Abstract
Introduction: The main aim in the management of diabetes mellitus is to prevent the development of its complications. Large fluctuations in glucose levels may increase the risk of complications, so improved control of glucose fluctuations, in addition to management of chronic hyperglycemia, could represent an important goal in diabetes pharmacotherapy.
Areas covered: Pre-clinical and clinical studies suggest that poor control of blood glucose fluctuations contributes to progression of diabetic vascular complications. Dipeptidyl peptidase (DPP)-4 inhibitors are one of several drug classes used to manage diabetes, and the potential vasoprotective effects of DPP-4 inhibition have attracted attention in recent years. The DPP-4 inhibitor teneligliptin was approved in Japan in 2012 and in Korea in 2014. Teneligliptin differs in its structural and pharmacokinetic characteristics compared with other drugs in the same class. It appears to have potent, sustained effects on glycemic control, thereby reducing the complications of hypoglycemia and postprandial hyperglycemia. Because of its effects on vascular function, teneligliptin may be beneficial in patients at high risk of cardiovascular disease.
Expert opinion: The possible pleiotropic effects of teneligliptin, such as those on endothelial function and metabolic syndrome, are of great interest. This review examines these effects and their potential clinical relevance.
1. Introduction
1.1 Diabetes mellitus and circulatory diseases
The overall goal in the management of diabetes mellitus is the prevention of complications and improvement in the individual’s quality of life. Diabetes greatly increases the risk of cardiovascular disease and mortality, and microvascular complications such as retinopathy, nephropathy, and neuropathy strongly affect the quality of life of affected patients. The glycosylated hemoglobin (HbA1c) level, which provides an indication of the average glucose level over a long period of time, is an established treatment target in diabetes. Glucose levels throughout the day, including those during fasting and between and after meals, contribute to HbA1c, which is a quantitative marker for overall glycemic control. Management of chronic hyperglycemia is important for preventing complications. However, it is vital to also consider fluctuations in glucose levels, so an assessment of glycemic variability should also be taken into account in strategies designed to reduce the complications associated with diabetes Citation[1]. Hyperglycemia increases the risk of developing complications associated with diabetes, with acute glucose fluctuations affecting endothelial function and triggering oxidative stress Citation[2]. Therefore, improvement in the control of postprandial glucose levels while avoiding inducing hypoglycemia–in other words, the control of fluctuations in blood glucose levels–has become increasingly important in the prevention of complications Citation[1-3].
An in vitro study by Risso and colleagues, which examined the effect of fluctuating glucose levels using human umbilical vein endothelial cells (HUVECs), reported enhanced apoptosis in endothelial cells exposed to intermittent high glucose levels. The cells also showed reduced expression of Bcl-2, a protein involved in regulating apoptosis, suggesting a role for acute increases in glucose levels in endothelial cells in the pathogenesis of diabetic vascular complications Citation[4].
Fluctuations in blood glucose levels have also been shown to induce inflammatory cytokines, oxidative stress, and the up-regulation of adhesion molecules. Another study using HUVEC lines found that fluctuating glucose levels had a greater effect on triggering nitrotyrosine (a marker of oxidative stress), adhesion molecules, and interleukin-6 than constantly high glucose levels, further supporting the deleterious role of glucose level fluctuations Citation[5].
The number of adherent macrophages was increased after 1 week of repetitive postprandial glucose spikes in apolipoprotein E-deficient mice, a model mimicking human atherosclerosis, and the size of atherosclerotic lesions was increased after 5 weeks, independent of serum cholesterol levels. These changes were not present in mice administered the α-glucosidase inhibitor miglitol, suggesting the importance of controlling postprandial glucose Citation[6]. These pre-clinical studies highlight the role of spikes in blood glucose in endothelial cell dysfunction, including apoptosis, inflammation, and atherosclerosis. Clinical studies have also suggested a role for fluctuating blood glucose levels in the development of complications. For example, glucose administered at oscillating levels during a 24-h period resulted in more deleterious effects on endothelial function and oxidative stress than consistently high glucose in both patients with (n = 27) and without (n = 22) type 2 diabetes mellitus (T2DM) Citation[7].
