Abstract
A body of evidence suggests that a mixture of therapeutic monoclonal antibodies (mAbs) may be better than a single antibody. Several strategies have been developed to achieve multiple targeting, including the administration of two or more mAbs to the patient, bispecific antibodies and antibody mixtures. Recently, new antibody technologies based on a diverse array of antibodies binding to several different epitopes on any given antigen or antigens have been developed. One of the most promising is the Sympress™ manufacturing technology, which allows the production of an antibody mixture in just one bioreactor as a single drug substance. Recombinant antibody mixtures may be applicable to therapy of neoplastic, autoimmune and infectious diseases.
Therapeutic monoclonal antibodies (mAbs) are a highly successful class of biological drugs, conventionally manufactured in mammalian cell lines, such as NS0 murine myeloma cells, PER.C6 human cells and Chinese hamster ovary (CHO) cells Citation[1]. In contrast to human monoclonal antibodies plasma-derived natural agents can be produced only in limited amounts with variable quality, low activity and potential danger of contamination with pathogens. Over the last few years, several mAbs and immunotoxins have been investigated in patients with malignancies and autoimmune disorders. Clinically effective anti-tumour mAbs, such as cetuximab, avastin and herceptin, work mainly by direct effects on their respective target. Moreover, clinically effective monoclonal anti-tumor antibodies can recruit Fc receptor (FcR)-positive accessory cells of the immune system via their constant fragment (Fc) domain and engage these cells additionally against the tumour Citation[2].
Different strategies have been developed recently to enhance the efficacy of antibody therapeutics. The newer generation of mAbs is humanised or fully human to reduce immunogenicity and/or have an engineered Fc region designed to increase their effector functions. However, engineering the natural mAb molecules increases the risk of increased immunogenicity and/or toxicity to the patient. More recently, small modular immunopharmaceuticals (SMIPTM) and recombinant immunotoxins have been developed that offer the promise of increasing both the efficacy and safety of therapeutic regimens by directing drugs specifically to tumour targets, and reducing systemic toxicity Citation[3,4]. Moreover, several bi-specific and pan-specific antibodies have been developed which represent alternative approaches Citation[5]. In these approaches, a single antibody binds to at least two molecular targets simultaneously. Recently, several bi-specific antibodies have been designed and produced in the laboratory and will undoubtedly undergo further testing, including administration to patients Citation[6,7].
Some evidence indicates that a mixture of therapeutic mAbs may be better than a single antibody. A recent approach to increase the therapeutic effectiveness of mAbs has been to combine two or more of them. The combination of antibodies can be used if they have a different molecular target, different mechanisms of action and/or complementary activity Citation[7,8]. Several authors reported that mAb combinations can increase the anticancer therapeutic potential in comparison with a single mAb Citation[8]. Using a mixture of therapeutic mAbs to simultaneously block different growth-factor receptors on tumour cells might result in less chance of target cell survival Citation[7,9]. In vitro studies have shown that a combination of anti-EGF receptor mAbs that engage distinct epitopes significantly accelerates receptor degradation Citation[8]. In addition, mAb combinations were found to be more effective than a single mAb in inhibiting HER2 signaling in vitro and in animals Citation[8]. Several strategies have been developed to achieve multiple targeting () Citation[10]. Two or more individual mAbs can be generated as individual drug products and then independently administered to the patient. Moreover, individual mAbs can be formulated into one drug product or they can be produced simultaneously using a single cell line. Finally, antibody mixtures can be produced by single-batch manufacturing of the antibody mixtures by cells originated from two or more cell clones.
Table 1. Strategies for generation of antibody mixtures with potential therapeutic value.
There are several examples of the use of two or more separately produced antibodies in the treatment of neoplastic or immune disorders. Preclinical and clinical data indicate that a combination of anti-CD23 mAb lumiliximab and anti-CD20 mAb rituximab has enhanced antitumor properties and an early clinical study suggests that the combination of these two antibodies with chemotherapy is more efficient than immunochemotherapy with rituximab alone in patients with chronic lymphocytic leukemia (CLL) Citation[11]. However, a Phase III international trial comparing lumiliximab combined with rituximab, fludarabine and cyclophosphamide (RFC) with RFC alone was recently prematurely closed because of the low benefit, demonstrated by this combination in relapsed CLL Citation[12]. Rituximab combined with the anti-CD52 antibody alemtuzumab were also used in the treatment of lymphoid malignancies and immune disorders. However, because of the limited duration of response, additional efforts are required to improve the efficacy of this therapy Citation[13]. Alemtuzumab-rituximab combination therapy appears to be an efficacious and safe treatment for steroid-refractory cGvHD Citation[14].
