Abstract
Objective: Investigate impact of 6-month earlier versus postponed initiation of rotigotine in patients with early Parkinson’s disease (PD) with mild symptom severity.
Background: Long-term benefit of rotigotine in early-PD has been demonstrated: SP702 (NCT00594165) and SP716 (NCT00599196) were long-term, open-label extensions of double-blind, placebo-controlled studies of 6-month maintenance; rotigotine was well tolerated for up to 6 years, and demonstrated efficacy (Unified Parkinson’s Disease Rating Scale [UPDRS] II + III below baseline) for ∼ 2 years (SP702) and ∼ 4 years (SP716).
Methods: Post hoc analysis of patients at Hoehn and Yahr 1–2; groups defined by treatment received in 6-month double-blind studies: ‘Rotigotine–Rotigotine’ received rotigotine (n = 221), ‘Placebo–Rotigotine’ received placebo (n = 125).
Results: At the start of open-label rotigotine maintenance, UPDRS II + III mean ± SD change from double-blind baseline was: −8.5 ± 10.6 ‘Rotigotine–Rotigotine’, −7.7 ± 9.0 ‘Placebo–Rotigotine.’ After this initial improvement scores gradually increased: It took ∼ 45 months for mean scores to cross baseline in ‘Rotigotine–Rotigotine’, and ∼ 21 months in ‘Placebo–Rotigotine.’ At the time mean UPDRS II + III had crossed baseline in ‘Placebo–Rotigotine’ (open-label week 84; ∼ 21 months), treatment difference (LS-mean) to ‘Rotigotine–Rotigotine’ change from baseline was −3.89 (95% CI −6.94, −0.84); p = 0.013.
Conclusions: In this post hoc analysis, 6-month earlier initiation of rotigotine resulted in slower return to baseline mean UPDRS II + III; initiation of rotigotine in patients with minimal/no functional disability or impairment may lead to an extended benefit.
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder; however, symptomatic therapy can provide benefit for many years. The progressive loss of dopaminergic neurons in the substantia nigra pars compacta of the basal ganglia and the corresponding loss of striatal dopamine levels, which define PD, is likely subject to compensatory mechanisms that help delay the emergence of symptoms and maintain motor function Citation[1-3]. Therefore, treatment early in the course of the disease may not only provide symptom relief, but also act to alleviate the stress on the intrinsic physiological compensatory mechanisms. However, in considering the potential for drug induced adverse events (AEs) and motor complications, the initiation of treatment is often delayed until symptoms of PD begin to limit the patient’s ability to function Citation[4,5]. There is increasing evidence to challenge this practice. First, the rate of clinical deterioration in PD may be rapid in the early stages, with a decline of ∼ 8 – 10 Unified Parkinson’s Disease Rating Scale (UPDRS) points in the first year after diagnosis Citation[6], and patients with lower Hoehn and Yahr (HY) or UPDRS motor scores at baseline have been shown to have greater progression of motor impairment than those with higher disease severity at baseline Citation[7], suggesting this early period after diagnosis is critical for treatment intervention. Moreover, evidence suggests that earlier intervention of PD treatment may lead to better long-term motor benefit Citation[8-10]; and has also been shown to maintain PD-related quality of life compared to postponing treatment, which may result in a deterioration of PD-related quality of life Citation[11]. While there are no established disease-modifying or ‘neuroprotective’ therapies Citation[12], timing of therapy initiation is thus of clinical importance. Postponing treatment may result in pathophysiological alterations in the basal–ganglia–cortex loops and, second, a loss of functional ability that cannot be regained later in the disease course. One might speculate that the restorative maintenance of dopaminergic tone might be beneficial in the first years of the disease manifestation, and that early treatment may, in turn, lead to an improved long-term benefit.
