Abstract
With the enormous success of recombinant monoclonal antibodies (rMAbs) as human therapeutics, there are increasing efforts underway to explore new molecular entities that mimic rMAbs to replicate this huge success. In addition to naked intact rMAbs, antibody drug conjugates (ADCs), FAb and F(ab′)2 fragments and also Fc fusion proteins have been developed and/or marketed as human therapeutics to treat different human diseases, including life-threatening diseases such as cancer. Several hundreds more intact rMAbs, ADCs, FAb, F(ab′)2 fragments and Fc fusion proteins are currently undergoing human clinical trials. In addition to these molecules, new type of antibody fragments such as single-chain Fvs (scFvs), VH, scFv-Fc, scFv-CH, scFAb, scFv-zipper, diabodies, bispecific antibodies and similar types of constructs are also being investigated to be developed as human monotherapeutics. Further, there are quite a few current examples of combinations of biologics being developed. For example, currently, several biopharmaceutical companies are developing combinations of antibody mixtures as human therapeutics. Accordingly, the question posed here is whether it is time to consider the possibility of developing a broader range of combinations of therapeutic biologics. Combinations of small organic molecules have been successfully used as therapeutics for many years to treat many diseases, so the context of using polypharmacology to treat human diseases is not novel. For the past several decades, intravenous immunoglobulins have successfully been used in treating various autoimmune diseases. In this context, several biotechnology companies are exploring the use of combinations of antibody mixtures as human therapeutics. This editorial discusses these current efforts and the potential future role of antibody mixtures as human therapeutics.
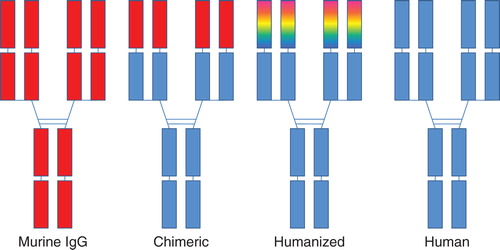
The US FDA approval of rituximab (Rituxan) for treatment of non-Hodgkin's lymphoma in 1997 started a wave of recombinant monoclonal antibodies (rMAbs) to be approved as human therapeutics Citation[1-3]. Today, more than two dozen full length intact rMAbs have been approved by FDA as human therapeutics to treat various diseases Citation[2]. With respect to the humanness of these rMAbs, there are currently four categories on the market; these include murine (no human sequences), chimeric (about two-thirds human sequences), humanized (typically > 95% human sequences) or fully human rMAbs (). These rMAbs are manufactured by expression in and purification from in vitro cell cultures, typically using mammalian cell lines such as Chinese hamster ovary cells or mouse myeloma cells Citation[1-3]. Muromonab-CD3 (Orthoclone OKT3), tositumomab and iodine I 131 tositumomab (Bexxar), and ibritumomab tiuxetan (Zevalin) are examples of murine antibodies; rituximab (Rituxan), basiliximab (Simulect), infliximab (Remicade®) and cetuximab (Erbitux) are chimeric molecules; daclizumab (Zenapax), palivizumab (Synagis), trastuzumab (Herceptin®), gemtuzumab ozogamicin (Mylotarg), alemtuzumab (Campath), efalizumab (Raptiva), omalizumab (Xolair), bevacizumab (Avastin), natalizumab (Tysabri), pertuzumab (Perjeta®) and eculizumab (Soliris) are humanized molecules; and adalimumab (Humira), golimumab (Simponi), canakinumab (Ilaris), ustekinumab (Stelara®), ofatumumab (Arzerra), denosumab (Prolia), belimumab (Benlysta) and ipilimumab (Yervoy) are human antibodies. Many of these rMAbs each have generated greater than $1 billion in annual revenue Citation[1-3].
Full length intact rMAbs generally show a very long serum half-life mainly due to FcRn binding to the Fc region of these molecules Citation[3,4]. The Fc region of rMAbs also plays an important role in the antibody cell killing mechanisms, including complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity, which may be important for antibodies used in treating tumors or infectious disease agents Citation[1,3]. The cell killing ability of naked intact antibodies, however, is somewhat limited and can be dependent on the highly heterogeneous glycosylation in the CH2 domain of the Fc Citation[1-3]. A different approach for the use of rMAbs to kill cancer cells is by utilizing the targeting ability of rMAbs to carry highly potent natural product cytotoxins directly to the tumor cells where they are taken up by cellular mechanisms and released from the antibody to kill the targeted cells Citation[4]. These molecules, called antibody drug conjugates (ADCs) Citation[4], typically link the cytotoxic drugs chemically through a stable or specifically cleavable linker to either Lys residues or Cys residues of rMAbs Citation[4]. The ADCs are engineered to deliver the cytotoxic payload to the specific tumor cell site to minimize the nonspecific toxicity of the drug. Currently, only a couple of ADCs, for example, brentuximab vedotin (Adcetris) and trastuzumab emtansine (Kadcyla), are approved for marketing. The recent approval of these two ADCs, however, has created a huge hope for these types of molecules. Accordingly, more than 30 ADCs are currently in human clinical trials and predictions are that many of these molecules have a very good chance to become successful drugs on the market Citation[1,4].
