.3 Pharmacokinetics and metabolism
3.3.1 Absorption
Dabigatran has a mean absolute bioavailability of 6.5%, which is independent of dose and not influenced by coadministration with food Citation[17]. The BISTRO I Citation[26] trial studied the postoperative absorption of dabigatran in total hip replacement patients. Dabigatran etexilate was administered using a tablet formulation in doses of 12.5, 25, 50, 100, 150, 200 and 300 mg twice daily, or 150 and 300 mg once daily. Peak plasma concentrations were reached at 6 h following administration in a postoperative period owing to contributing factors such as anesthesia, gastrointestinal paresis, and surgical effects causing delay in absorption independent of the oral formulation. Slow and delayed absorption is only present on the day of surgery. On subsequent days, absorption of dabigatran is rapid with peak plasma concentrations attained 2 h after administration Citation[27].
3.3.2 Distribution
In healthy men, dabigatran plasma concentrations decline in a biphasic manner characterized by a rapid distribution phase, with a decrease in dabigatran plasma concentrations to < 30% of Cmax within 4 – 6 h of dosing. This is followed by a prolonged elimination phase, resulting in a mean plasma terminal half-life of 12 – 14 h. Steady state dabigatran concentrations are achieved approximately 3 days after multiple-dose administration, with no evidence of significant accumulation Citation[17]. Dabigatran exhibits plasma protein binding of about 35%, implying that displacement interactions are unlikely to affect its pharmacokinetics and pharmacodynamics. The volume of distribution of dabigatran is 60 – 70 liters, which exceeds the volume of total body water, indicating moderate tissue distribution Citation[17].
3.3.3 Metabolism and elimination
In healthy men, dabigatran was the predominant compound in plasma, urine and feces following both oral administration of dabigatran etexilate and intravenous administration of dabigatran Citation[28]. The hydrolysis of dabigatran etexilate and BIBR 1087 SE is mediated by microsomal-carboxylesterases, which are members of the family of serine-esterases. Dabigatran is not metabolized by P450-isoenzymes to any significant extent Citation[28].Therefore, the risk of drug interactions is low. Renal excretion of unchanged dabigatran is the predominant elimination pathway, with about 80% of an intravenous dose excreted unchanged in the urine Citation[28]. The remainder is conjugated with glucuronic acid to form acylglucuronides, which are predominantly excreted via the bile with only very small amounts of conjugates found in urine Citation[28].
Bioconversion of prodrug to dabigatran was slightly slower in patients with moderate hepatic impairment Citation[29]. Compared with those without renal impairment, the bioavailability of dabigatran was 2.7-fold higher in patients with moderate renal impairment and 6-fold greater in patients with severe renal impairment. Therefore, use of dabigatran is contraindicated in patients with severe renal impairment Citation[17].
In healthy older adults (aged ≥ 65 years) who received dabigatran 150 mg twice daily, plasma dabigatran concentrations reached steady state in 2 – 3 days. However, in comparison with historical data for healthy young adults, bioavailability increased 1.7- to 2-fold in elderly subjects, probably attributable to lower renal clearance Citation[25].
Dabigatran systemic availability is 20 – 30% higher in elderly women than elderly men, but dosage adjustments are not required Citation[25]. Similarly, limited available data indicate that no dosage adjustment is necessary in patients with bodyweight < 50 or > 110 kg, although close surveillance is recommended in patients outside these weight limits Citation[17,30].
3.4 Clinical efficacy
The efficacy of dabigatran for the prevention of stroke in atrial fibrillation was studied in two randomized, double-blinded trials ().
Table 1. Summary of clinical trials evaluating the role of dabigatran etexilate in atrial fibrillation.
