Abstract
Stem cell transplantation is a promising approach for improving cardiac function after severe myocardial damage for which use of autologous cells have been preferred to avoid immune rejection. Recently, however, rodent as well as human mesenchymal stromal cells (MSCs) have been reported to be uniquely immune tolerant, both in in vitro as well as in vivo transplant models. In this editorial, we briefly summarize the current understanding of the underlying immunologic mechanisms, which can facilitate the use of such cells as “Universal Donor Cells.”
Myocardial infarction remains a widespread and important cause of morbidity and mortality among adults. A promising approach currently under intensive investigation is transplantation of mesenchymal stromal cells (MSCs) in order to improve the function of the injured myocardium through several mechanisms aimed at attenuating left ventricular dysfunction Citation[1]. The observed beneficial effects of cell transplantation have led to numerous human clinical trials in the past several years Citation[2]. Although most studies have focused on the role of MSCs after a severe ischemic injury, other groups have reported promising results in cases of idiopathic or dilated cardiomyopathies Citation[3].
The current preferred approach of using autologous stem cells taken from the same donor aims to avoid immune rejection of transplanted cells, which can be expected after allogeneic transplantation, in which cells from a different donor within the same species are used. However, harvesting autologous cells from individual patients still poses significant logistic, economic and timing constrains. Furthermore, most patients who could benefit from such therapy are elderly patients with multiple medical comorbidities. Unfortunately a number of recent studies have documented that MSCs obtained from elderly donors, and those with diabetes, renal failure or severe ischemic heart disease, demonstrate significantly reduced capacity for proliferation, differentiation and neovascularization, with increased levels of apoptosis in vitro and in vivo Citation[4]. Such impaired autologous cells from sick elderly patients could therefore limit their therapeutic potential. Thus there would be obvious clinical advantages if “universal donor cells” from healthy young donors could be used for stem cell transplantation without the need for immunosuppressive therapies.
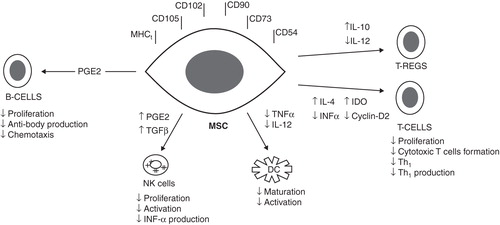
In this emerging field of cell transplantation, it is generally taken for granted that such donor cells will behave immunologically like any other mature cells, when transplanted in histocompatibility-mismatched recipients. However, in the last few years, increasing experimental findings have pointed towards a unique immunomodulatory property of the MSCs both in the in vitro and in vivo settings Citation[5]. The mechanisms of such immunotolerance have been the subject of intense studies and three interrelated candidate mechanisms are emerging. MSCs appear to survive within the foreign environment by: (1) being hypoimmunogenic; (2) modulating T-cell phenotype; and (3) immunosuppressing the local environment Citation[6].
A large body of in vitro experiments supports the view that MSCs avoid allogeneic response by being MHC class-I positive and MHC class-II negative Citation[5]. In addition to this, MSCs do not appear to express Fas-ligand nor costimulatory molecules such as B7-1 (CD80), B7-2 (CD86), CD40 for effector T-cell induction Citation[6]. There is also good in vitro evidence that MSCs can directly modulate the function of T cells by constitutively secreting PGE2, inhibiting TNF-α and interferon-γ and stimulating IL-10 secretion, hence altering the cytokine secretion profile of dendritic cells (DCs), cytotoxic T lymphocytes, and NK cells Citation[7]. By doing so, they inhibit the maturation and migration of various antigen-presenting cells, suppress B-cell activation, induce suppressor T-cell formation and alter the expression of several receptors necessary for antigen processing Citation[8]. Furthermore, through the release of IL-4, they accelerate a shift from a majority of pro-inflammatory Th1 cells towards an increase in the anti-inflammatory Th2 cells, evade NK-cell targeting mechanisms and inhibit the formation of CD8+ T cells. Citation[7,8].
In addition to the proposed role of indoleamine 2,3-dioxygenase (IDO)-mediated tryptophan degradation, MSCs can also secrete other peptides such as hepatocyte growth factor (HGF), which may contribute to the creation of a local immunosuppressive environment Citation[5]. Similarly, transforming-growth factor β1 seems also involved in T-cell suppression by working with HGF in promoting the allo-escaping phenotype. Others provided further evidence that MSCs interfere with DC maturation, differentiation and function by down-regulating the expression of CD1a, CD40, CD80, CD86, and HLA-DR Citation[6,7].
Finally, another level at which MSCs may modulate immune responses is through the inhibition of B-cells proliferation as well as their chemotactic behavior and antibody production Citation[5,6,8]. Taken together, numerous studies provide strong evidence that MSCs are able to modulate the function of different immune cells in vitro through several interrelated mechanisms as summarized in .
Figure 1. Proposed mechanisms of the immunomodulatory effects of MSCs. Tregs indicates regulatory T cells.