Fc fusion proteins are another type of IgG-like molecules comprising a protein, peptide or receptor exodomain fused to the Fc portion of an IgG molecule Citation[5,6]. In these molecules, the Fc portion typically contains the hinge region along with CH2 and CH3 domains. Hence, the Fc fusion proteins also contain the conserved N-glycosylation site in the CH2 domain Citation[6]. In these antibody-like molecules, the Fc portion provides the much-needed boost to the increased serum half-life of smaller proteins/receptors, without much affecting their biological activity Citation[3]. Etanercept (Enbrel) is an Fc fusion protein which consists of the tumor necrosis factor receptor (TNFR) subunit p55 fused to IgG1Fc; hence, it is sometimes referred to as TNFR-IgG Citation[7]. TNFR-IgG reduces inflammation by binding to active TNF present at elevated level in people with autoimmune diseases Citation[7]. Etanercept, approved by the US FDA to treat rheumatoid arthritis and other autoimmune disorders, is a very highly successful drug, generating more than $7 billion in annual revenue in 2011 Citation[2,3].
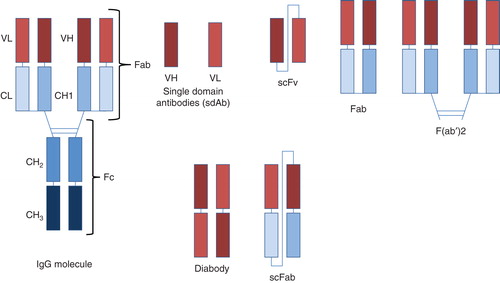
To treat some human diseases, the longer serum half-life and/or cell killing mechanisms of rMAb-based therapeutics are not required. Thus, antibody fragments such as FAb fragments that show reduced serum half-life have been also developed as human therapeutics Citation[8]. Ranibizumab (Lucentis), a FAb fragment of an anti-VEGF antibody that was approved in 2007 to treat advanced macular degeneration, is a very highly successful marketed drug, with revenue in 2011 of over $3.7 billion Citation[1-3]. Because of the success of antibody and antibody fragments as human therapeutics, efforts are underway to develop additional potential formats of antibodies, including VHH (single-domain antibodies), single-chain Fv (scFv), scFv-Fc, diabodies, scFv-CH, scFAb, scFv-zipper and such similar human antibody constructs (), as human therapeutics Citation[1,8]. Many of these monoclonal antibody fragment-based drug candidates are currently in human clinical trials.
Over the past few years, the interest in developing bispecific and multispecific antibodies as human therapeutics has significantly increased Citation[9]. Bispecific antibodies, which can be generated by a variety of different approaches Citation[9], may be intact rMAbs that contain two or more variable domains with specific affinity to bind different antigens. One example of a bispecific antibody format is the DuoBody developed by Genmab, which is formed by combining two antibodies, each separately mutated in the CH3 domain, resulting in a more favorable recombination over formation of the parental antibodies when reduced and re-oxidized Citation[9]. Another example of a bispecific antibody format is the dual-variable domain-immunoglobulin (DVD-Ig) Citation[10]. Unlike DuoBody, in DVD-Ig, each heavy and light chains contain two variable fused domains and hence each IgG molecule of DVD-Ig carries four variable-binding domains. Currently, there is a rapidly increasing interest by many companies to develop bispecific and/or multispecific antibodies as human therapeutics because of their significant therapeutic potential Citation[9,10]. At least seven new bispecific antibodies are in early stages of clinical development, with many more in preclinical studies.
Intravenous immunoglobulins (IVIGs) have long been used in treating many autoimmune diseases Citation[10,11]. IVIG is a highly heterogeneous mixture of polyclonal antibodies derived from the serum of multiple healthy human donors Citation[10]. IVIG preparations, which have been very successful on the market, are being used to treat many human diseases throughout the world. However, the limitation of IVIG is that patients need to be injected with several grams of IVIG to obtain any clinical benefits. Recently, it was shown that specifically glycosylated IVIG fractions have anti-inflammatory activity Citation[12]. This specific glycosylation has been identified as α-2,6-sialylation of Fc glycans, which results in the binding to DC sign on dendritic cells, imparting an anti-inflammatory activity to those IVIG fractions Citation[12]. This specifically sialylated fraction found in IVIG, however, is very small and hence the efficacy of the IVIG drug is limited.
