Abstract
Biological drugs, derived from living organisms, have immensely helped millions of patients across the globe. Biosimilars, although are the copies of the existing innovator biological products, cannot be substituted like the generic drugs owing to several differences. The final products in case of biosimilars are not identical as they are prepared from different cell lines by using different production methodologies and purification processes. Due to these differences, several challenges exist for entering into the biosimilar market. Many regulatory guidelines are now available for the approval of biosimilars. This renders an opportunity for healthcare community and industries to develop biosimilars in order to provide cost-effective treatment to the patients suffering from serious and life-threatening disorders. Despite the fact that entry of any biosimilar into the market would offer a competitive price advantage, large controversy exists regarding merits and demerits of biosimilars. In the present Editorial, we have carried out strength, weakness, opportunities and threats analysis of biosimilars so as to assist the readers in having a better understanding with broader perspective of biosimilars.
1. Introduction
Products of biological origin have a successful record in treating serious and chronic diseases. The recent expiry of data protection/patents for the first original biotherapeutics has led to the development and authorization of copy versions, termed ‘similar biological medicinal products’ (biosimilars) by the European Medicines Agency (EMA) in the European Union. The definition of biosimilars differs among the various regulatory agencies across the world. The US FDA's The Biologics Price Competition and Innovation Act defines Biosimilar or Biosimilarity as ‘the biological product that is highly similar to the reference product notwithstanding minor differences in clinically inactive components,’ and that ‘there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.’ Internationally, different names are used for them, for example, they are known as follow-on protein products or follow-on biologics by the US FDA and Ministry of Health, Labor and Welfare; and as subsequent entry biologics by Health Canada; as similar biological medicinal products (biosimilars) by the EMA and Korea Food and Drug Administration.
Biological drugs are derived from living organisms. The recombinant DNA technology facilitates production of biotechnology-based drugs on a large commercial scale. There are three main sources from which biological products are produced, viz. Escherichia coli, Yeast cells, and Chinese Hamster Ovary cells Citation[1-3]. Biological drugs that are commercially available are human growth hormone, insulin, monoclonal antibodies like rituximab and trastuzumab, granulocyte colony-stimulating factor (G-CSF), erythropoietin, interferon-α, etc. Citation[2].
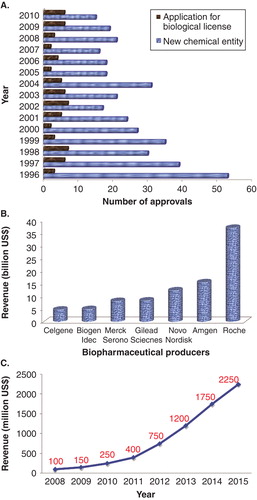
It has been estimated that biotechnology-based products have helped almost 500 million patients worldwide Citation[4,5]. Global sales of biologic products are growing rapidly with several numbers of drugs receiving approval from US FDA from 1996 to 2010 () Citation[6]. Several biopharmaceutical producers have generated > 5 billion USD revenues in 2011 from these products () Citation[7]. Biological drugs constitute one of the largest growing sectors of the pharmaceutical industry and a total global sale in 2015 is expected to reach nearly 2250 million USD () Citation[8]. It is predicted that in coming next 10 years more agents with increased complexity will be entering global markets Citation[3]. Over long term, the emergence of biosimilars from low-cost manufacturing sites plus the next generation of biopharmaceuticals (biobettters) is also set to drive the market growth.
Figure 1. (A) US FDA-approved products (from 1996 to 2010), (B) Top global biopharmaceutical producers in 2011 (based on revenue), and (C) Global revenue forecast for biosimilars.
Several regulatory guidelines are now available for the approval of biosimilars. This renders an opportunity for healthcare community and industries to develop biosimilars in order to provide cost-effective treatment to the patients suffering from serious and life-threatening disorders. This Editorial article aims to put forth the fundamentals of biosimilars along with strength, weakness, opportunities and threats (SWOT) analysis of the same so as to assist the readers in obtaining better and deeper perspectives of biosimilars.