Rotigotine is a non-ergoline dopamine receptor agonist (DA) with activity across D1 through D5 receptors as well as select adrenergic and serotonergic sites Citation[13]. It is applied once daily using a transdermal patch, enabling continuous drug delivery and stable plasma levels over a 24-h period Citation[14]. The benefit of treating patients with early PD with rotigotine has been demonstrated in both short- and long-term studies. Two randomized, placebo-controlled studies of 6-month maintenance (SP512 and SP513) have shown rotigotine transdermal patch to be an efficacious therapy in early-stage PD, producing significant improvements in activities of daily living and motor symptoms, as assessed by UPDRS II + III total score Citation[15-17]. The SP702 and SP716 studies were long-term, single-arm, open-label extensions of the SP512 and SP513 studies. Continuous transdermal delivery of rotigotine was well tolerated for up to 6 years, and demonstrated sustained efficacy (UPDRS II + III below double-blind baseline) for ∼ 2 years in the SP702 study Citation[18] and 4 years in the SP716 study Citation[19]. Moreover, the incidence of dyskinesia was low in these long-term studies of rotigotine Citation[20].
The objective of this post hoc analysis of these long-term open-label extension studies was to investigate the impact of 6-month earlier double-blind initiation of treatment with rotigotine transdermal patch in patients with early PD with mild symptom severity and disability, as defined by the HY scale. The analysis was restricted to a subgroup of patients at HY stage 1 – 2, in order to focus specifically on the timing of rotigotine initiation patients with minimal/no functional disability or impairment. We hypothesized that a 6-month earlier double-blind initiation of treatment with rotigotine may preserve motor function and activities of daily living longer than postponing treatment.
2. Patients and methods
2.1 Design
Patients completing the 6-month maintenance period of one of two Phase III, randomized, double-blind studies of rotigotine transdermal patch in patients with early-stage PD (SP512 and SP513) had the option of enrolling into prospective, single-arm, open-label extension studies of up to 6 years’ duration: SP702 (participants from SP512; ClinicalTrials.gov NCT00594165) and SP716 (participants from SP513; ClinicalTrials.gov NCT00599196). Patients enrolled in each double-blind study were aged 30 years or older, had been diagnosed with idiopathic PD for up to 5 years, had a UPDRS Part III score of ≥ 10, and were HY stage 1 – 3 (mild-to-moderate PD). Treatment with DAs or levodopa was not permitted during the double-blind studies or in the 1 month prior to enrollment. Levodopa (in combination with benserazide or carbidopa) was allowed, if required, after 1 month of open-label rotigotine maintenance. In addition, the following other anti-Parkinson medications were allowed after 1 month of open-label rotigotine maintenance: monoamine oxidase B inhibitors, anticholinergic agents, N-Methyl-D-aspartate receptor-antagonists (e.g., amantadine), entacapone and modafinil. Complete inclusion and exclusion criteria are published Citation[15-19].
2.2 Procedures
In the double-blind studies, patients were randomized to receive once-daily transdermal patches of placebo or rotigotine (2 – 6 mg/24 h) in SP512, and placebo, rotigotine (2 – 8 mg/24 h), or oral ropinirole (up to 24 mg/day) in SP513. At the end of double-blind maintenance, patients underwent de-escalation to 2 mg/24 h rotigotine/placebo, while remaining blinded, prior to commencing the open-label extension studies (i.e., neither the study investigators nor the patients were aware of the previous treatment). All patients entering the open-label studies then received open-label rotigotine titrated to their optimal dose (up to 6 mg/24 h in SP702, up to 8 mg/24 h in SP716). The optimal dose was defined following discussion between the patient and the investigator, taking into account the potential for improvement of disease symptoms and the patient’s AE profile. After the first year of open-label maintenance, up-titration to a maximum dose of 16 mg/24 h was permitted. During open-label maintenance, dose adjustments of rotigotine were permitted at any time at the discretion of the investigator. The investigator was encouraged to increase rotigotine to the maximum allowed dose before adding/increasing levodopa or other permitted anti-Parkinson medications. Patients were maintained at their optimal dose until rotigotine became commercially available or the sponsor closed the trial, whichever came first. Maintenance visits were scheduled at the end of the first month of open-label maintenance and at 3-month intervals thereafter Citation[18,19].