Recombinant antibody mixtures represent a new antibody technology which consists of a diverse array of antibodies binding to several different epitopes on any given antigen or more than one antigen. They are produced using either in vitro cell culture processes or transgenic technology in animals or plants Citation[15,16]. Antibody mixtures can be produced either by individually manufacturing the constituent antibodies or producing a single batch of two or more antibodies Citation[10]. The antibody mixtures have several advantages over individual mAbs, which include increased immune effector functions, increased target elimination by receptor cross-linking and target degradation, synergy by simultaneous binding of more than one target and superior systemic clearance of soluble toxins due to complex formation Citation[10]. In consequence, they have higher therapeutic effectiveness and/or broader reactivity than a single monoclonal antibody Citation[17-20]. Mixtures of recombinant antibodies consisting of a defined number of well-characterised mAbs have better therapeutic efficacy in disorders with heterogenic endogenous targets including neoplastic and infectious diseases.
Several companies are currently using a manufacturing strategy of simple mixtures of independently produced antibodies, produced and characterized as individual drug substances. These antibodies are subsequently mixed into one drug product administered to the patient Citation[10]. An example of a simple mixture of two antibodies is Combotox (National Cancer Institute). It is a 1:1 mixture of two immunotoxins, prepared by coupling deglycosylated ricin, a chain (dgRTA) to monoclonal antibodies directed against CD22 (RFB4-dgRTA) and CD19 (HD37-dgRTA) Citation[21]. Combotox has been studied in adults and children with B-lineage haematologic malignancies Citation[22,23]. The drug was well-tolerated and proved efficacious in these neoplasms.
An alternative approach to target these two antigens has been to make an improved bivalent recombinant immunotoxin (DT2219ARL), in which one Fv binds to CD19 and the other to CD22 Citation[24]. DT2219ARL has shown excellent activity in preclinical models Citation[24]. The ability of the agent to kill malignant B cells is greater than that of its monospecific counterparts and has the ability to produce long-term cancer survivors in two highly aggressive systemic human B cell malignancy models in scid mice Citation[24]. DT2219ARL is now in a Phase I trial in patients with B-cell NHL or CLL that have relapsed or not responded to treatment (ClinicalTrials.gov Identifier:NCT00889408).
Another example of a simple mixture of antibodies is CL184, which was designed for postexposure prophylaxis against rabies. CL184 is a 1:1 equipotent mixture of two human monoclonal antibodies (CR57 and CR4098), each directed against a distinct, non-overlapping rabies virus epitope Citation[25]. CR57 and CR4098 are fully human monoclonal IgG1 antibodies produced on the PER.C6 human cell line directed against different rabies virus glycoprotein epitopes (antigenic site I and III, respectively) and are capable of neutralising the virus. These two antibodies have been developed as replacements of human rabies immune globulin and equine rabies immune globulin in post-exposure prophylaxis. The CL184 antibody cocktail is currently being tested in clinical trials as a replacement for human rabies immune globulin in post-exposure prophylaxis. In Phase I study, CL184 was found to be safe, well-tolerated and demonstrated the expected rabies virus neutralising activity Citation[26]. CL184 seems to be a more optimised product than the plasma-derived, polyclonal products obtained from rabies-vaccinated human donors or horses Citation[27].
XOMA 3AB is a mixture of three antibodies in one anti-botulinum neurotoxin product Citation[28]. XOMA 3AB was designed for the treatment and prevention of type A botulism poisoning. It is an equimolar mixture of three IgG1 mAbs, referred to as NX01, NX02 and NX11. The intact XOMA 3AB antibodies are composed of two IgG1 gamma heavy chains and two kappa light chains with a single asparagine-linked site of glycosylation located in the CH2 region of the Fc Citation[29,30]. These antibodies target different epitops of botulinum toxin type A. Each mAb comprising XOMA is individually expressed by separate stable Chinese hamster ovary (CHO) cell lines. XOMA 3AB was investigated in rats as a single-dose for intravenous and intramuscular administration. A Phase I, placebo-controlled, double-blinded, dose escalation study of XOMA 3AB in healthy adult volunteers is ongoing (ClinicalTrials.gov Identifier:NCT01357213).