Postprandial glucose fluctuations and glucose swings generally resulted in a more specific triggering effect on oxidative stress (as measured using urine 8-iso prostaglandin F2α) compared with sustained chronic hyperglycemia in a case-control study of 21 patients with T2DM and 21 age- and sex-matched controls Citation[8]. These authors showed significant linear correlations between 8-iso prostaglandin F2α levels and both the mean postprandial incremental area under the curve for glucose concentration and the mean amplitude of glycemic excursions (MAGE).
Blood glucose levels, HbA1c, and glycemic variability were measured in 222 consecutive acute myocardial infarction patients admitted to a Chinese hospital. Glycemic variability was estimated using the MAGE, as determined using continuous glucose monitoring, and the relationship between major adverse cardiac events and glycemic variation, glucose, and HbA1c levels was also investigated. Glycemic variability, but not HbA1c levels on admission, was shown to be a strong predictor for adverse cardiac outcomes during a 12-month follow-up Citation[9].
Taken together, these pre-clinical and clinical studies imply a role for poor control of fluctuations in blood glucose levels in the progression of vascular complications in patients with diabetes Citation[4-9].
1.2 Dipeptidyl peptidase-4 inhibitors and circulatory diseases
Dipeptidyl peptidase (DPP)-4 inhibitors are one of several classes of oral anti-hyperglycemic drugs used in the management of T2DM. Their mechanism of action is to increase levels of the active forms of the incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide in response to meal intake, which in turn results in insulin secretion and reduces glucagon secretion. DPP-4 inhibitors are not associated with hypoglycemia or weight gain. These advantages mean that they have the potential to prevent complications associated with T2DM.
The potential vasoprotective effects of DPP-4 inhibition have attracted attention, and beneficial cardiovascular effects of GLP-1 and other DPP-4 substrates such as stromal cell-derived factor-1α, a chemokine that promotes homing of endothelial progenitor cells to areas of cellular injury, have been reported Citation[10,11].
Furthermore, pleiotropic actions of DPP-4 inhibitors not mediated by incretin have also been documented, including effects on lipid profile and blood pressure, as well as anti-inflammation/anti-oxidant effects, attracting attention from circulatory specialists regarding the effects of these agents on the overall cardiovascular risk profile Citation[12,13].
Evaluating the cardiovascular safety of anti-hyperglycemic drugs is important, particularly in patients who might be at increased risk of cardiovascular events. In the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) study of 5380 T2DM patients who had experienced a recent acute coronary syndrome, alogliptin significantly reduced HbA1c Citation[14]. Importantly, the investigators observed no increase in the rates of major cardiovascular events in patients administered alogliptin compared with those administered placebo. The large-scale SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) trial included 16,492 T2DM patients who had a history of or were at risk for cardiovascular events Citation[15]. Patients received saxagliptin or placebo and were followed for a median of 2.1 years. Saxagliptin improved glycemic control, but whereas the rate of ischemic events was not changed with saxagliptin treatment, the rate of hospitalization for heart failure was increased. The study investigators concluded that other approaches to decrease cardiovascular risk in T2DM patients are needed, and the study highlights that ongoing monitoring of the cardiovascular safety of DPP-4 inhibitors is prudent, particularly in those patients at increased cardiovascular risk.
The DPP-4 inhibitor teneligliptin was approved in 2012 in Japan Citation[16] and in 2014 in Korea for the treatment of T2DM. The following sections provide an overview of the available data and characteristics of teneligliptin, which has a different structure and pharmacokinetic characteristics to other drugs in the same class. The possible role these differences have in the potential pleiotropic effects of teneligliptin is also discussed.
2. Pharmacological profile of teneligliptin
The pharmacological profile of teneligliptin has been examined both in vitro and in vivo Citation[17]. In vitro, teneligliptin inhibited recombinant human DPP-4, DPP-4 from human plasma, and DPP-4 from rat plasma with IC50 (nmol/L) values of 1.01, 1.45, and 1.14, respectively. Comparative inhibition studies showed that teneligliptin exhibited more potent inhibition of the DPP-4 enzyme than sitagliptin, vildagliptin, and alogliptin, perhaps because of the unique structure of teneligliptin, namely, its J-shaped structure and anchor lock domain Citation[18]. In vivo, plasma DPP-4 inhibition was sustained at 24 h after oral administration of teneligliptin in Wistarrats. However, DPP-4 inhibition was no longer observed at 24 h with the administration of sitagliptin or vildagliptin in this model. A single dose of teneligliptin after fat loading in Zucker fatty rats, an obesity model characterized by insulin resistance and dyslipidemia, resulted in reduced postprandial triglyceride and free fatty acid levels, in addition to increased GLP-1 and insulin levels Citation[17]. Taken together with the effects on body weight and triglyceride levels observed in obese mice models Citation[19], these findings point to potentially beneficial effects on metabolic syndrome beyond those of improving glycemic control and warrant further investigation. DPP-4 has been shown to exist in tissues, especially in the kidneys Citation[20], where it is suggested to be related to pathologies Citation[21]. Because teneligliptin has hydrophobic properties and is distributed in tissues Citation[22], it may demonstrate a tissue-protective effect. Further research is needed to explore these issues.