3.4.1 PETRO
PETRO (dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation) was an open-label Phase II trial, designed to identify a safe dose of dabigatran in patients with atrial fibrillation Citation[31]. Safety of a dose was determined by the occurrence of bleeding and clinical events. Oral dabigatran doses assessed included 50, 150 and 300 mg twice daily. Study participants were monitored for safety at 1, 4, 8 and 12 weeks after randomization. Dabigatran monotherapy was compared with dabigatran plus aspirin 81 or 325 mg once daily and with warfarin monotherapy (dosed to reach a target INR of 2 – 3) in a 3 × 3 factorial design. The choice of aspirin dose was selected by the investigator. Plasma dabigatran concentrations, aPTT, D-dimer concentrations and liver function tests were measured at baseline and at 1, 2, 4, 8 and 12 weeks. Inclusion criteria mandated that patients have a median of three risk factors for stroke: patients had to have atrial fibrillation (median duration was 4 years), coronary artery disease (later removed to facilitate recruitment), and at least one of hypertension, diabetes mellitus, heart failure (or left ventricular dysfunction, LVEF < 40%), previous stroke or transient ischemic attack (TIA) or age > 75 years. Exclusion criteria were the presence of mitral-stenosis, prosthetic heart valves, planned cardioversion, recent (≤ 1 month) myocardial infarction, stroke or TIA, intracoronary stent placement within 6 months, contraindications or additional indications for anticoagulation, major hemorrhage in the past 6 months, severe renal impairment (estimated glomerular filtration rate < 30 ml/min), abnormal hepatic function, risk of pregnancy and investigational drug use within 30 days. The median aPTT with dabigatran 150 mg twice/day was comparable to therapeutic doses of ximelagatran 36 mg twice a day, which was studied previously for stroke prevention in atrial fibrillation. Trough plasma concentrations of dabigatran were noted to be higher in women (12 – 20%) and patients with reduced creatinine clearance. Trough aPTTs demonstrated low variability, with coefficients of variation of 13 – 21%, indicating that no routine monitoring of aPTT need be performed. Major hemorrhages were noted only in the dabigatran 300 mg plus aspirin group (4 of 64). This rate was significantly higher compared with the 300 mg-treated patients (p < 0.02); thus aspirin was discontinued in that group, and the patients were merged with the 300-mg monotherapy group. Alanine aminotransferase (ALT) levels greater than three times the upper limits of normal (ULN) range were observed in 0.9% of the dabigatran groups. Increases in ALT levels greater than the ULN range were reported in 10% of warfarin-treated patients.
3.4.2 RE-LY
RE-LY (randomized evaluation of long-term anticoagulation therapy) was a randomized noninferiority trial designed to compare two fixed doses of dabigatran, with open-label use of warfarin in patients with atrial fibrillation and were at increased risk for stroke Citation[32]. Patients were recruited from 951 clinical centers in 44 countries. Patients were eligible if they had atrial fibrillation documented on electrocardiography performed at screening or within 6 months and at least one of the following characteristics: previous stroke or TIA, LVEF < 40%, New York Heart Association heart failure class II or higher, and an age of at least 75 years or an age of 65 – 74 years plus diabetes mellitus, hypertension or coronary artery disease. Reasons for exclusion were the presence of a severe heart-valve disorder, stroke within 14 days or severe stroke within 6 months before screening, a condition that increases the risk of hemorrhage, a creatinine clearance < 30 ml/min, active liver disease or pregnancy. All trial participants were randomly assigned to receive one of two doses of dabigatran, or warfarin, by means of a central, interactive, automated telephone system. Dabigatran was administered, in a blinded fashion, in capsules containing either 110 or 150 mg of the drug, to be taken twice daily. Warfarin was administered, in an unblinded fashion, adjusted locally to an INR of 2.0 – 3.0. Concomitant use of aspirin (at a dose of < 100 mg per day) or other antiplatelet agents was permitted. Follow-up visits occurred 14 days after randomization, at 1 and 3 months, every 3 months thereafter in the first year, and then every 4 months until the study ended. Liver function testing was performed monthly during the first year of the follow-up period.
The three treatment groups were well balanced with respect to major baseline characteristics including age, sex, types of atrial fibrillation (persistent, paroxysmal and permanent) and CHADS2 (Congestive heart failure, hypertension, Age > 75, Stroke or TIA) scores. The rate of the primary outcome (stroke and systemic embolization) was significantly lower with dabigatran at a dose of 150 mg twice daily (1.1%) than with either dabigatran at a dose of 110 mg twice daily (1.5%; RR = 0.73, 95% CI 0.58 – 0.91, p = 0.005) or warfarin (1.7%; RR = 0.66, 95% CI 0.53 – 0.82, p < 0.001, for noninferiority < 0.001).
