In the article “Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study”, by Buynak et al. (Expert Opin. Pharmacother. 2010;11:1787-804) an error has been identified. The lines in that illustrate the percentage of patients who received placebo or tapentadol ER and experienced treatment-emergent adverse events that led to study discontinuation were not labeled correctly. The figure should have appeared as shown below.
Figure 7. Distribution of time to onset of TEAEs leading to treatment discontinuation (safety population).
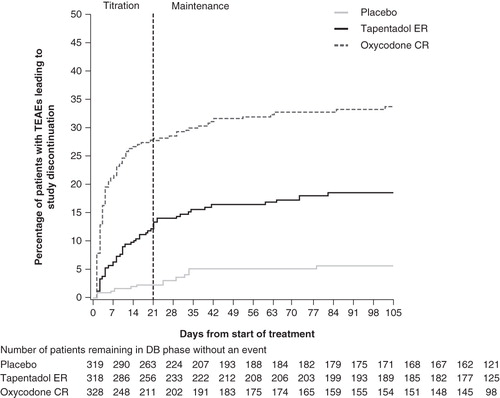
Informa Healthcare and the authors of this article would like to apologies for any confusion caused.