It is however to be noted that some in vitro studies have showed that the administration of allogeneic MSCs without immunosuppression has resulted in an increase the memory T-cell response as well as activation of NK cells leading to their rejection Citation[9,10]. Although it is likely that MSCs can effectively modulate the properties of different immune cells in vitro, particularly by actively suppressing T-cell proliferation and inhibiting DC differentiation, the exact mechanisms underlying these immunotolerant properties are still unclear and some contradictory findings have been reported Citation[5,6,10]. Furthermore, subtle differences related to the route of administration, the exact phenotype of the cell injected and the timing of injection could partially explain some of the different results reported by different groups Citation[6]. It is also important to note that there is typically a low retention yield when MSCs are directly injected within the myocardium, or given intravenously. This is mostly attributed to the mechanical loss secondary to leakage and washout or the passage through the lungs Citation[11]. This could also partially explain the different results sometimes observed among different studies.
Although considerable data of in vitro findings support the immunomodulatory properties of MSCs, relatively little evidence is available on the immunogenicity of MSCs in vivo. Despite this, there is growing evidence that the in vitro observations may translate to the in vivo setting. Bartholomew and colleagues Citation[12] first demonstrated that the in vivo administration of allogeneic MSCs prolonged third party skin graft survival in immunocompetent baboons. This study, as well as many others, paved the way for the use of these cells in immune-mediated disorders such as Hurler's syndrome, metachromatic leukodystrophy, or osteogenesis imperfect Citation[6,8]. Other groups have reported that it can also prevent the rejection of allogeneic B16 mouse melanoma cells in immunocompetent mice, successfully engraft in brains of albino rats, lead to significant improvement in symptoms in mice with autoimmune encephalomyelitis through the induction of peripheral tolerance and attenuate GVHD in humans with grade IV acute GVHD Citation[6,13].
Allogeneic MSCs transplant into the myocardium between unrelated porcine donors and recipients was first successfully reported in a swine and a rat model Citation[5]. Moreover, these cells were shown to differentiate and contribute to the functional improvement of the host myocardium Citation[6].
In November 2000, an important study by Liechty and coworkers was published in Nature Medicine Citation[14]. Well-characterized human MSCs were implanted into fetal sheep early in gestation. In this xenogeneic system, the human MSCs engrafted and persisted in multiple tissues even after maturation of the fetal immune system. Furthermore, these cells underwent site-specific differentiation into multiple cell lineages including cardiomyocytes. Nevertheless, these experiments were carried out in fetal recipients.
In a series of studies at our laboratory, Saito and associates injected intravenously labeled mice MSCs into fully immunocompetent adult rats, successfully producing stable cardiac chimeras for at least 12 weeks without any immunosuppression and with no evidence of rejection Citation[13]. In the following 4 to 6 weeks, the labeled cells were seen to differentiate into various phenotypes. In subsequent studies, MacDonald and colleagues showed that not only stable chimeras were formed, but also the overall ventricular function was significantly improved Citation[15]. These findings were replicated by our group and confirmed the survival of pig MSCs implanted into fully immunocompetent rat myocardium for up to 6 months after xenotransplantation. More recently, Atoui and associates were able to confirm the engraftment of human MSCs within the rat myocardium for at least 8 weeks after myocardial infarction, and without the use of any immunosuppression Citation[16]. Such xenotransplant significantly contributed to the improvement in the overall cardiac function and in attenuating left ventricular remodeling.
However, tolerance of MSCs across the MHC barrier might not be absolute. Grinnemo and colleagues Citation[17] demonstrated that although the MSCs successfully engraft across allogeneic barriers, rejection occurs when a xenotransplant model is used. These findings were in direct contrast to those obtained in our laboratory. Despite the similarities between our two studies, there seems to be nonetheless subtle differences in the experimental designs. For instance, in their study, MSCs were harvested from the sternum of patients undergoing cardiac surgery. These cells, taken from elderly donors, were previously shown to have a significantly lower capacity for differentiation, angiogenesis, survival and even proliferation Citation[4]. It is of interest to note that in the in vitro studies, human MSCs used were instead harvested from young healthy donors. Furthermore, other experimental differences linked to the amount of fetal calf serum present in the culture media could also partially explain these differences Citation[6]. Still, further studies are needed to better clarify these contradictory findings.
Such contradictory findings are perplexing, but not unique in this rapidly developing field of stem cell biology and regenerative medicine. Despite the substantive body of evidence from the in vitro literature confirming the immunomodulatory properties of MSCs, their importance in the in vivo setting remains controversial. Nevertheless, MSCs have already been introduced to clinical practice, especially in the autoimmune and hematological fields Citation[18].