2. Biologics versus small-molecule drugs
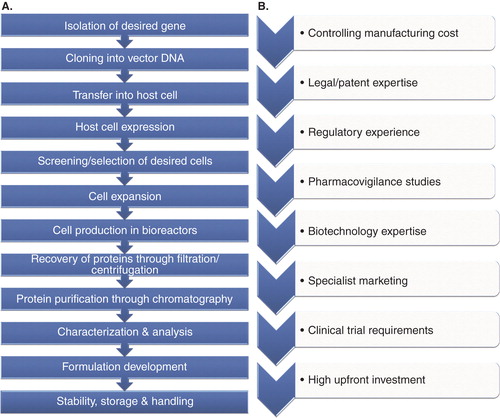
The origin of biologics is different as compared to typical small-molecule drugs and hence they differ significantly. Small-molecule drugs are usually prepared by chemical synthesis. On the contrary, biological products are prepared usually by cells or living organisms with a complex procedure involving several steps of cloning, transfection, amplification, purification, and validation (). Due to this, there is a difference in structure, composition, manufacturing methods and equipment, intellectual property, formulation, handling, dosing, regulation, and marketing of biologics Citation[9,10]. Despite the fact that biologics are prepared from same living cell, there are batch-to-batch variations even in the innovator product. This problem exists with biosimilars too, making them even more difficult to produce as an identical product. Owing to these differences, several challenges exist for entering into the biosimilar market ().
Figure 2. (A) Steps involved in the manufacturing process of biosimilars, and (B) Challenges involved in developing biosimilars.
The molecular size of biologics and small molecules is also different. For example, the size of paracetamol is 151 Da, while that of insulin is 6000 Da. Monoclonal antibodies are even larger with sizes being in the range of 145,000 – 160,000 Da Citation[3,11]. Chemical drugs have a well-defined structure and chemical formula, which makes them easy to reproduce. In contrast to this, biologics have complicated mechanism of action and unique multi-dimensional structures, which makes them difficult to reproduce. Biotechnological medicines (including both biologics and biosimilars) as compared to small molecules possess different pharmacokinetic and pharmacodynamic properties even though they have the same molecular weight and are produced by the same type of cells or microorganisms Citation[12].
In the developed markets, the official regulatory approval process for biosimilars is distinctly different from that of the generic small molecules. In case of generics, the manufacturers have to prove only pharmacokinetic comparability with respect to the innovator products. Once a generic is comparable in terms of pharmacokinetics, it can be considered as completely interchangeable with the innovator product with similar therapeutic efficacy. Moreover for generics, pharmacodynamic or clinical equivalence is not required to be established. However, due to the several differences mentioned above, biosimilars are not considered interchangeable with the reference products even after regulatory approval Citation[3]. provides an overview of difference between small molecular drugs/generics and biologics/biosimilars.
Table 1. Differences between small-molecule drugs/generics and biologics/biosimilars Citation[9].
3. Biosimilar or ‘Bio-different’
Biosimilars cannot be termed as (bio)generics. They are copied versions of the innovator biological drugs. The final products in case of biosimilars are not identical as they are prepared from different cell lines by using different production methodologies and purification processes Citation[5]. In the case of generics, only the chemical structure and formula need to be reproduced, while in the case of biosimilars, in addition to the protein structure, its folds and three-dimensional (3D) orientation also need to be the same. By the process of enzymatic glycosylation the polysaccharides are attached to proteins. The pattern of glycosylation determines the protein folds and overall stability of biosimilars, and hence they are important for the development of biopharmaceuticals. In addition, they may have a function in cell-cell adhesion and can be affected by the production methodology Citation[3,11]. For biopharmaceutical companies involved in manufacturing of biosimilars, developing an identical product with the same glycosylation pattern is very difficult as they do not have access to proprietary manufacturing data from the innovator company. Looking to the above-mentioned points, it appears that a completely interchangeable biological drug is impossible to produce with the help of existing technologies. Hence, the biopharmaceutical companies are now focusing on developing biological agents which are not exactly same as that of innovator, but are either biologically and clinically comparable (biosimilars) or have better profiles than that of innovator (biobetters) Citation[2].
4. Biobetters
While biosimilars have structural limitations over the innovator, biobetters (also known as biosuperiors or second-generation biosimilars or next-generation biosimilars) are the improvements to the innovator molecules with similar active ingredient but better profile. For example, an antibody which acts on a particular target, the biobetter of that antibody will act on the same target but with better bioavailability or reduced immunogenicity. This is possible because of the molecular or chemical modifications made in that antibody compared to that of the innovator. Biobetters can easily be patented as they are improvised versions of the innovator unlike the biosimilars. Due to the molecular or chemical modification, biobetters may have enhanced efficacy, or a longer half life which leads to lowering the dosing frequencies of the drug and reduces the risks of immunogenicity, toxicity, and side effects. These improvements are made by identifying how the changes in protein folding create a change in the effects of the drugs Citation[13].