3. Structural and pharmacokinetic characteristics of teneligliptin
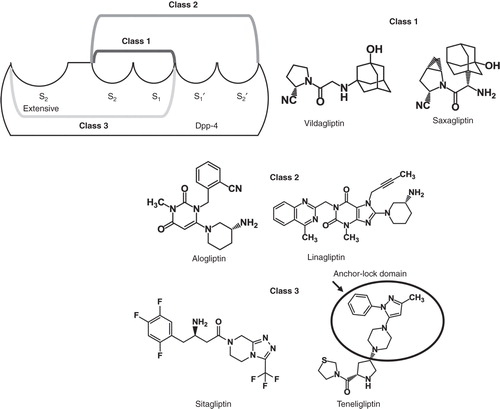
DPP-4 inhibitors are structurally diverse and can be classified into three classes based on their interaction with DPP-4 binding subsites Citation[18]. Teneligliptin is grouped into class 3, owing to its interaction with S1, S2, and S2 extensive subsites Citation[18]. It is a potent, selective, and long-lasting inhibitor of DPP-4 and exhibits strong binding via its J-shaped structure and ‘anchor lock domain’ () Citation[18,23].
Figure 1. Structure and classification of the DPP-4 inhibitors, showing the J-shaped structure and anchor-lock domain of teneligliptin.
Teneligliptin is administered once daily at a dose of 20 mg, which can be increased to 40 mg if the response is insufficient. It’s elimination half-life in plasma is 24.2 and 20.8 h in 20 and 40 mg doses, respectively, with resulting DPP-4 inhibition through the day Citation[16].
Teneligliptin is eliminated via both the liver and kidney, meaning its use can be considered in patients with hepatic or renal impairment. A Phase I mass balance study using a single oral 20 mg dose of [14C]-teneligliptin showed that, in contrast to other DPP-4 inhibitors, 20 – 34% of the absorbed teneligliptin was excreted via the kidneys and 66 – 80% was eliminated via multiple drug metabolic enzymes Citation[16,24].
In a study of patients with mild, moderate, severe, and end-stage renal impairment, a single 20 mg dose of teneligliptin had no effect on maximum plasma concentration (Cmax) in those with mild, moderate, and severe renal impairment. The area under the concentration–time curve to infinite time (AUC0–INF) was increased in all groups compared with healthy controls, but the degree of increase was no larger than twofold. Overall, the results suggested that teneligliptin was well tolerated in patients with renal impairment, including those with end-stage renal disease Citation[25].
In patients with hepatic impairment, Cmax and AUC0–INF were increased in patients with mild and moderate hepatic impairment, but were below FDA-recommended thresholds for dose adjustment. Teneligliptin was generally well tolerated in these patients, but caution in its use is needed, particularly in patients with severe hepatic impairment Citation[26].
4. Effect of teneligliptin on blood glucose levels
Teneligliptin has been shown to produce clinically significant improvements in HbA1c, fasting plasma glucose (FPG), and postprandial glucose levels in patients with T2DM, both alone and in combination with other oral anti-hyperglycemic drugs Citation[27-29].