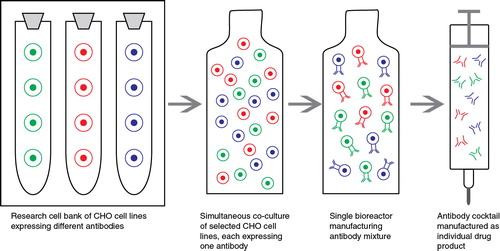
Researchers from the Danish biotechnology company Symphogen A/S have developed a single batch manufacturing approach using the Sympress™ method for the manufacture of recombinant antibody mixtures (). This technology allows the production of any number of specific antibodies in a single bioreactor as one drug substance Citation[10]. To produce all the desired antibodies by this method, seed material from the polyclonal working cell bank (pWCB) is prepared for the bioreactor by mixing the individual stable cell lines Citation[18]. In the Sympress™ system, mixtures of recombinant antibodies can be produced under predictable, reproducible and stable conditions based on expression in the genetically modified dihydrofolate reductase (DHFR) negative CHO cell line DG44 Citation[31]. Individual manufacturing cell lines expressing a target antibody are initially generated by vector construction and transfection into a chosen cell and integrated into the host genome. A bidirectional CMV promoter construct enabling co-expression of the IgG light and heavy chains from one plasmid is used for stable transfection Citation[17,18]. Parental cells are transfected separately with individual antibody expression vectors using standard transfection technology, and high-expressing clones are expanded and frozen as individual monoclonal cell lines. The relative antibody ratio in antibody mixtures can be controlled effectively by mixing the individual monoclonal cell lines appropriately before generation of the polyclonal master cell banks (pMCB). The relevant monoclonal cell lines are then mixed in predefined ratios and frozen as pMCB. The single batch manufacturing approach which expresses a mixture of full-length IgG provides a robust and reproducible platform that can be used for manufacturing recombinant antibody mixtures. In addition, this system is cost-efficient and comparable to costs for monoclonal antibodies.
Figure 1. Schematic presentation of the Sympress™ technology for single-batch manufacturing of antibody mixtures.
A Sym004 antibody mixture directed against two distinct epitopes on the extracellular domain of EGFR has been produced using the Sympress™ technology Citation[19,32]. In an efficacy-based approach, more than 400 different antibody combinations were tested in a standard viability assay in vitro, and then three candidate mixtures were selected and tested in vitro for their ability to inhibit tumour growth in vivo Citation[33]. The most potent and having the highest efficacy of all combinations both in vitro and in vivo had a combination of two antibodies, 992 and 1024. The combination of 992 and 1024 works highly synergistically due to very efficient induction of EGFR internalisation and subsequent superior growth inhibitory activity with their ability to induce efficient EGFR degradation. Sym004 has superior anticancer efficacy than existing mAbs. In vitro experiments with Sym004 showed that the antibody mixture was able to induce ADCC at levels similar to mAb cetuximab, as well as high levels of CDC, which is not induced by cetuximab Citation[34]. Importantly, Sym004 also induces superior apoptosis compared with cetuximab in vitro Citation[32,33]. The results of in vitro experiments indicate that Sym004 can overcome resistance from the competing ligand by effectively removing the receptor from the cell surface. Moreover, Sym004 was superior to cetuximab at inhibiting EGFR phosphorylation and downstream signaling to ERK1/2, PDK1, AKT and S6R in human cancer cell lines Citation[17]. Sym004 was shown to be superior to its component mAbs alone in inhibiting cancer cell growth in vivo. In addition, Sym004 exhibits more pronounced growth inhibition in vitro and superior efficacy in vivo than reference anti-EGFR monoclonal antibodies and has a mechanism of action that differs from other mAbs currently used in the clinic. Moreover, this recombinant antibody mixture is active in tumours with acquired resistance to other EGFR-targeted agents, including both mAbs, like cetuximab and selective tyrosine kinase inhibitor AG1478 Citation[19,33].