In contrast to the regulations of the biosimilars, approval routes for the biobetters are clear. Biosimilars have efficacy and safety concerns which govern their success rate. However, since biobetters have better efficacy than the innovator product, their rate of success is better. The only risk associated with these types of products would be safety concerns, for which preclinical and clinical studies would be required. Hence, the overall costs for development of a biobetter will be closer to the estimated direct cost for a single product which is nearly $375 million Citation[14].
Opportunities are offered by biobetters to establish brand-to-brand competition within treatment indications. Since innovator product usually identifies the specific target receptor, biobetters would get an impulse for development. With the modification in the profile of the innovator products, various industries will be able to target the same indication with new products and this will create competition within the market Citation[14].
5. Expert opinion
The biosimilar industry is a fast-growing and lucrative business. There has been a huge dispute about the pros and cons of biosimilars. This dispute has even questioned the utility of biosimilars inspite of them being cheaper than the innovator and even beneficial in treating patients with several life-threatening disorders including cancer, hematological diseases, and diabetes. SWOT analysis is a method used to evaluate the SWOT of any product/business venture. It is a detailed analysis of identifying products'/business's characteristics pertaining to advantages (strengths), disadvantages (weakness), elements which could be explored (opportunities), and elements which could be troublesome (threats). We have carried out a SWOT analysis which would enable the readers to have a look at the bigger picture of the biosimilars.
Strength
Biologics are a new class of drugs that are inevitable in many diseased conditions especially in areas of rheumatology, oncology, cardiology, dermatology, neurology, etc. These are mainly recombinant proteins and have bigger structure and high molecular weight. Presently available biologics include insulin, human growth factor, erythropoietin, interleukins, G-CSF, alteplase, interferon-α, interferon-β, low-molecular-weight heparins etc. It is predicted that by 2015 nearly 50% of all new approved pharmaceuticals will be biopharmaceuticals Citation[5].
Similar efficacy as that of innovator product is obtained at a lower cost.
Weakness
Biosimilars although ‘similar’ to the innovator product are not identical. Safety is a major concern for biosimilars. Safety of any biotechnological product depends on various factors. While preparing a replica of an innovator biotechnological product, any minor modification in the method of manufacturing like change of cell line, modification of the enzyme used, or alterations in the process of purification may lead to a biosimilar which may not be identical to the innovator product. This means that even if the units of the protein molecules may be the same, however, due to the differences in their folding and 3D structure, safety issues arise.
All the biotechnological products have the potential to induce immune reactions with production of antibodies. The antibodies may initiate an immune response to the therapeutic or endogenous protein which may be life threatening. Further, being a biological response, the immunogenicity of the biosimilars becomes difficult to be predicted in preclinical studies. This thus makes it extremely important to carry out clinical immunogenicity studies before their approvals which is again next to impossible. The well-known example of this has been reported following treatment with epoetin, which induced neutralizing antibodies against recombinant erythropoietin, producing a life-threatening antibody reaction Citation[5,15].
After the expiration of any patent for chemical molecules, generics can enter into the market allowing competition and thereby reduction in prices. However, many biologics are currently under patent and hence competitive biosimilars are yet to come. Moreover, till 2009 there were no clear regulatory guidelines to approve generic versions, that is biosimilars which prevented competition from pushing down prices of biosimilars. Additionally, biosimilars are marginally cheaper than the innovators due to which market penetration becomes difficult.
Excessive funding is required since rigorous guidelines have come up for biosimilars.
Opportunities
Although safety of the biosimilar will be a question if there are slight alterations in the manufacturing process, its safety profile can be established and separate labeling can be provided. For example, valtropin is a biosimilar of humatrope, a growth hormone. Valtropin is manufactured using yeasts while humatrope uses Escherichia coli and hence valtropin has different precautions and warnings compared to humatrope Citation[5]. Thus, although limitations of safety profile exist, use of separate labeling serves as opportunity for entering into the market.
In various diseases like diabetes and cancer when the conventional drugs do not work, the patients are shifted on therapies involving biotechnological products like insulin. This serves as a positive sign for the development and market of biosimilars.
The market for biopharmaceutical products is large and growing rapidly and thus there is a potential for growth of biosimilars too.