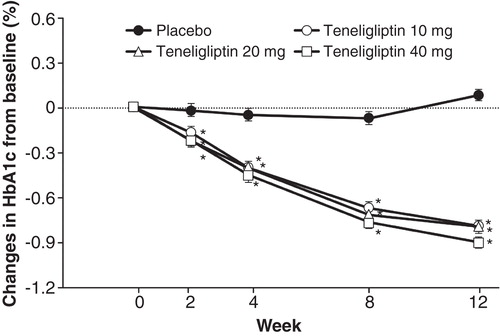
The effect of teneligliptin monotherapy was assessed in a double-blind, randomized, placebo-controlled trial of 324 Japanese patients with T2DM that was inadequately controlled with lifestyle changes Citation[27]. Participants were randomized to receive placebo or 10, 20, or 40 mg doses of teneligliptin once daily for 12 weeks. The primary endpoint was change in HbA1c from baseline over the study period, and secondary endpoints included FPG and 2-h postprandial glucose. Significant improvements in HbA1c were observed over the 12-week study period. The differences between the teneligliptin and the placebo group for the change in HbA1c were (least squares [LS] mean, 95% CI) −0.9 (−1.0, −0.7), −0.9 (−1.1, −0.7), and −1.0 (−1.2, −0.9) % for the 10, 20, and 40 mg dosage groups, respectively (all p < 0.001). (). Although the authors pointed out the need for studies of longer duration, it is noteworthy that significant and clinically relevant reductions in FPG and 2-h postprandial glucose levels were also observed in each of the dosage groups. Differences between the teneligliptin and placebo groups for the 10, 20, and 40 mg dosage groups were −17.8 (−23.4, −12.1), −16.9 (−22.6, −11.2), and −20.0 (−25.7, −14.3) mg/dl, respectively, for the change in FPG, and −50.6 (−62.8, −38.4), −56.8 (−69.2, −44.3), and −58.6 (−71.1, −46.1) mg/dl, respectively, for the change in 2-h postprandial glucose (all p < 0.001). The incidences of adverse events (AEs) and adverse drug reactions (ADRs) were similar between the four groups, including the rate of hypoglycemia Citation[27].
Figure 2. Effect of teneligliptin on glycosylated hemoglobin (HbA1c) levels in type 2 diabetic patients (n = 324) in Japan in a double-blind placebo-controlled trial. Values are least square means ± standard error.
In a placebo-controlled trial of teneligliptin in Japanese patients with inadequate glycemic control on glimepiride monotherapy, 194 patients on stable glimepiride therapy were randomized to receive 20 mg teneligliptin or placebo for 12 weeks. The primary endpoint was change in HbA1c, and FPG and 2-h postprandial glucose were among the secondary endpoints. The 12-week double-blind period was followed by a 40-week open-label phase in which all subjects received teneligliptin once daily. Teneligliptin significantly (p < 0.001) reduced HbA1c (placebo-subtracted change in HbA1c [LS mean ± standard error] −1.0 ± 0.1%), FPG (−27.1 ± 3.2 mg/dl), and 2-h postprandial glucose (−49.1 ± 6.2 mg/dl), and the glucose-lowering effects were maintained throughout the open-label phase of the study. Rates of AEs and ADRs including episodes of hypoglycemia were similar between the groups Citation[28].
Similar results were obtained when teneligliptin was assessed in patients inadequately controlled on pioglitazone alone. In this study, 204 patients on stable pioglitazone therapy received 20 mg teneligliptin or placebo in a 12-week double-blind phase, followed by a 40-week open-label phase in which all subjects received teneligliptin. Again, significant improvements in HbA1c, FPG, and 2-h postprandial glucose were observed with teneligliptin, an effect maintained in the 40-week extension phase. AEs and ADRs occurred slightly more frequently in the teneligliptin group than in the placebo group, although the incidence of hypoglycemia remained low Citation[29].
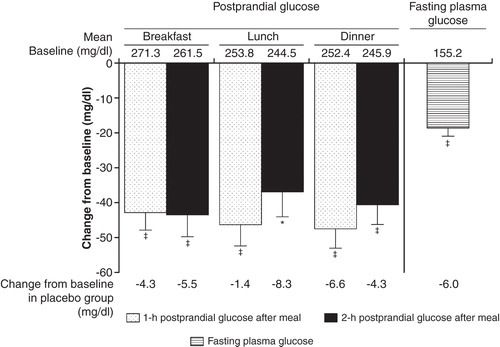
Teneligliptin has been shown to stabilize blood glucose levels throughout the day. In a 4-week placebo-controlled trial of 99 Japanese T2DM patients inadequately controlled with diet and exercise, both 10 and 20 mg doses of teneligliptin resulted in significantly lower postprandial glucose, 24-h mean glucose, and FPG levels than those achieved with placebo, and no patients in any of the groups experienced hypoglycemia. The postprandial glucose-lowering effects of teneligliptin administered before breakfast were sustained throughout the day, with the effects observed after dinner being similar to those observed after breakfast () Citation[16,30].