Currently under development by Symphogen A/S is the first in a new class of a recombinant anti-D antibody mixture: rozrolimupab (SYM-001, Symphoglobulin-D) Citation[35,36]. It comprises 25 genetically unique fully human IgG1 mAbs produced by a single-batch manufacturing strategy, which were designed to capture the natural diversity of the human antibody response to RhD Citation[35]. They all are specific for the RhD erythrocyte antigen derived from the plasma of eight RhD-negative female donors with high antibody titres against RhD. The manufacturing of rozrolimupab is based on the Flp-In system in CHO cells. This system enables integration of the recombinant expression cassette at the same genomic position earlier defined by the presence of integrated Flp-In recombination target site Citation[36]. The technology enables highly consistent and reproducible manufacture of recombinant antibody mixtures from batch to batch. It has been documented in preclinical studies that compositions of rozrolimupab are consistently obtained when cells are cultured in shaker flasks and in bioreactors. In addition, different batches of rozrolimupab bind to RhD-positive erythrocytes with similar potency Citation[35,36]. Reaserchers from Symphogen documented selected quality attributes of rozrolimupab based on a battery of assays at the genetic-, protein- and functional-levels. They demonstrated that the manufactured rozrolimupab batches are highly pure and very uniform in their composition Citation[35]. The advantage of rozrolimupab over polyclonal anti-D immunoglobulin is unlimited supply, high and reproducible specificity and activity and an improved safety profile. Rozrolimupab is intended for the treatment of ITP in RhD-positive patients and the prevention of hemolytic disease of the fetus and newborn. In October 2010, the US FDA granted Orphan status to the drug for the treatment of ITP. Recently, the results of a Phase I/II of rozrolimupab dose escalation study in ITP patients have been reported Citation[37,38]. The objective of the study was to evaluate safety and efficacy of the drug in adult RhD-positive patients with ITP. Rozrolimupab was well-tolerated with no unexpected toxicities. The mostfrequent adverse events were headache, pyrexia, chills and fatigue. Haemoglobin decreased in 8% of the patients and direct antiglobulin test (DAT) positivity was noted in 6%. Serious adverse events defined as rozrolimupab dependent were observed in 4 patients. The best response was noted in patients treated with rozrolimupab administered at doses of 250 and 300 µg/kg. Among the 13 patients treated with the 300 µg/kg dose, response was observed in 8 patients. Median time to response was 2 days and median duration of response was 14 days. These results indicate that rozrolimupab is safe, well-tolerated and effective in patients with ITP.
Recently, de Kruif et al. developed a method to efficiently express multiple monoclonal antibodies from a single cell. Using this technology, the generation of stable cell clones that express high levels of a human monoclonal antibody mixture has became possible Citation[39,40]. PER.C6 cells were transfected with a combination of plasmids containing genes encoding three different antibodies. The majority of subclones expressed all three antibody specificities in constant ratios resembling those of single monoclonal antibody cell lines from conventional clone generation programs. This methodology is applicable to the generation of stable PER.C6(R) clones for industrial scale production of mixtures of antibodies. Recombinant antibody mixtures present a new therapeutic strategy that can be more effective in the management of autoimmune and neoplastic diseases than existing single antibodies.
The single-batch manufacture of the antibody mixtures is sufficiently robust for commercial manufacturing scales. Developing more-potent therapeutic human antibody mixtures based on ease and cost of development and production, regulatory approvability and unmet medical needs will change the therapeutic-antibody landscape.
In the future these new agents will be probably used in combination with emerging promising compounds like kinase inhibitors and other agents. Further studies exploring the toxicity profile and efficacy of these new therapeutic agents, alone or in combination with standard treatments are required to address these issues in well designed clinical trials.
Declaration of interest
This work was supported in part by grant from the Medical University of Lodz, Poland (No 503/1-093-01/503-1). The author received research grant and travel grant from Symphogen A/S.