2.3 Patients included in this post hoc analysis
In this post hoc analysis of pooled data from SP702 and SP716 and the preceding double-blind studies (SP512 and SP513), data are reported for patients at HY stage 1 or 2 at double-blind baseline (HY stage 1: Unilateral involvement only, usually with minimal or no functional disability; HY stage 2: Bilateral or midline involvement, without impairment of balance). Analyses were performed on patients who had at least one UPDRS II + III assessment at double-blind baseline, at the start of the open-label maintenance period, and at least one UPDRS II + III assessment in the open-label maintenance period.
2.3.1 Patient groups assessed
In this post hoc analysis, patient groups are defined based on whether they received rotigotine or placebo in the 6-month double-blind studies. Patients who received rotigotine in the preceding 6-month, double-blind study (i.e., 6-month earlier initiation of rotigotine), and continued rotigotine treatment in the long-term open-label extension study are referred to as the ‘Rotigotine–Rotigotine’ group. Patients who received placebo in the preceding 6-month, double-blind study, and thus initiation of rotigotine treatment started in the long-term open-label extension study are referred to as the ‘Placebo–Rotigotine’ group.
2.4 The effect of 6-month earlier double-blind initiation with rotigotine on long-term efficacy: UPDRS II + III total score
Change in UPDRS II + III total score from double-blind baseline over the course of the double-blind and open-label studies are reported for the ‘Rotigotine–Rotigotine’ and ‘Placebo–Rotigotine’ treatment groups. In addition, the time taken for the mean UPDRS II + III total scores to return to double-blind baseline values (i.e., at what time point the mean scores are no longer improved relative to double-blind baseline) are reported. Furthermore, in order to explore the influence of the burden of activities of daily living and motor impairment in the time taken for the mean UPDRS II + III total scores to return to double-blind baseline values, we also looked at the UPDRS II (activities of daily living) and UPDRS III (motor) subscores over time.
2.5 Rotigotine and levodopa use over the course of the open-label studies
Rotigotine dose during the open-label studies is reported for two groups. In addition, use of levodopa during the open-label studies (number of patients receiving, time to initiation and dose) is reported.
2.6 Statistical analyses
Analyses of pooled data from the two open-label studies and preceding double-blind studies are reported. Analysis for the 6-month double-blind period is restricted to patients who also participated in the open-label extensions. All data are reported as observed, and were analyzed in a descriptive manner only. Thus, the statistical tests applied are considered exploratory in nature and p-values < 0.05 do not infer statistical significance. The rotigotine–placebo treatment difference for the improvement in UPDRS II + III from baseline to end of double-blind maintenance, as well as the ‘Rotigotine–Rotigotine’ – ‘Placebo–Rotigotine’ treatment difference for the change from double-blind baseline to open-label week 84 (the time point where mean UPDRS II + III total scores had crossed baseline in the ‘Placebo–Rotigotine’ group), was evaluated using an analysis of covariance with treatment and study as factors and baseline UPDRS II + III score as the covariate. The time to: i) first return to double-blind baseline UPDRS II + III score; and ii) first levodopa use, during the open-label maintenance period was analyzed using survival analysis methods; Kaplan–Meier plots were generated and the likelihood ratio test was applied to compare differences between the groups.
3. Results
3.1 Patients
A total of 346 patients (221 ‘Rotigotine–Rotigotine’ and 125 ‘Placebo–Rotigotine’) were included in the current analyses; these were HY stage 1 or 2 at double-blind baseline and defined as having at least one UPDRS II + III assessment at baseline, at the start of open-label maintenance period, and at least one UPDRS II + III assessment in the open-label maintenance period. Premature discontinuations from the open-label extensions were similar between the treatment groups: 114/221 (52%) ‘Rotigotine–Rotigotine’ patients and 64/125 (51%) ‘Placebo–Rotigotine’ patients were still in the study when rotigotine became commercially available or at the time of closure by the sponsor (4 – 6 years after the onset of open-label rotigotine treatment). 44/221 (20%) ‘Rotigotine–Rotigotine’ and 31/125 (25%) ‘Placebo–Rotigotine’ patients withdrew prematurely due to AEs, and 9/221 (4%) ‘Rotigotine–Rotigotine’ and 5/125 (4%) ‘Placebo–Rotigotine’ withdrew due to lack of efficacy. Demographic and baseline characteristics at double-blind study baseline were similar between the treatment groups and are reported in (); the mean age at double-blind baseline was slightly numerically higher in the ‘Placebo–Rotigotine’ group (63.0 ± 10.3 vs 60.9 ± 9.7).