Figure 3. Effect of teneligliptin on 1- or 2-h postprandial glucose levels and fasting plasma glucose at week 4 in type 2 diabetic patients in Japan in a placebo-controlled trial. Values are least square means ± standard error. Teneligliptin 20 mg group: n = 33; placebo group: n = 32.
In a study of the effects of teneligliptin on glucose fluctuations in 26 Japanese T2DM patients receiving insulin therapy, with or without other anti-diabetes drugs, and using continuous glucose monitoring, the standard deviation (SD) of 24-h glucose levels and MAGE both improved following administration of teneligliptin Citation[31]. Postprandial glucose levels after breakfast, lunch, and dinner also improved in these patients. There were no significant differences in the proportion of time spent in hypoglycemia. In another report Citation[32], 10 Japanese T2DM patients treated for 3 days with teneligliptin also showed improved 24-h glucose levels, SD of 24-h glucose levels, and MAGE (). These results suggest that teneligliptin improves 24-h glucose fluctuations.
Table 1. Indices of daily blood glucose fluctuations measured by continuous glucose monitoring in patients before or 1 – 3 days after the start of teneligliptin treatment.
4.1 Efficacy in special patient groups
The pharmacokinetic properties of teneligliptin have been studied in patients with renal and hepatic impairment Citation[25,26]. Given that nephropathy is a common complication in patients with diabetes, elucidating its utility in hemodialysis patients is important. A prospective study in hemodialysis patients with poor glycemic control was carried out in two centers in Japan. Fourteen patients received teneligliptin (seven newly started; seven switching from other medications), and 29 patients continued with their existing medication (the control group). Blood glucose levels were significantly decreased from week 4 in the teneligliptin group. The differences in glycated albumin (at week 28) and HbA1c (at week 24) between the teneligliptin group and the control group were −3.1% (p < 0.05) and −0.57% (p = 0.057), respectively. One patient experienced constipation, but there were no discontinuations as a result of adverse effects, indicating that teneligliptin was well tolerated and effective in diabetic patients undergoing dialysis Citation[33].
5. Potential pleiotropic effects of teneligliptin
5.1 Effectiveness in obesity and metabolic syndrome
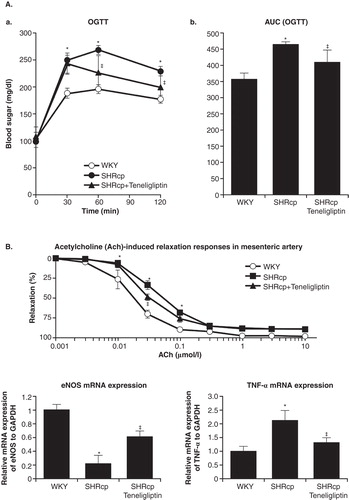
Obesity is one of the most important risk factors for T2DM. Fukuda-Tsuru et al. Citation[19] recently examined the effect of teneligliptin on obesity-related factors in an obese mouse model. Ten mice were fed a normal diet, and thirty were fed a high-fat diet for 10 weeks. Five days after the initiation of high-fat feeding, the mice were randomized to receive teneligliptin 30, 60 mg/kg, or vehicle alone. Mice fed a high-fat diet showed accelerated body weight increases, but this was attenuated in the 30 and 60 mg/kg teneligliptin groups to 88 and 71%, respectively, compared with the vehicle group. Calorimetry studies showed that 60 mg/kg teneligliptin increased oxygen consumption, and mean adipocyte size and hepatic triglyceride levels were 44 and 34%, respectively, of those in the vehicle group.
T2DM, hypertension, and metabolic syndrome are all major risk factors for cardiovascular events. Spontaneous hypertensive rats with severe obesity, hypertension, and endothelial dysfunction (SHR/NDmcr-cp) were used as a model for the metabolic syndrome. Teneligliptin treatment resulted in significant improvements in hyperglycemia assessed by the oral glucose tolerance test (), and insulin resistance assessed by the homeostasis model assessment for insulin resistance. The vasodilatory response of the aorta to acetylcholine was also improved, as reflected by increased endothelium-derived nitric oxide synthase mRNA expression. Teneligliptin-treated rats showed significantly decreased tumor necrosis factor-α expression compared with untreated rats () Citation[34].