Interestingly, and based on the clinical and experimental data discussed previously, there have been two clinical trials so far that looked at the allogeneic use of MSCs in patients post myocardial infarction. The FDA recently approved an Osiris, Inc. sponsored Phase-I multi-center clinical trial in which allogeneic human MSCs were given intravenously, without immunosuppression, to patients following an acute myocardial infarction. The preliminary results after 6 months were presented and are highly encouraging with evidence of significant improvement in the ventricular function Citation[19]. Similarly, the Revascor trial is another randomized, placebo-controlled multicenter trial that assesses the safety and feasibility of three different doses of allogeneic MSCs delivered into the myocardium of patients with chronic heart failure. After 3 months, their preliminary results were also reported online (http://www.angioblast.com) and described a significant improvement in the overall ventricular function as well as a reduction in left ventricular end systolic volumes, after a mean follow-up of 22 months. More importantly, promising results of the Phase-II trial were recently reported at the American Heart Association (Florida, 2011) that confirmed that, in addition to the improvement in the overall function, MSCs treatment significantly delayed the time to a first Major Adverse Cardiac Event (MACE) and reduced the overall risk for MACE by up to 78%. Based on this, Phase-III trials are expected to start within the upcoming 6 months.
Finally, it is important to raise a safety concern with the use of MSCs. Although most studies performed to date confirm the overall safety of the infused MSCs, concerns remain over the risk of ectopic tissue formation or malignant transformation Citation[20]. Further studies are therefore needed to address these concerns.
Expert opinion
The potential importance of these findings for the treatment of ischemic heart disease is apparent. In addition to their powerful replicative capacity, MSCs can easily be harvested from bone marrows, expanded ex vivo, and differentiated into many cell-type lineages, if desired. Due to their immunotolerance property, the establishment of MSCs as effective “universal donor cells” Citation[6] could then significantly expand the therapeutic potential for cellular cardiomyoplasty. From a clinical perspective, these cells could be isolated and expanded from donors irrespective of their MHC haplotype, tested for their functional capabilities well in advance, and stored as an “off-the-shelf” cell population for immediate use when needed on any patient after an acute myocardial infarction. Such logistic advantages are not available with the use of autologous MSCs which is currently the cell source of choice. Perhaps more importantly, since such allogeneic MSCs can be obtained from young healthy donors, they could be of great value in patients with genetic cardiomyopathies and in elderly patients with multiple medical comorbidities whose own MSCs could be dysfunctional.
Despite the exciting preliminary results, further investigations are required to address many of the remaining controversial findings, as well as the important question of chronic rejection after cell transplantation. Although the preliminary results of allogeneic MSC transplantation described above seem quite promising, the trials enrolled only a limited number of patients who were evaluated relatively shortly after the treatment. Moreover, further mechanistic studies and more quantitative assessment of MSCs engraftment are still needed before the therapeutic promise of these cells can be fully achieved.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Notes
Bibliography
- Williams AR, Trachtenberg B, Velazquez DL, Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011;108(7):792-6
- Kinkaid HY, Huang XP, Li RK, Weisel RD. What's new in cardiac cell therapy? Allogeneic bone marrow stromal cells as “universal donor cells”. J Card Surg 2010;25:359-66
- Chin SP, Poey AC, Wong CY, Intramyocardial and intracoronary autologous bone marrow derived mesenchymal stromal cell treatment in chronic severe dilated cardiomyopathy. Cytotherapy 2011;13(7):814-21
- Zhuo Y, Li SH, Chen MS, Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg 2010;139(5):1286-94
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110(10):3499-506
- Atoui R, Shum-Tim D, Chiu RCJ. Myocardial regenerative therapy: immunologic basis for the potential “universal donor cells”. Ann Thorac Surg 2008;86:327-34
- Aggarwal S, Pittenger M. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22
- Kassis I, Vaknin-Dembinsky A, Karussis D. Bone marrow mesenchymal stem cells: agents of immunomodulation and neuroprotection. Curr Stem Cell Res Ther 2011;6(1):63-8
- Nauta AJ, Westerbuis G, Kruisselbrink AB, Donor-dereived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006;108(6):2114-20
- Spaggiari GM, Capobianco A, Becchetti S, Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2 induced NK-cell proliferation. Blood 2006;107(4):1484-90
- Al Kindi AH, Asenjo JF, Ge Y, Microencapsulation to reduce mechanical loss of microspheres: implications in myocardial cell therapy. Eur J Cardiothorac Surg 2011;39(2):241-7
- Bartholomew A, Sturgeon C, Satskas M, . Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42-8
- Saito T, Kuang JQ, Bittira B, Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg 2002;74:19-24
- Liechty KW, Mackenzie TC, Shaaban AF, Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 2000;11:1282-6
- MacDonald D, Saito T, Shum-Tim D, Persistence of marrow stromal cells implanted into acutely infarcted myocardium: observations in a xenotransplant model. J Thorac Cardiovasc Surg 2005;130:1114-21
- Atoui R, Asenjo JF, Duong M, Marrow stomal cells as “universal donor cells” for myocardial regenerative therapy: their unique immune tolerance. Ann Thorac Surg 2008;85:571-80
- Grinnemo K, Mansson A, Dellgren D, Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infracted rat myocardium. J Thorac Cardiovasc Surg 2004;127:1293-300
- Prasad VK, Lucas KG, Kleiner GI, Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 2011;17(4):531-41
- Trachtenberg B, Velazquez DL, Williams AR, Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (tac-hft) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J 2011;161(3):487-93
- Breitbach M, Bostani T, Roell W, Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007;110(4):1362-9