Table 1. Demographic and baseline characteristics (double-blind baseline).
3.2 The effect of 6-month earlier double-blind initiation with rotigotine on long-term efficacy: UPDRS II + III total score
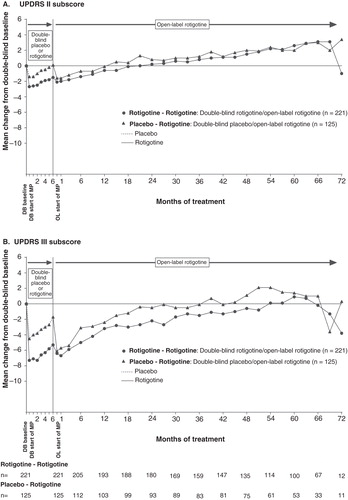
Patients were treated with double-blind rotigotine or placebo for 6 months, and then open-label rotigotine for up to 6 years (72 months) (). Mean ± SD UPDRS II + III total scores at double-blind baseline were similar between the different treatment groups: ‘Rotigotine–Rotigotine’ (29.3 ± 10.5) and ‘Placebo–Rotigotine’ (27.8 ± 10.1). In the 6-month double-blind studies, for patients that also participated in the open-label studies, the improvement in UPDRS II + III from baseline to end of double-blind maintenance was greater in those receiving rotigotine versus those receiving placebo; LS mean (95% CI) treatment difference: −5.01 (−7.28, −2.75), p < 0.001 (exploratory analysis). At start of open-label rotigotine maintenance, mean change from double-blind baseline UPDRS II + III were −8.5 ± 10.6 in the ‘Rotigotine–Rotigotine’ group and −7.7 ± 9.0 in ‘Placebo–Rotigotine’ group (). After this initial decrease (i.e., improvement) in UPDRS II–III total score, mean scores gradually increased: It took 180 weeks (∼ 45 months) for mean scores to cross double-blind baseline (i.e., worsened relative to double-blind baseline) in the group of patients who initiated treatment with rotigotine 6-months earlier (i.e., the ‘Rotigotine–Rotigotine’ group), whereas it took 84 weeks (∼ 21 months) in the group of patients whose initiation of rotigotine treatment started 6-months later (i.e., the ‘Placebo–Rotigotine’ group) (). At the time the mean UPDRS II + III had crossed baseline in the ‘Placebo-Rotigotine’ group (open-label week 84), the LS mean ± SE change from baseline to open-label week 84 was −2.85 ± 0.91 in the ‘Rotigotine–Rotigotine’ group and 1.03 ± 1.25 in the ‘Placebo–Rotigotine’ group; LS mean (95% CI) treatment difference −3.89 (−6.94, −0.84), p = 0.013. Mean UPDRS III (motor) subscores remained improved (i.e., below double-blind baseline values) for longer than mean UPDRS II (activities of daily living) subscores in both treatment groups ().
Figure 1. Mean change from double-blind baseline in UPDRS II + III for patients with early PD (HY 1–2). UPDRS II + III total score possible range 0 – 160.
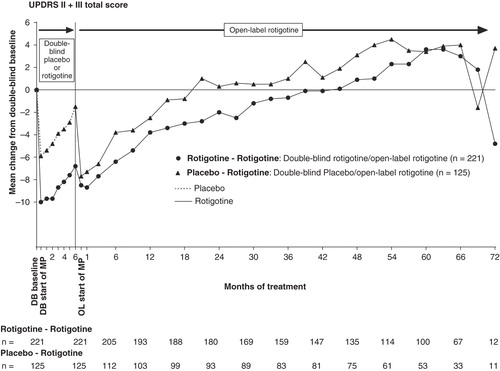
Figure 2. Mean change from double-blind baseline in (A) UPDRS II and (B) UPDRS III for patients with early PD (HY 1–2). UPDRS II is measured on a scale from 0 to 52 points, and UPDRS III measured on a scale from 0 to 108 points.