The rate of nonhemorrhagic (i.e., ischemic or unspecified) stroke also was significantly lower with 150 mg of dabigatran (0.92%) than with either 110 mg of dabigatran (1.34%; RR = 0.76, 95% CI 0.60 – 0.98, p = 0.03) or warfarin (1.20%; RR = 0.69, 95% CI 0.54 – 0.88, p = 0.002).The rates of hemorrhagic stroke with the 110-mg and 150-mg dabigatran doses were significantly lower than that with warfarin. The rate of extracranial hemorrhage was similar in all three groups. Myocardial infarction and gastrointestinal side effects were significantly more frequent with dabigatran 150 mg (compared with warfarin). The proportion of participants whose ALT levels were elevated more than three times the ULN was similar to warfarin group.
3.5 Safety and tolerability
3.5.1 Lethal and toxic doses
Safety and tolerability of dabigatran is evaluated in many animal studies. Single-dose oral administration of dabigatran did not increase mortality in mice and rats at doses up to 2000 mg/kg and up to 600 mg/kg in Rhesus monkeys Citation[20].
The toxicity observed in the repeat-dose toxicity studies was associated to the exaggerated pharmacological activity of dabigatran Citation[20].
3.5.2 Bleeding risk
The most commonly reported adverse reaction in four actively controlled venous thrombo-embolism (VTE) prevention trials was bleeding Citation[20]. In Phase II and Phase III trials evaluating the role of dabigatran in atrial fibrillation, risk of major hemorrhages was higher with higher doses of dabigatran Citation[31,32]. When compared with warfarin, rate of major hemorrhage was comparable in patients who received 150 mg of dabigatran but significantly lower in patients who received 110 mg of dabigatran daily. Rates of life-threatening bleeding, intracranial bleeding, and major or minor bleeding were higher with warfarin than with either the 110-mg dose of dabigatran or the 150-mg dose of dabigatran (p < 0.05 for all comparisons of dabigatran with warfarin; Table 3). There was a significantly higher rate of major gastrointestinal bleeding with 150-mg daily dosing of dabigatran compared with INR-based warfarin.
3.5.3 Other adverse events
The rate of myocardial infarction was higher with both doses of dabigatran compared with warfarin (). The most frequent adverse events in the pivotal studies including both prevention of VTE and stroke in atrial fibrillation were dyspepsia (RE-LY) and nausea, vomiting and constipation Citation[20,32].
Table 2. Major efficacy and safety outcomes in RE-LY Citation[32].
3.5.4 Drug interactions
Dabigatran etexilate is a substrate for the efflux transporter P-glycoprotein transporter. Drugs such as amiodarone, verapamil, clarithromycin, rifampin and St John's wort, which act on the P-glycoprotein, may alter the plasma concentration of dabigatran and dose adjustment may be needed. The use of quinidine, a strong inhibitor of P-glycoprotein transporter is contraindicated Citation[30].
4. Regulatory affairs
On 18 March 2008, the European Medicines Agency granted marketing authorization for dabigatran use in preventing VTE Citation[20]. Dabigatran has not been approved for use in patients with atrial fibrillation.
5. Conclusion
Dabigatran in doses of 110 mg and 150 mg daily reduces the risk of ischemic stroke among patients with atrial fibrillation who are at high risk for such events. Its advantages include oral administration, predictable pharmacokinetics and pharmacodynamics, minimum food and drug interactions, a wide therapeutic window, and no requirement for regular monitoring. Disadvantages include increased risk of myocardial infarction, gastrointestinal bleeding and dyspepsia Citation[32]. The rates of myocardial infarction were higher in both dabigatran- versus warfarin-treated groups; though these rates were similar to what would be expected in the UK for a general population of similar age Citation[33]. One explanation to this finding is that warfarin provides additional protection against coronary ischemia, which is evident in previously published large randomized trials Citation[34]. The increased incidence of dyspeptic symptoms with both dabigatran doses and the increased risk of gastrointestinal bleeding with the 150-mg dose may partly be explained by the peculiar formulation of dabigatran capsule. To enhance its absorption, a tartaric acid core was used Citation[32]. Other disadvantages include lack of specific antidote in the event of overdose and hemorrhagic complications. Supportive therapy such as fresh frozen plasma may be indicated in case of severe bleeding. Thrombin time and aPTT can be used to monitor the severity of bleeding diasthesis. However, their correlation with thrombosis and bleeding in a population with atrial fibrillation has not yet been clearly established Citation[35].