For several human therapies, therapeutic rMAbs may be used either alone or in combination with small molecule-based drugs to treat patients. One classic example of this paradigm is the treatment of rheumatoid arthritis patients with the small molecule, methotrexate, and an anti-TNF-α antibody such as infliximab, or adalimumab, or golimumab. Combinations of small molecule drugs are also successfully used in treating many human diseases Citation[13]. One such example is using a cocktail of drugs including a protease inhibitor, a nucleoside-based reverse transcriptase inhibitor, a non-nucleoside reverse transcriptase inhibitor and/or an integrase inhibitor to treat patients infected with HIV Citation[14]. Similar to these approaches, mixtures of rMAbs are now being developed as human therapeutics Citation[15]. These antibody mixtures contain two or more antibodies directed against either a single antigen or different antigens Citation[16]. Antibody mixtures against a single antigen may recognize different binding epitopes and may exhibit synergies in neutralizing and/or cell killing, whereas antibody mixtures against different antigens is perhaps more similar to IVIG antibodies, with the exception that the recombinant antibody mixtures contain well-defined and well-characterized antibodies that target well-defined antigens. There are at least eight different examples of antibody mixtures that are currently in clinical development Citation[1]. These antibody mixtures may provide advantages over antibody monotherapy because the individual antibody components present in the mixture may complement each other to target the diseases () Citation[17]. For example, Xoma is developing an antibody mixture containing three antibodies to target and neutralize the toxin from botulism poisoning Citation[15-17]. Xoma 3AB, which is a lyophilized formulation of three different antibodies in equimolar proportions directed against three distinct domains on the BoNT/A neurotoxin protein, is an example of an antibody mixture against a single antigen Citation[15-17].
Table 1. Advantages of antibody mixtures.
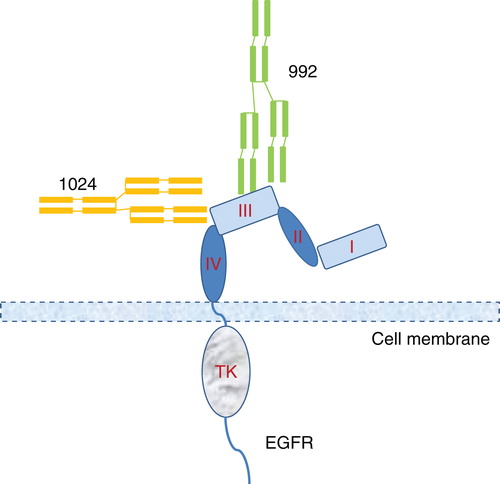
Symphogen A/S is currently developing several antibody mixtures that are directed against either a single antigen or multiple antigens Citation[16]. Sym004 is a mixture of two chimeric IgG1 antibodies against non-overlapping epitopes on EGFR domain III () Citation[15-17]. This antibody mixture seems to possess a unique mechanism of action which induces more rapid internalization and degradation of EGFR than single anti-EGFR antibodies (). Sym004 is currently in Phase II human clinical trials Citation[17]. Sym013, or Pan-HER antibody mixture, contains six humanized antibodies targeting three different antigens, that is, EGFR, HER2 and HER3 Citation[15-17]. The Pan-HER antibody mixture has been shown to simultaneously downmodulate the EGFR, HER2 and HER3 antigens and appears to circumvent the acquired resistance to anti-HER2 drugs due to upregulation of other receptor tyrosine kinases. Several other similar antibody mixtures targeting a single antigen or multiple antigens are being developed by other companies (). Also, new platform technologies have been developed that makes it easier to produce antibody mixtures either using multiple cell lines or by using one cell line for each antibody molecule in order to reduce the effect of cost of goods (COGs) on the marketed products Citation[15,16].
Table 2. Some antibody mixtures that are currently in human clinical trials.
Similar to IVIG and small-molecule drug combinations, antibody mixtures may have a very significant future. Emerging evidence suggests that antibody mixtures may provide benefits over a single antibody in some circumstances Citation[18]. Early human clinical data shows that a combination of anti-CD20 and anti-CD23 rMAbs may have better antitumor activity than either of the rMAbs alone Citation[17,18]. Also, rituximab in combination with an anti-CD52 antibody appears to have better cell killing properties than rituximab alone Citation[15-18]. Hence, the combination of antibodies with complementary activity may be a potential approach to treat complex human diseases. Many human diseases are highly complex due to the influence of multiple signaling pathways. For example, psoriasis is very complex skin disorder for which the US FDA has approved several different antibodies targeting multiple different targets, for example, TNF-α and IL-12/23 Citation[19]. In addition to the overexpression of TNF-α, IL-12 and IL-23, there is evidence to suggest that other antigens pathways may be involved in psoriasis Citation[19]. Although monotherapies with either anti-TNF-α (Remicade) or anti-IL12/23 (Stelara) alone is effective, a combination therapy containing a mixture of antibodies against IL-12, IL-17, IL-23 and/or possibly TNF-α may eventually prove to be more effective. Similarly, cancer is a very complex disease with multiple causes that may be due to the overexpression of many different antigens. Although, several rMAbs have been approved to treat different types of cancer, none of them has been shown to effectively cure 100% of the treated patients. More effective treatment of cancer patients may be achieved using a combination of highly potent rMAbs that are currently in the market. One example of this paradigm is the mixture of the anti-HER2 antibodies, Herceptin and Perjeta with docetaxel, which provided more survival benefit to patients in a Phase III clinical trial of metastatic breast cancer than did Herceptin and docetaxel alone Citation[19,20]. This general phenomenon might be one reason why Symphogen A/S has developed Symselect™, a novel platform technology to select functional lead combination antibodies intended for antibody mixtures Citation[19,20].