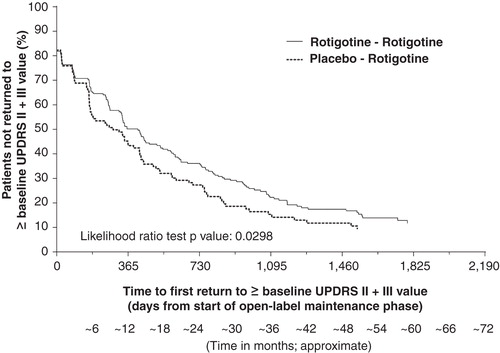
The survival curve for time to return to baseline UPDRS II + III scores (i.e., no longer improved relative to double-blind baseline) over the duration of the open-label maintenance period was in favor of those patients who had received initiation of treatment with rotigotine 6 months earlier (‘Rotigotine–Rotigotine’ group) compared with the ‘Placebo–Rotigotine’ group (p = 0.0298, likelihood ratio test) ().
Figure 3. Kaplan–Meier plot for time to first return to double-blind baseline UPDRS II + III for patients with early PD (HY 1–2). The Kaplan–Meier curve is calculated from the total number of patients at risk of returning to ≥ double-blind baseline UPDRS II + III value at each time point. Patients at risk are those remaining in the study and who have not returned to ≥ double-blind baseline UPDRS II + III value.
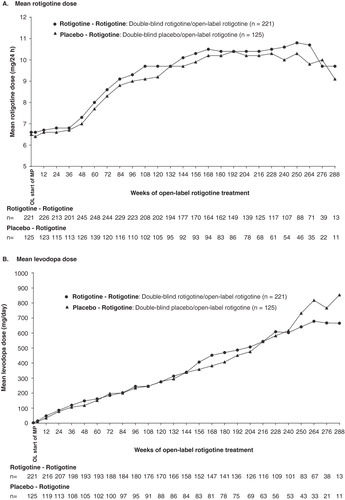
3.3 Rotigotine and levodopa use over the course of the open-label studies of up to 6 years’ duration
3.3.1 Rotigotine
Six-month earlier initiation of treatment with rotigotine did not appear to influence the dose of rotigotine in the open-label studies; the mean ± SD dose of rotigotine during the open-label studies was similar between the ‘Rotigotine–Rotigotine’ and ‘Placebo–Rotigotine’ treatment groups (). The mean dose of rotigotine increased over the course of the open-label studies of up to 6 years’ duration; however, there was no apparent difference in the increase over time between the groups ().
Table 2. Rotigotine and levodopa exposure over the course of the open-label studies.
3.3.2 Concomitant levodopa
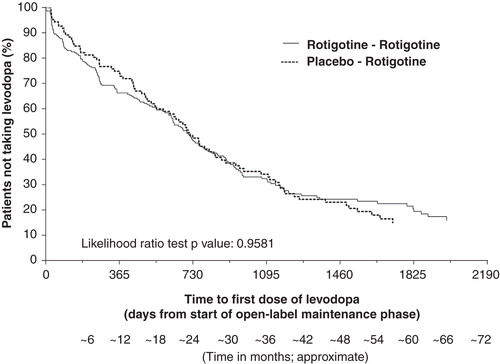
Six-month earlier initiation of treatment with rotigotine did not appear to influence the use of concomitant levodopa during the open-label studies. During up to 6 years of open-label rotigotine treatment, 254 of 346 patients (73.4%) included in these analyses started concomitant levodopa treatment: this was similar between the ‘Rotigotine–Rotigotine’ and ‘Placebo–Rotigotine’ treatment groups (). The distribution in time to first levodopa use was similar between the treatment groups; there was no apparent difference between the treatment groups in the time to first levodopa use (p = 0.9581, likelihood ratio test) (). The mean ± SD dose of concomitant levodopa used during the open-label study was similar between groups (). The mean dose of levodopa increased over the course of the open-label studies; however, there was no apparent difference in the increase over time between the groups ().