5.2 Improvement in vascular endothelial function in T2DM patients
The effects of teneligliptin on vascular endothelial function were examined in an uncontrolled study of 11 patients with T2DM Citation[35]. Two methods of determination were used: the Endo-PAT 2000 method measuring arterial pulse waves at a finger artery, and the flow-mediated vasodilatation (FMD) method measuring the flow-dependent vasodilation reaction of the brachial artery after occlusion of blood flow. Patients were aged 73 ± 11 years and included those with ischemic heart disease. The change in vascular endothelial function was assessed using both techniques before and after a 2-week administration of teneligliptin at 20 mg/day. In addition, both the reactive hyperemia index (the index of measurement for the Endo-PAT 2000) and FMD were significantly improved (p < 0.05 and p < 0.01, respectively). The results suggested that in this group of elderly patients with T2DM, including those with cardiac complications, teneligliptin may show benefit in improving vascular endothelial function within 2 weeks of administration Citation[35].
6. Conclusions
Fluctuations in blood glucose levels have been shown to cause oxidative stress and induce inflammatory markers leading to endothelial dysfunction and arteriosclerosis. There is also evidence that the postprandial glycemic state contributes to atherosclerotic risk Citation[36]. The DPP-4 inhibitor teneligliptin appears to have potent, sustained effects on glycemic control, which are beneficial in ameliorating the effects of hypoglycemia and postprandial hyperglycemia on the development of diabetes complications. Owing to its effects on vascular function, teneligliptin may show benefits in obese patients and others at high risk of cardiovascular disease, and animal models have shown effects on body weight and lipid levels Citation[17,19,34,35]. Teneligliptin was recently approved in Japan and Korea for the management of T2DM, and further studies in higher-risk groups of patients, in particular head-to-head studies of the effects of different DPP-4 inhibitors on cardiovascular outcomes, are needed Citation[37,38]. These studies, combined with the evidence emerging from the real-life use of this drug, will be useful in shedding further light on the potential benefits of teneligliptin beyond those based on glycemic control.
7. Expert opinion
Glucose fluctuations can cause endothelial dysfunction and may contribute to complications in diabetic patients. DPP-4 inhibitors are expected to help prevent these complications because of their effect on postprandial glucose levels and lower likelihood of inducing hypoglycemia. The DPP-4 inhibitor teneligliptin is relatively new to the market and evidence for its long-term efficacy and usability will be beneficial. The main goal in this field is improved quality of life for diabetic patients. To achieve this, in relation to teneligliptin, studies of longer duration designed to measure parameters other than glycemic control are needed. Over the coming years, rates of obesity and associated T2DM will increase globally, with subsequent lowering of quality of life and increased complications and costs to healthcare systems. Therefore, there is a pressing need for treatments that are effective and well tolerated with long-term use. Pleiotropic effects of teneligliptin such as those on endothelial function and metabolic syndrome are currently of particular interest because skeletal muscle insulin resistance may be caused by impaired insulin signaling not only in myocytes, but also in endothelial cells Citation[39]. Thus, the importance of endothelial function to glucose metabolism needs to be considered. Differences in structural and pharmacokinetic characteristics between teneligliptin and other DPP-4 inhibitors may underlie its potency and sustained effects on glycemic control, which make it potentially beneficial for patients with a high risk of cardiovascular disease. This review serves to highlight some of these effects and their possible clinical relevance.
Box 1. Drug summary.