Antibody mixtures using a combination of highly successful marketed rMAbs or developing new combinations of novel rMAbs may prove to be a useful alternative to monotherapy to treat complex human diseases. Since new technologies have been developed to produce novel combinations of antibody mixtures at potentially reasonable COGs, it may be possible to develop highly successful antibody mixtures to treat complex human diseases. Although regulatory avenue for approval of antibody mixtures is not yet clear, this should not hinder the progress of developing successful antibody mixtures as human therapeutics. Regulatory agencies may take more time, initially, to approve antibody mixtures as human therapeutics. This is because; the characterization of each individual component will take time to assess. However, once the regulatory path is clear, the approval process will be easier and may be similar to rMAbs' approval. Hence, antibody mixtures may have a significant role as future potentially successful human therapeutics.
Declaration of interest
All authors are employed by Janssen R&D, LLC and have no conflict of interest.
Bibliography
- Strohl WR, Strohl LM. Therapeutic antibody engineering: current and future advances driving the strongest growth area in the pharma industry. Woodhead Publishing Series in Biomedicine No. 11 Woodhead Publishing; Cambridge: 2012
- Reichert JM. Marketed therapeutic antibodies compendium. MAbs 2012;4:413-15
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol 2008;20:471-8
- Govindan SV, Goldenberg DM. Designing immunoconjugates for cancer therapy. Expert Opin Biol Ther 2012;12:873-90
- Weidle UH, Schneider B, Georges G, Brinkmann U. Genetically engineered fusion proteins for treatment of cancer. Cancer Genomics Proteomics 2012;9:357-72
- Raju TS, Briggs JB, Chamow SM, et al. Glycoengineering of therapeutic glycoproteins: in vitro galactosylation and sialylation of glycoproteins with terminal N-acetylglucosamine and galactose residues. Biochemistry 2001;40:8868-76
- Spadaro A, Lubrano E, Ferrara N, Scarpa R. Etanercept in psoriatic arthritis. J Rheumatol Suppl 2012;89:74-6
- Dimitrov DS. Therapeutic proteins. Methods Mol Biol 2012;899:1-26
- Labrijn AF, Meesters JI, de Goeij BE, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci USA 2013;110:5145-50
- Jakob CG, Edalji R, Judge RA, et al. Structure reveals function of the dual variable domain immunoglobulin (DVD-Ig™) molecule. MAbs 2013;5:358-63
- Günther G, Dreger B. Post-marketing observational study on 5% intravenous immunoglobulin therapy (Alphaglobin®/Flebogamma®) in secondary immunodeficiency with recurrent serious bacterial infections. Microbiol Immunol. 2013; doi:10.1111/1348-0421.12060
- Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol 2010;30(Suppl 1):S9-14
- Polat ZA, Walochnik J, Obwaller A, et al. Miltefosine and polyhexamethylene biguanide: a new drug combination for the treatment of Acanthamoeba keratitis. Clin Experiment Ophthalmol 2013; doi:10.1111/ceo.12120
- Olender S, Wilkin TJ, Taylor BS, Hammer SM. Advances in antiretroviral therapy. Top Antivir Med 2012;20:61-86
- Meng Q, Garcia-Rodriguez C, Manzanarez G, et al. Engineered domain-based assays to identify individual antibodies in oligoclonal combinations targeting the same protein. Anal Biochem 2012;430:141-50
- Haurum JS. Recombinant polyclonal antibodies: the next generation of antibody therapeutics? Drug Discov Today 2006;11:655-60
- Skartved NJ, Jacobsen HJ, Pedersen MW, et al. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res 2011;17:5962-72
- Byrd JC, Kipps TJ, Flinn IW, et al. Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood 2010;115(3):489-95
- Raut AS, Prabhu RH, Patravale VB. Psoriasis clinical implications and treatment: a review. Crit Rev Ther Drug Carrier Syst 2013;30:183-216
- Koefoed K, Steinaa L, Søderberg JN, et al. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs 2011;3:584-95