6. Expert opinion
Dabigatran etexilate is very close to the definition of an ideal anticoagulant, which makes it a potentially suitable new alternative for prevention of stroke in atrial fibrillation in appropriate settings. Based on the results of PETRO and RE-LY, we think that dabigatran can have therapeutic value in patients with atrial fibrillation under two conditions: i) a 150-mg twice-daily dose for patients with recurrent ischemic stroke or embolic events despite therapeutic doses of warfarin; and ii) a 110-mg twice-daily dose for patients who are not good candidates for warfarin because of higher risk of fall and hemorrhagic complications; though additional studies are needed to support this hypothesis. Future subgroup analyses are needed to explore dabigatran's use in elderly, patients with acute stroke and patients with low creatinine clearance as these patients were excluded from RE-LY. Dabigatran's twice-daily dosing, increased risk of nonhemorrhagic side effects like dyspepsia and a likely higher cost may reduce compliance if used as an alternative to warfarin in the future.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N
- Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin 2009;27:13-24, vii
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003;22:118-23
- Lin HJ, Wolf PA, Kelly-Hayes M, Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760-4
- Feinberg WM, Blackshear JL, Laupacis A, Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155:469-73
- Go AS, Hylek EM, Phillips KA, Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5
- Glazer NL, Dublin S, Smith NL, Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med 2007;167:246-52
- Fuster V, Ryden LE, Cannom DS, ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006;48:854-906
- Hylek EM, Skates SJ, Sheehan MA, An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996;335:540-6
- Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol 2008;31:55-62
- Newman DH, Zhitomirsky I. The prevalence of nontherapeutic and dangerous international normalized ratios among patients receiving warfarin in the emergency department. Ann Emerg Med 2006;48:182-9, 189 e1
- Bousser MG, Bouthier J, Buller HR, Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet 2008;371:315-21
- Bates SM, Weitz JI. The status of new anticoagulants. Br J Haematol 2006;134:3-19
- Turpie AG. New oral anticoagulants in atrial fibrillation. Eur Heart J 2008;29:155-65
- Albers GW. Stroke prevention in atrial fibrillation: pooled analysis of SPORTIF III and V trials. Am J Manag Care 2004;10(14 Suppl):S462-9, discussion S469-73
- Hauel NH, Nar H, Priepke H, Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem 2002;45:1757-66
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009
- Eriksson BI, Dahl OE. Prevention of venous thromboembolism following orthopaedic surgery: clinical potential of direct thrombin inhibitors. Drugs 2004;64:577-95
- Eriksson BI, Smith H, Yasothan U, Kirkpatrick P. Dabigatran etexilate. Nat Rev Drug Discov 2008;7:557-8
- European Medicines Agency (EMEA). Committee for Medicinal Products for Human Use (CHMP) Assessment Report for Pradaxa. 2008. Available from: http://www.emea. europa.eu/humandocs/PDFs/EPAR/pradaxa/H-829-en6.pdf [Last accessed 28 December 2009]
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285-95
- Wienen W, Stassen JM, Priepke H, In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost 2007;98:155-62
- Sanford M, Plosker GL. Dabigatran etexilate. Drugs 2008;68:1699-709
- Stangier J, Rathgen K, Stahle H, The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292-303
- Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47-59
- Eriksson BI, Dahl OE, Ahnfelt L, Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost 2004;2:1573-80
- Stangier J, Eriksson BI, Dahl OE, Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol 2005;45:555-63
- Blech S, Ebner T, Ludwig-Schwellinger E, The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008;36:386-99
- Stangier J, Stahle H, Rathgen K, Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol 2008;48:1411-19
- Boehringer Ingelheim Limited Pradaxa. Summary of product characteristics. 2009. Available from: http://emc.medicines.org.uk/medicine/20760 [Last accessed 18 March 2010]
- Ezekowitz MD, Reilly PA, Nehmiz G, Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100:1419-26
- Connolly SJ, Ezekowitz MD, Yusuf S, Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Goldacre MJ, Roberts SE, Yeates D, Gill LE. Myocardial infarction: an investigation of measures of mortality, incidence and case-fatality. Report to the Department of Health. Oxford: National Centre for Health Outcomes Development; 2001
- Connolly S, Pogue J, Hart R, Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12
- RELY Trial Emergency Information. 2009. Available from: https:// www.rely-trial.com/RelyWeb/resources/jsp/emergency/intro.jsp [Last accessed 21 March 2010]