Figure 5. Kaplan–Meier plot for time to first levodopa use during open-label study for patients with early PD (HY 1–2). The Kaplan–Meier curve is calculated from the total number of patients at risk of receiving their first dose of levodopa at each time point. Patients at risk are those remaining in the study and who have not received levodopa.
4. Discussion
The results of this exploratory post hoc analysis suggest that a 6-month earlier double-blind initiation of rotigotine treatment in patients with early PD with mild symptom severity and disability (HY stage 1–2) may result in a slower return to double-blind baseline UPDRS II + III total scores, suggesting that initiation of rotigotine in PD patients during the early stages of this disease, when patients are presenting with minimal/no functional disability or impairment, may be associated with additional long-term clinical benefit.
As expected, UPDRS scores indicated disease progression over the course of the long-term studies. UPDRS III (motor) subscores remained improved relative to double-blind baseline for longer than mean UPDRS II (activities of daily living) subscores in both treatment groups, suggesting that motor function may have been better controlled over the long-term compared with the ability to control activities of daily living. Of note, mean UPDRS II + III total scores remained improved relative to double-blind baseline for ∼ 3.5 years in the patients who initiated treatment with rotigotine 6 months earlier, whereas in the patients who started rotigotine 6 months later, the UPDRS II + III total scores remained improved relative to baseline for ∼ 1.5 years. These results suggest there may have been a potential increase of up to 2 years longer sustained efficacy in some patients with early PD when treatment with rotigotine was started only 6 months earlier. At the time point when the mean UPDRS II + III total scores had crossed baseline scores (i.e., worsened relative to double-blind baseline) in the patients who started rotigotine 6-months later, the mean scores were still improved relative to baseline in the patients who initiated treatment with rotigotine 6-months earlier (LS-mean treatment difference of −3.89; p = 0.013). The Kaplan–Meier plot and analysis for time to first return to double-blind baseline UPDRS II + III total score was in support of a difference between the two groups (p = 0.0298). Of note, there were no apparent clinically relevant differences between the treatment groups over the course of the long-term open-label studies in the rotigotine dose, number of patients who received concomitant levodopa, time to levodopa or levodopa dose, suggesting that these factors did not contribute to the observed numerical differences in UPDRS II + III total scores between the two groups. The results of the current post hoc analyses do not provide information on the mechanism/s for the potential additional long-term benefit of 6-month earlier treatment with rotigotine. However, the data are in support of the concept that treatment intervention in the early stages of PD, when there may be a strain on the intrinsic compensatory mechanisms counteracting the dopaminergic deficit and a rapid rate of deterioration, may be beneficial due to supplementation of dopaminergic activity Citation[1,2,6]. Early restoration of the dopaminergic ‘tone’ may thus help support the compensatory events; if initiation of treatment is delayed, patients may not have the full opportunity to support the compensatory mechanisms and may be less able to stabilize the symptoms of PD. There are preclinical data which suggest that rotigotine may offer neuroprotection in dopaminergic cell cultures Citation[21,22] and in dopamine neurons in a mouse model of PD Citation[23]. However, our current exploratory analyses to assess the impact of 6-month earlier treatment with rotigotine on long-term efficacy cannot be interpreted to support a neuroprotective or disease-modifying effect of rotigotine in patients with PD.