Several regulatory guidelines are now available and many biosimilars have entered into the market after the approvals. For example, Omnitrope was the first biosimilar version of genotropin to be approved in 2006. Binocrit, Retacrit, Silapo, Epoetin alfa HEXAL, and Abseamed are biosimilar versions of Eprex, that is epoetin alfa, which are approved in European Union. Filgrastim, Biograstim, Nivestim, Zarzio, Ratiograstim, and Tevagrastim are biosimilars of Neupogen which were approved after 2008. Thus, several biosimilars have been successfully approved in Europe and the experience with them has been impressive. This has elevated the stature of the European regulatory approach and has allowed penetration of the biosimilars into the European market.
Currently in the developed markets, the manufacturing and commercialization are centered among a bunch of medium- and large-sized companies. However, these companies are now entering into acquisitions, mergers, or partnerships so as to accelerate the business. This would lead to emergence of more and more cost-effective biosimilar products in the market.
In the near future, due to increase in accessing of biosimilar products and rapid growth, globally it appears that biosimilar would rise by leaps and bounds.
Threats
In cases of generics involving chemical drugs, the substances are identical and can be easily substituted with other drugs as this substitution involves lower risks compared to that of the biologics. Moreover, generics have the same therapeutic effect. In case of the biosimilars the same substitution rule cannot be applied as the biotechnological drugs are not identical.
Concerns revolving comparability, brand loyalty, dearth of substitutes, and competition from second-generation brands are the crucial matters of contention for biosimilar market.
As per World Health Organization (WHO), International Nonproprietary Names (INN) is a unique, globally recognized generic name of any medicinal product. INN provides health professionals with a unique and universally available designated name to identify each pharmaceutical substance. Currently, WHO is considering biosimilars for this purpose and is deciding to assign a different INN than the innovator biotechnological product. This implies that since INN is different, the biosimilar becomes different and hence its labels should also be different than the innovator product. For preparing appropriate labeling, thus, proper preclinical and clinical studies' data should be available Citation[5].
Biologics, biosimilars, and biobetters will dominate the pharmaceutical market and provide treatment for several diseases which are now difficult to treat with conventional drugs. Although biosimilars seem to be lucrative, various issues and concerns have been raised and need to be addressed. With the help of SWOT analysis, it would be easier to weigh the pros and cons and thereby develop effective products for the benefit of the patients.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- Woodcock J, Griffin J, Behrman R, et al. The FDA's assessment of follow on protein products: a historical perspective. Nat Rev Drug Discov 2007;6:437-42
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol 2008;19:411-19
- Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies: regulatory, clinical and commercial considerations. Drugs 2011;71:1-10
- Karpusas M, Whitty A, Runkel L, et al. The structure of human interferon-beta: implications for activity. Cell Mol Life Sci 1998;54:1203-16
- Nowicki M. Basic facts about biosimilars. Kidney Blood Press Res 2007;30:267-72
- Kling J. Fresh from the biologic pipeline—2010. Nat Biotechnol 2011;29:197-200
- Available from: http://www.statista.com/statistics/241962/top-producers-of-biopharmaceuticals-based-on-revenue-2011/ [Accessed on 7 June 2013]
- Available from: http://www.stellarix.com/blog/review-on-the-current-and-future-trends-of-biosimilar-market-in-usa-and-india/ [Accessed on 7 June 2013]
- Sekhon BS, Saluja V. Biosimilars: an overview. Biosimilars 2011;2011:1-11
- Marshall SA, Lazar GA, Chirino AJ, et al. Rational design and engineering of therapeutic proteins. Drug Discov Today 2003;8:212-21
- Beck A. European medicines agency workshop on biosimilars monoclonal antibodies: perspective from the EU. MAbs 2009;1:406-10
- Schellekens H. Follow-on biologics: challenges of the ‘next generation’. Nephrol Dial Transplant 2005;20:iv31-6
- Available from: http://www.gabionline.net/Biosimilars/Research/Biosimilars-or-biobetters-what-does-the-future-hold/%28highlight%29/%20Biosimilars%20or%20biobetters%E2%80%93what%20does%20the%20future%20hold [Accessed on 7 June 2013]
- Dinwoodie N. Biobetters and the Future Biologics Market. BioPharm International 2011;24:31-5
- Casadevall N, Rossert J. Importance of biologic follow-ons: experience with EPO. Best Pract Res Clin Haematol 2005;18:381-7

