Abstract
Introduction: Stroke is a major worldwide cause of death and disability. Currently, intravenous thrombolysis and reperfusion therapies, but not the so-called neuroprotectant drugs, have been shown to be effective for acute ischemic stroke. Thus, new strategies to promote brain plasticity are necessary. Stem cell administration is an attractive future therapeutic approach.
Areas covered: Brain protection and repair mechanisms are activated after stroke. This article is focused on the capacity of stem cell-based therapy to enhance this postinfarct brain plasticity and recovery. Future therapeutic considerations and prospects for stroke are discussed.
Expert opinion: Although cell therapy is promising in stroke treatment, mechanisms of action need to be characterized in detail. Further, the different mechanisms of axonal plasticity and remodeling involucrated in brain repair, not only in the gray but also in white matter, must be investigated through noninvasive techniques, and a multidisciplinary approach is fundamental in this.
1. Introduction
A serious public health problem, stroke is one of the most important causes of death and disability worldwide and also results in significant comorbidities for patients. To date, the therapeutic strategies include nonpharmacological measures to maintain homeostasis of physiological parameters in Stroke Units, and rehabilitation Citation[1]. Intravenous thrombolysis and reperfusion therapies are also proving helpful, but are limited by their narrow therapeutic time window Citation[2].
Most of the drugs called neuroprotectants have failed in clinical trials. Some of the reasons the drugs have not been found effective in clinical trials are probably due to a failure in the development and evaluation of the animal models for cerebral infarct. Guidelines are needed to unify the methodology used in the development of experimental models, clarify and establish the critical factors to optimize the results and improve research in the field of stroke Citation[3,4]. What is more, the existing neuroprotectant therapies are only focused on saving neural cells instead of being designed to preserve all components of the neurovascular unit. Stroke produces disability not only as a result of synaptic and neuronal dysfunction but also through primary or secondary damage to white matter: axons and glia Citation[5]. The repair capacity of the brain is limited, so new strategies are required to enhance brain plasticity by the use of drugs with trophic effects, cell therapy and a combination of the two Citation[6].
Stem cells are immature cells with a self-renewal capacity and, depending on their origin, the ability to differentiate into multiple cell types. A variety of these cells types have been proposed for the treatment of stroke, including embryonic stem cells (ESCs), neural stem cells (NSCs), bone marrow mononuclear cells (BMMCs), mesenchymal stem cells (MSCs), induced Pluripotent stem (iPS) cells and some immortalized cell lines. Because of their relatively low immunogenicity, ready availability and good results in experimental animals, MSCs are especially promising candidates for cell therapy after stroke. These cells are defined by their adherence to plastics, fibroblast-like morphology, expression of surface antigens (CD44+, CD90+, CD29+), differentiation into mesodermal cells, and different lineages in vitro, including neural cells. What is more, they can be obtained from different sources: bone marrow, adipose tissue, peripheral blood and other adult tissues.
This article describes how cell therapy has gained importance as a new method to develop therapeutic strategies that enhance brain plasticity, and it also focuses on possible approaches to be considered in future translational research. In particular and in addition to gray matter damage, it would be interesting to analyze the effects of stem cell therapy on white matter injury, something that has yet to receive sufficient attention. In addition, it is important to analyze brain response in the contralesional area after stroke and how this may be affected after cell therapy.
2. Mechanisms of brain repair and recovery after stroke
Brain plasticity contributes to neurovascular unit repair by helping to reorganize structure and function in response to behavior, environment or damage. Brain repair is a natural mechanism that is spontaneously activated for days and weeks after damage.
As a compensatory endogenous process, stroke spontaneously induces neurogenesis, gliagenesis, oligodendrogenesis, remyelination, synaptic plasticity and angiogenesis – all processes that can be enhanced by cell therapy and trophic factors.
After cerebral ischemia, adult neurogenesis is activated in the subventricular zone and dentate gyrus causing the proliferating cells to migrate toward the ischemic lesion. As well, vessel remodeling in the peri-infarct zone may play a role in the recruitment of newly-born neurons to the peri-infarct region. This is probably the reason for observing neurogenesis and neuroblast migration being highly associated with the vasculature after a stroke Citation[7]. In fact, experimental evidence has indicated a close link between neurogenesis and angiogenesis in the adult brain Citation[8]. Even though most neuroblasts do not survive in the long-term because of the unfavorable microenvironment that limits survival of newborn neurons, their downstream pathways may provide a new target for stroke treatment Citation[9]. In addition, it is still unclear whether the abundance of newborn neurons generated after stroke is properly integrated into the hippocampal network since some of them display aberrant features involving bipolar dendritic arborizations and ectopic locations Citation[10].