The issue of when to initiate treatment in patients with PD remains somewhat controversial. Multiple studies have demonstrated the efficacy and tolerability of levodopa and other, non-levodopa, strategies for early and advanced PD symptom control, although data are still lacking in support of a particular strategy that definitively changes the ultimate outcome of disease progression. It is intriguing to consider that, in up to 25% of patients, the use of levodopa was not initiated in the open-label studies, potentially reducing the development of motor complications and avoiding the need to consider surgical options such as deep brain stimulation. The optimal timing of dopaminergic therapy initiation is influenced by clinical judgment, and may be withheld in some clinical practices. The current findings suggest that delaying treatment initiation with DAs, even in patients with only mild symptom severity (minimal/no functional disability/impairment), may result in loss of functional ability that cannot be regained.
There are a number of limitations to consider. First, the studies included in the current analyses were not prospectively designed or powered to assess the timing of initiation of rotigotine treatment on the outcome of UPDRS scores, and the data are of a descriptive nature only; this limits the interpretation of these analyses. Second, it is worth noting the difference in mean age between the two groups of ∼ 2 years. However, although a higher age is a risk factor for faster disease progression in PD, we do not consider this relatively small difference to influence the current findings. Third, it is difficult to evaluate the long-term efficacy of rotigotine monotherapy, as the majority of patients started treatment with concomitant levodopa during the long-term open-label extension studies. However, as the proportion of patients who received levodopa was similar in each treatment group (i.e., ‘Rotigotine–Rotigotine’ 74% vs ‘Placebo–Rotigotine’ 73%), this does not appear to explain the longer sustained efficacy observed in the group of patients who received rotigotine treatment 6 months earlier. Finally, as approximately half of the patients in the open-label studies did not have the opportunity to complete the full 6 years of study participation Citation[18,19], this prevents a comprehensive longitudinal analysis of the entire study cohort. In studies comparing the long-term outcome of an active treatment group versus a group receiving initial placebo treatment, the ‘lessebo-effect’ (the negative expectation related to receiving a placebo) in the latter group should be considered Citation[24]. However, in the current studies neither patients nor physicians were aware of the double-blind treatment arm, thus this phenomenon cannot contribute to the differences observed between the groups.
5. Conclusions
Whilst recognizing the limitations of this post hoc exploratory analysis, the data suggest that a 6-month earlier initiation of rotigotine treatment may be associated with long-term benefits in patients with early PD with mild symptom severity and disability; postponing treatment may result in loss of functional ability that cannot be regained. Consideration of treatment initiation in patients with minimal/no functional disability or impairment may lead to an extended clinical benefit for the patient.
Declaration of interest
This study was supported by UCB Pharma, Monheim am Rhein, Germany. The sponsors were involved in the design of the study, the analysis and interpretation of data and in the decision to submit the paper for publication. The authors acknowledge Emily Thompson, PhD (Evidence Scientific Solutions, London, UK) for writing assistance which was funded by UCB Pharma, Brussels, Belgium, and Cédric Laloyaux, PhD (Global Publications Manager, UCB Pharma, Brussels, Belgium) for publication coordination. L Timmermann has received payments as a consultant for Medtronic Inc., Boston Scientific, SAPIENS, St Jude Medical, Bayer Healthcare, UCB Pharma, Archimedes Pharma; has received honoraria as a speaker on symposia sponsored by TEVA Pharmaceuticals, Lundbeck Pharma, Bracco, Gianni PR, Medas Pharma, UCB Pharma, Desitin Pharma, Boehringer Ingelheim, GlaxoSmithKline, Eumecom, Orion Pharma, Medtronic, Boston Scientific, Cephalon, Abbott, GE Medical, Archimedes, Bayer. The institution of L Timmermann, not L Timmermann personally, received funding by the German Research Foundation, the