Bibliography
- Li F, Vijayasankaran N, Shen AY, et al. Cell culture processes for monoclonal antibody production. MAbs 2010;2:466-79
- Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist 2007;12:1084-95
- Robak T. Emerging monoclonal antibodies and related agents for the treatment of chronic lymphocytic leukemia. Future Oncol 2013;9(1):69-91
- Pastan I, Hassan R, FitzGerald DJ, et al. Immunotoxin treatment of cancer. Annu Rev Med 2007;58:221-37
- Fagète S, Fischer N. Smarter drugs: a focus on pan-specific monoclonal antibodies. BioDrugs 2011;25:357-64
- Thakur A, Lum LG. Cancer therapy with bispecific antibodies: clinical experience. Curr Opin Mol Ther 2010;12:340-9
- Choi BD, Kuan CT, Cai M, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci USA 2013;110:270-5
- Fuentes G, Scaltriti M, Baselga J, et al. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 2011;13:R54
- Fournier P, Schirrmacher V. Bispecific antibodies and trispecific immunocytokines for targeting the immune system against cancer: preparing for the future. BioDrugs 2013;27:35-53
- Rasmussen SK, Næsted H, Müller C, et al. Recombinant antibody mixtures: production strategies and cost considerations. Arch Biochem Biophys 2012;526:139-45
- Byrd JC, Kipps TJ, Flinn IW, et al. Phase ½ study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood 2010;115:489-95
- Robak T. Improving FCR immunochemotherapy in CLL. Blood 2010;115:437-8
- Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer 2008;113:2110-18
- Gutiérrez-Aguirre CH, Cantú-Rodríguez OG, Borjas-Almaguer OD, et al. Effectiveness of subcutaneous low-dose alemtuzumab and rituximab combination therapy for steroid-resistant chronic graft-versus-host disease. Haematologica 2012;97:717-22
- Raju TS. Assessing Fc Glycan Heterogeneity of therapeutic recombinant monoclonal antibodies using NP-HPLC. Methods Mol Biol 2013;988:169-80
- Yusibov V, Streatfield SJ, Kushnir N, et al. Hybrid viral vectors for vaccine and antibody production in plants. Curr Pharm Des 2013; Epub ahead of print
- Rasmussen SK, Nielsen LS, Müller C, et al. Recombinant antibody mixtures; optimization of cell line generation and single-batch manufacturing processes. BMC Proc 2011;5(Suppl 8):O2
- Frandsen TP, Naested H, Rasmussen SK, et al. Consistent manufacturing and quality control of a highly complex recombinant polyclonal antibody product for human therapeutic use. Biotechnol Bioeng 2011;108:2171-81
- Skartved NJ, Jacobsen HJ, Pedersen MW, et al. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res 2011;17:5962-72
- Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010;70:588-97
- Messmann RA, Vitetta ES, Headlee D, et al. A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin Cancer Res 2000;6:1302-13
- Schindler J, Gajavelli S, Ravandi F, et al. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol 2011;15:471-6
- Herrera L, Bostrom B, Gore L, et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2009;31:936-41
- Vallera DA, Chen H, Sicheneder AR, et al. Genetic alteration of a bispecific ligand-directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res 2009;33:1233-42
- Bakker AB, Marissen WE, Kramer RA, et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J Virol 2005;79:9062-8
- Bakker AB, Python C, Kissling CJ, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine 2008;26:5922-7
- Kostense S, Moore S, Companjen A, et al. Validation of the rapid fluorescent focus inhibition test for rabies virus-neutralizing antibodies in clinical samples. Antimicrob Agents Chemother 2012;56:3524-30
- Meyer K, Ng H, Parman T, et al. Nonclinical safety evaluation of XOMA 3AB, a novel triple monoclonal antibody drug product targeting botulinum toxin type A, in Sprague-Dawley Rats. Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Meeting; 10 – 12 January 2011; Washington
- Teshima G, Li MX, Danishmand R, et al. Separation of oxidized variants of a monoclonal antibody by anion-exchange. J Chromatogr A 2011;1218:2091-7
- Meng Q, Li M, Silberg MA, et al. Domain-based assays of individual antibody concentrations in an oligoclonal combination targeting a single protein. Anal Biochem 2012;421:351-61
- Nielsen LS, Baer A, Müller C, et al. Single-batch production of recombinant human polyclonal antibodies. Mol Biotechnol 2010;45:257-66
- Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: A novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010;70:588-97
- Koefoed K, Steinaa L, Søderberg JN, et al. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs 2011;3:584-95
- Dechant M, Weisner W, Berger S, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res 2008;68:4998-5003
- Andersen PS, Haahr-Hansen M, Coljee VW, et al. Extensive restrictions in the VH sequence usage of the human antibody response against the Rhesus D antigen. Mol Immunol 2007;44:412-22
- Wiberg FC, Rasmussen SK, Frandsen TP, et al. Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol Bioeng 2006;94:396-405
- Stasi R. Rozrolimupab, symphobodies against rhesus D, for the potential prevention of hemolytic disease of the newborn and the treatment of idiopathic thrombocytopenic purpura. Curr Opin Mol Ther 2010;12:734-40
- Robak T, Windyga J, Trelinski J, et al. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood 2012;120:3670-6
- de Kruif J, Kramer A, Nijhuis R, et al. Generation of stable cell clones expressing mixtures of human antibodies. Biotechnol Bioeng 2010;106:741-50
- Rosati S, Thompson NJ, Barendregt A, et al. Qualitative and semiquantitative analysis of composite mixtures of antibodies by native mass spectrometry. Anal Chem 2012;84:7227-32