Declaration of interest
The Department of Clinical Gene Therapy is financially supported by Mitsubishi Tanabe Pharma Corp., AnGes MG, Inc., Novartis, Shionogi, Boehringer Ingelheim and Rohto. The Division of Vascular Medicine and Epigenetics (to which Hironori Nakagami belongs) is financially supported by Bayer. Ryuichi Morishita is a founder of AnGes MG, Inc., and holds stock in the company. Editorial support for the preparation of this manuscript was provided by H Roberton. Publication support was funded by Mitsubishi Tanabe Pharma Corp. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Del Prato S. In search of normoglycaemia in diabetes: controlling postprandial glucose. Int J Obes Relat Metab Disord 2002;26:S9-S17
- International Diabetes Federation. Guideline for management of post meal glucose in diabetes. International diabetes federation. 2011. Available from: http://www.idf.org/2011-guideline-management-postmeal-glucose-diabetes
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010;33(7):1389-94
- Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab 2001;281(5):E924-30
- Piconi L, Quagliaro L, Da Ros R, et al. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly (ADP-ribose) polymerase. J Thromb Haemost 2004;2(8):1453-9
- Mita T, Otsuka A, Azuma K, et al. Swings in blood glucose levels accelerate atherogenesis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun 2007;358(3):679-85
- Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57(5):1349-54
- Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295(14):1681-7
- Su G, Mi SH, Tao H, et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care 2013;36(4):1026-32
- Balakumar P, Dhanaraj SA. Cardiovascular pleiotropic actions of DPP-4 inhibitors: a step at the cutting edge in understanding their additional therapeutic potentials. Cell Signal 2013;25(9):1799-803
- Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33(2):187-215
- Aroor AR, Sowers JR, Jia G, et al. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart Circ Physiol 2014;307(4):H477-92
- van Genugten RE, Möller-Goede DL, van Raalte DH, et al. Extra-pancreatic effects of incretin-based therapies: potential benefit for cardiovascular-risk management in type 2 diabetes. Diabetes Obes Metab 2013;15(7):593-606
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369(14):1327-35
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14):1317-26
- Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc) 2013;49(10):615-29
- Fukuda-Tsuru S, Anabuki J, Abe Y, et al. A novel, potent, and long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, improves postprandial hyperglycemia and dyslipidemia after single and repeated administrations. Eur J Pharmacol 2012;696(1–3):194-202
- Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun 2013;434(2):191-6
- Fukuda-Tsuru S, Kakimoto T, Utsumi H, et al. The novel dipeptidyl peptidase-4 inhibitor teneligliptin prevents high-fat diet-induced obesity accompanied with increased energy expenditure in mice. Eur J Pharmacol 2014;723:207-15
- Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul Pept 1999;85(1):9-24
- Liu WJ, Xie SH, Liu YN, et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther 2012;340(2):248-55
- Pharmaceuticals and Medical Devices Agency. Tenelia application document. 2012. Available from: http://www.pmda.go.jp/english/service/pdf/drugs/tenelia_jun2012_e.pdf
- Yoshida T, Akahoshi F, Sakashita H, et al. Discovery and preclinical profile of teneligliptin(3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): a highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem 2012;20(19):5705-19
- Nakamaru Y, Hayashi Y, Ikegawa R, et al. Metabolism and disposition of the dipeptidyl peptidase IV inhibitor teneligliptin in humans. Xenobiotica 2014;44(3):242-53
- Halabi A, Maatouk H, Siegler KE, et al. Pharmacokinetics of teneligliptin in subjects with renal impairment. Clin Pharm Drug Dev 2013;2:246-54
- Halabi A, Maatouk H, Siegler KE, et al. Pharmacokinetics and safety of teneligliptin in subjects with hepatic impairment. Clin Pharm Drug Dev 2014;3(4):290-6
- Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013;15(9):810-18
- Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab 2014;16(5):418-25
- Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013;4(6):576-84
- Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2012;14(11):1040-6
- Tanaka S, Suzuki I, Aoki C, et al. Add-on treatment with teneligliptin ameliorates glucose fluctuations and improves glycemic control index in Japanese patients with type 2 diabetes on insulin therapy. Diabetes Technol Ther 2014;16(12):840-5
- Tsuchimochi W, Ueno H, Yamashita E, et al. Teneligliptin improves glycemic control with the reduction of postprandial insulin requirement in Japanese diabetic patients. Endocr J 2014; 10.1507/endocrj.EJ14-0393
- Otsuki H, Kosaka T, Nakamura K, et al. Safety and efficacy of teneligliptin: a novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int Urol Nephrol 2014;46(2):427-32
- Nakagami H, Pang Z, Shimosato T, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin improved endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp rat model of metabolic syndrome. Hypertens Res 2014;37:629-35
- Takase B, Nagata M. Subacute effect of a novel dipeptidyl peptidase-4 inhibitor teneligliptin on endothelial function in humans. J N Remedies Clin 2013;50:698-704
- Ceriello A. The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev 2000;16(2):125-32
- Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother 2013;14(15):2047-58
- Scheen AJ. A review of gliptins for 2014. Expert Opin Pharmacother 2014;10:1-20
- Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 2011;13(3):294-307