On the other hand, little interest has been dedicated to the study of white matter after stroke, and it is very important to note that this tissue is also damaged Citation[5]. Astrocytes and oligodendrocytes provide basic trophic support that is independent of either the brain region or the surrounding neuronal populations, thus setting the stage for brain plasticity processes Citation[11]. However, several intrinsic myelin-associated neurite growth inhibitors, including Nogo-A, have important roles in blocking fiber regeneration, sprouting and new network formation, and they prevent the recovery of lost function after central nervous system damage. Treatment with anti-Nogo-A immunotherapy has improved functional outcome and been associated with neuroanatomical plasticity when administered after ischemic stroke Citation[12].
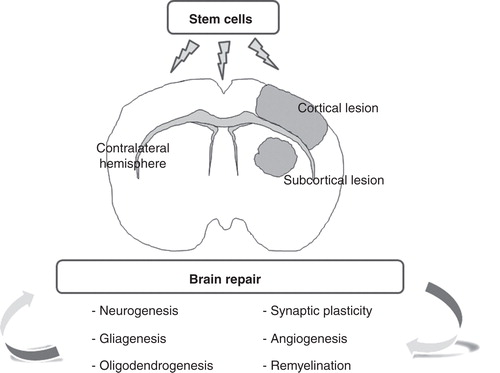
In general, all these mechanisms are implicated in brain plasticity and can be stimulated by cell therapy in order to promote remodeling and functional recovery after a stroke ().
Figure 1. Stem cell administration enhances brain repair mechanisms after cerebral infarct. Cell therapy is an attractive therapeutic approach to enhance brain plasticity that improves neurogenesis, gliagenesis, oligodendrogenesis, remyelination, synaptic plasticity and angiogenesis, not only in gray but also in the white matter. Additionally, stem cells are able to stimulate compensatory processes in the contralateral hemisphere after stroke.
3. Stem cell-derived therapy in stroke
Many studies in experimental animal models have shown that stem cell administration can improve function after stroke by stimulating a number of mechanisms such as neuron replacement or trophic action, inflammation modulation, angiogenesis promotion, induction of remyelination and axonal plasticity, as well as protection Citation[13].
Currently, three main theories have attempted to explain MSC-mediated brain repair: i) ‘trans’-differentiation, ii) cell fusion and iii) paracrine activity through the release of soluble factors. While there is evidence for all of these phenomena, there is debate over the contribution that each can realistically make to recovery Citation[10]. In this sense, trophic factor secretion seems to be especially relevant in the MSC-mediated brain repair response after stroke. The effects of these factors can be broadly classified as angiogenic (the formation of new blood vessels), neurogenic (the formation of new neural tissue), protective (the protection of neural tissue from degeneration and apoptosis), synaptogenic (the formation of synapses and synaptic contacts) and inhibition of scarring (prevention of scarring that would prevent reconstruction of neural circuitry following injury or damage) Citation[14].
In our opinion, cell-based treatment is a very interesting area for developing a new approach to cerebral infarct. However, at present there are still some open questions: the appropriate cell type, timing of administration, delivery route, optimal dose and MSC action mechanisms, in both experimental animals and clinical research. The best therapy requires that the maximum therapeutic effect would be provided to patients using minimally invasive protocols and therefore presenting the least possibility of risk.
Regarding optimum delivery, it has been posited that less-invasive routes can be as effective as more aggressive routes and would therefore be more appropriate for clinicaluse. An experimental animal study has compared two different administration routes—intracarotid and intravenous—for allogenic BM-MSCs after cerebral infarct. Both routes showed the same effectiveness for promoting functional recovery through brain protection (decreased cell death) and brain repair [increased cell proliferation and vascular endothelial growth factor (VEGF)] mechanisms. Interestingly, no stem cell migration or implantation into the ischemic lesion was observed when the MSCs were administered intravenously, indicating that the formation of cell niches is not required to achieve good functional recovery Citation[15].
Although it does not seem necessary for cells to have reached the injury site to exert a beneficial effect when administered intravenously, an interesting line of research is whether greater efficacy could be achieved by improving cell engraftment in the injury site. However, most of the administered cells are known to not survive in the environment of a brain lesion. However, a structural support like biodegradable scaffolds would allow NSCs to remain within the cavity and interconnect to form a de novo tissue Citation[16], helping to replicate the conditions and functions of the healthy tissue. Recently, axonal plasticity has been demonstrated to play an important role in recovery after ischemic brain injury. Transplanting MSCs with scaffolds enhances the effect of MSCs on axonal sprouting by corticospinal tract fibers (CTS) from the contralateral intact cortex into the denervated side of the spinal cord after damage Citation[17]. However, although exciting, the use of this approach has presented several disadvantages for translational application. It is a very hazardous, high risk technique because treatment requires these patients undergo craniotomy.
In regard to the most suitable cell types and sources, a variety of cells have been proposed to treat cerebral ischemia. A comparison of the effects of allogenic BM-and-adipose tissue-derived MSCs on functional recovery and brain repair markers in an experimental model of ischemic stroke found that both cell types showed similar efficacy in achieving a good functional recovery, as well as reducing cell death, and increasing cellular proliferation, neurogenesis, oligodendrogenesis, synaptogenesis and angiogenesis markers at 14 days post infarct Citation[18]. These good results in experimental animals have led to the start of a Phase IIa clinical trial, AMASCIS-01 (NCT01678534) investigating the safety and efficacy of intravenous administration of allogenic MSCs from adipose tissue within the first two weeks post stroke.