German Ministry of Education and Research, Manfred und Ursula Müller Stiftung, Klüh Stiftung, Hoffnungsbaum e. V., NBIA DISORDERS SOCIETY USA, Köln Fortune, Medtronic, Deutsche Parkinson Vereinigung. Archimedes Pharma, Abbott, Bayer, UCB Pharma, Zur Rose Pharma, TEVA Pharmaceuticals. LW Elmer has received speaker’s bureau honoraria or consulting fees from Lundbeck, Novartis, TEVA Pharmaceuticals, and UCB Pharma; and has received research or unrestricted educational grant support from TEVA Pharmaceuticals. M Asgharnejad, B Boroojerdi, E Dohin, and F Woltering are salaried employees of UCB Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, et al. Imaging of compensatory mechanisms in Parkinson’s disease. Cur Opin Neurol 2010;23(4):407-12
- Maetzler W, Hausdorff JM. Motor signs in the prodromal phase of Parkinson’s disease. Move Disorders 2012;27(5):627-33
- Herz DM, Florin E, Christensen MS, et al. Dopamine replacement modulates oscillatory coupling between premotor and motor cortical areas in Parkinson’s disease. Cereb Cortex 2014;24(11):2873-83
- Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the quality standards subcommittee of the american academy of neurology. Neurology 2002;58(1):11-17
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311(16):1670-83
- Schapira AH, Obeso J. Timing of treatment initiation in Parkinson’s disease: a need for reappraisal? Ann Neurol 2006;59(3):559-62
- Schrag A, Dodel R, Spottke A, et al. Rate of clinical progression in Parkinson’s disease. A prospective study. Mov Disord 2007;22(7):938-45
- Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Eng J Med 2009;361(13):1268-78
- Rascol O, Fitzer-Attas CJ, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease (the ADAGIO study): prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol 2011;10(5):415-23
- Parkinson Study Group. A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Archiv Neurol 2004;61(4):561-6
- Grosset D, Taurah L, Burn DJ, et al. A multicentre longitudinal observational study of changes in self reported health status in people with Parkinson’s disease left untreated at diagnosis. J Neurol Neurosurg Psychiat 2007;78(5):465-9
- AlDakheel A, Kalia LV, Lang AE. Pathogenesis-targeted, disease-modifying therapies in Parkinson disease. Neurotherapeutics 2014;11(1):6-23
- Scheller D, Ullmer C, Berkels R, et al. The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol 2009;379(1):73-86
- Elshoff JP, Braun M, Andreas JO, et al. Steady-state plasma concentration profile of transdermal rotigotine: an integrated analysis of three, open-label, randomized, phase I multiple dose studies. Clin Ther 2012;34(4):966-78
- Watts RL, Jankovic J, Waters C, et al. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 2007;68(4):272-6
- Jankovic J, Watts RL, Martin W, et al. Transdermal rotigotine: double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol 2007;64(5):676-82
- Giladi N, Boroojerdi B, Korczyn AD, et al. Rotigotine transdermal patch in early Parkinson’s disease: a randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord 2007;22(16):2398-404
- Elmer LW, Surmann E, Boroojerdi B, et al. Long-term safety and tolerability of rotigotine transdermal system in patients with early-stage idiopathic Parkinson’s disease: a prospective, open-label extension study. Parkinsonism Relat Disord 2012;18(5):488-93
- Giladi N, Boroojerdi B, Surmann E. The safety and tolerability of rotigotine transdermal system over a 6-year period in patients with early-stage Parkinson’s disease. J Neural Transm 2013;120(9):1321-9
- Giladi N, Ghys L, Surmann E, et al. Effects of long-term treatment with rotigotine transdermal system on dyskinesia in patients with early-stage Parkinson’s disease. Parkinsonism Relat Disord 2014;20(12):1345-51
- Oster S, Radad K, Scheller D, et al. Rotigotine protects against glutamate toxicity in primary dopaminergic cell culture. Eur J Pharmacol 2014;724:31-42
- Radad K, Scheller D, Rausch WD, et al. Neuroprotective effect of rotigotine against complex I inhibitors, MPP(+) and rotenone, in primary mesencephalic cell culture. Folia Neuropathol 2014;52(2):179-86
- Scheller D, Stichel-Gunkel C, Lubbert H, et al. Neuroprotective effects of rotigotine in the acute MPTP-lesioned mouse model of Parkinson’s disease. Neurosci Lett 2008;432(1):30-4
- Mestre TA, Shah P, Marras C, et al. Another face of placebo: the lessebo effect in Parkinson disease: meta-analyses. Neurology 2014;82(16):1402-9