4. Expert opinion: future considerations and prospects for stroke
Stroke produces disability not only as a result of neuron and synapse dysfunction but also through primary or secondary damage to white matter: axons and glia, something still poorly understood. Up to 25% of ischemic strokes in humans are lacunar and confined to white matter areas such as the internal capsule Citation[5]; however, most studies only focus on neural cells after stroke and do not address the role of white matter damage. The exact mechanisms of white matter lesion formation are much less well-understood and, in our opinion, this aspect and the white matter response to MSCs administration merit serious research.
Another aspect to consider is the possibility that the contralateral area to the lesion may partly compensate for the loss of function after ischemic stroke. Recent studies have shown that astrocytes may play critical roles in functional remodeling/recovery in the area contralateral to the lesion with functional compensation after a stroke, thus providing new insights into the mechanisms underlying synaptic remodeling, thus contributing to the development of effective therapeutic approaches for patients after a stroke Citation[19]. A study observed that MSC administration after experimental ischemic stroke in adult rats significantly enhanced neuronal remodeling in the intact contralesional hemisphere through CST axonal sprouting into the impaired side of the spinal cord, thereby possibly partially contributing to functional recovery Citation[20].
Additionally, new technologies may allow advances in this field. The use of new noninvasive imaging techniques can increase our understanding of structure, morphology and brain function after cell therapy and also improve monitoring of cells after treatment. Different disciplines, such as biomedicine and computing science, are most coupled to better understand brain architecture, organization and functional activities. Therefore, the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative should develop new information about this organ and the possibilities of finding an effective treatment for stroke.
In summary, cell therapy is a good approach in stroke treatment, as it enhances repair and recovery in the damaged brain through plasticity, but mechanisms of action need to be characterized in detail. Further, it is necessary to investigate the different mechanisms of axonal plasticity and remodeling involved in brain repair, not only in the gray but also in the white matter employing noninvasive techniques, so a multidisciplinary approach is fundamental.
Article highlights.
Cell therapy is a promising therapy in stroke treatment that enhances brain plasticity and recovery.
The exact mechanisms of stem cells are less well-understood. It is necessary to investigate this aspect in depth.
Analyzing the effect of stem cell administration on white matter and the contralateral hemisphere could allow advances in this field.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of the manuscript.
Notes
This box summarizes key points contained in the article.
Bibliography
- Díez-Tejedor E, Fuentes B. Homeostasis as basis of acute stroke treatment: stroke units are the key. Cerebrovasc Dis 2005;20:129-34
- Jauch EC, Saver JL, Adams HP Jr, American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870-947
- STAIR. Recommendations for standards regarding preclinical neuropro-tective and restorative drug development. Stroke 1999;30:2752-8
- García-Bonilla L, Rosell A, Torregrosa G, et al. Recommendations guide for experimental animal models in stroke research. Neurología 2011;26:105-10
- Matute C, Domercq M, Perez-Samartin A, Ransom BR. Protecting white matter from stroke injury. Stroke 2013;44:1204-11
- Gutiérrez-Fernández M, Fuentes B, Rodríguez-Frutos B, et al. Trophic factors and cell therapy to stimulate brain repair after ischaemic stroke. J Cell Mol Med 2012;16:2280-90
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006;26:13007-16
- Thored P, Wood J, Arvidsson A, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007;38:3032-9
- Cui L, Qu H, Xiao T, et al. Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia. Restor Neurol Neurosci 2013;31:239-51
- Niv F, Keiner S, Krishna et al. Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke 2012;43:2468-75
- Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol 2012;11:369-80
- Tsai SY, Papadopoulos CM, Schwab ME, Kartje GL. Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke 2011;42:186-90
- Lindvall O, Kokaia Z. Stem cell research in stroke: how far from the clinic? Stroke 2011;42:2369-75
- Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int 2011;59:347-56
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Álvarez-Grech J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2011;175:394-405
- Bible E, Dell'Acqua F, Solanky B, et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials 2012;33:2858-71
- Mahmood A, Wu H, Qu C, et al. Effects of treating traumatic brain injury with collagen scaffolds and human bone marrow stromal cells on sprouting of corticospinal tract axons into the denervated side of the spinal cord. J Neurosurg 2013;118:381-9
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, et al. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 2013;4:11
- Takatsuru Y, Eto K, Kaneko R, et al. Critical role of the astrocyte for functional remodeling in contralateral hemisphere of somatosensory cortex after stroke. J Neurosci 2013;33:4683-92
- Liu Z, Li Y, Zhang X, et al. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke 2008;39:2571-7
