Abstract
The notion that the immune system can act as a key factor in controlling cancer cell proliferation and thus its stimulation may be an important resource for cancer therapy has long been known. Tumors can elude the immune system by deploying proteins that shut down the immune response by binding to specific surface receptors on immune cells. Several promising strategies have been designed to overcome cancer cells’ ability to suppress the immune surveillance. Immune checkpoint molecules that block cytotoxic T-lymphocyte associated antigen-4 (ipilimumab) or the programmed death-1/programmed death-ligand 1 axis (i.e., nivolumab and pembrolizumab) promote antitumor immunity, reactivating T-cell proliferation and activity. This efficient strategy currently represents one of the major oncological breakthroughs, with impressive clinically durable responses observed in cancer patients, particularly in melanoma, renal cell carcinoma, NSCLC and more recently in bladder cancer patients. Fifteen years ago, we replaced the IL-2 and INF-α for molecular targeted therapies. Today, we believe that immune therapy will represent the future, perhaps as part of a combination of different therapeutic strategies that act synergistically in each tumor and individual patient.
It has been about 60 years since Dr Farber first intuited the role of a folic acid antagonist as an anti-metabolite to inhibit DNA synthesis in leukemia Citation[1]. In the following years, chemotherapy has galvanized oncologists, convinced that the indiscriminate destruction of abnormally proliferating cells could be the turning point for defeating cancer. Since the late 90s, the focus shifted to the molecular agents targeting a specific driver mutation, aimed to enhance the antitumor efficacy towards the so-called ‘stupid’ tumors (dependent on a single mutation) and to reduce systemic side effects. The peculiar wretched ability of cancer cells to develop resistance mechanisms to treatments and the preponderance of ‘smart’ diseases with a high mutational load, however, makes the battle against cancer far from being over and letting cancer to remain the untamed plague of our century.
The notion that the immune system can act as a key factor in controlling cancer cell proliferation and thus its stimulation may be an important resource for cancer therapy has long been known.
Cytokines (IL-2 and IFN-α) have been used for decades, principally in patients with renal cell carcinoma (RCC) and melanoma, albeit with a limited activity and a significant toxicity profile. Until recently, cancer immunotherapy was considered as an overpromising but underdelivering strategy.
Tumors arise from an uncontrolled proliferation of transformed cellular clones harboring a remarkable number of somatic mutations. Cancer cells express antigens recognized from the immune system as being foreign (non-self). Tumor antigens are products of mutated, deregulated or viral transforming genes, not expressed in autologous tissues prior to tumor development or expressed at levels unlikely to induce tolerance, which may elicit specific immune responses. According to the profile of expression, tumor antigens are distinguished in tumor-specific antigens – exclusively expressed on tumor cells – and tumor-associated antigens – shared by normal and tumor cells and expressed on tumor cells surface abnormally for quantity, site and time. Therefore, although originating from host cells, cancer cells are able to evoke an immune response. The immune system should inhibit tumor growth through the recognition of tumor antigens by the suitably activated immune-competent cells (concept known as immune-surveillance). Surprisingly, it seems that neo-antigens encoded by ‘passenger’ mutations – cancer cell mutations not directly contributing to carcinogenesis – play a key role in tumor immunity Citation[2]. Tumor antigens have been explored as vaccines, as targets for monoclonal antibodies, and as targets for adoptively transferred cytotoxic T cells Citation[3].
Unfortunately, cancer cells develop different mechanisms to escape from the immune response (a process defined as immunoediting) Citation[4]. Several mechanisms contribute to cancer immunoediting: i) a decreased expression of the MHC class I molecules on tumor cells, so as to preclude their recognition by cytotoxic T lymphocytes (CTL); ii) the inability of the tumor to evoke a CTL-mediated response, since most of the tumor cells do not express co-stimulatory (CD80 or CD86) or MHC class II molecules; iii) the production of tumor factors (i.e., TGF-β) that negatively regulate the antitumor immune response; iv) the induction by tumor antigens of immunological tolerance; v) the loss of tumor immunogenic antigens; vi) the M2 polarization of tumor-associated macrophages, which enhances the expression of programmed death-1 (PD-1) and promotes the development of regulatory T cells (Treg) Citation[5]. It is important to emphasize that mutations or deletions in genes encoding tumor antigens are quite common, given the high rate of mitosis and the genetic instability that characterize cancer cells, especially in case of tumor neo-antigens not essential for the initiation and maintenance of the tumor phenotype.
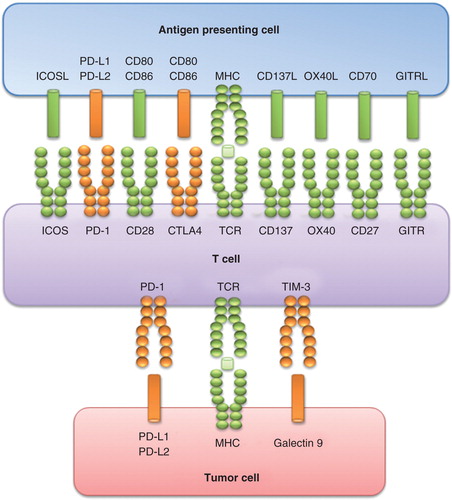
Usually, clinically apparent cancers arise in immunocompetent patients in part as a consequence of the cancer-induced immunosuppression. Immunosuppression is partly mediated by cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and PD-1, two immunomodulatory receptors expressed on T cells that trigger inhibitory pathways dampening T-cell activity (). CTLA-4 is expressed on recently activated T CD4+ and CD8+ lymphocytes. It inhibits T-cell activation upon binding with B7-1 and B7-2 (co-stimulatory molecules present on antigen-presenting cell surface), counteracting CD28-mediated signals. Similarly, the interaction between the receptor PD-1 (expressed on T cells and on other immune cells of the inflamed tumor microenvironment) and its ligands PD-L1/L2 (expressed on myeloid dendritic cells, activated T cells, some non-hematopoietic tissues and tumor cells) determines a downregulation of T-cell effector functions: this event minimizes the tissue damages, prevents the development of auto-immunity through the promotion of tolerance to self-antigens and, in cancer patients, inhibits the antitumor immune response.
Tumors can elude the immune system by deploying proteins that shut down the immune response by binding to specific surface receptors on immune cells (). Several promising strategies have been designed to overcome cancer cells ability to suppress the immune surveillance. Immune checkpoint molecules that block CTLA-4 (ipilimumab) or the PD-1/PD-L1 axis (i.e., nivolumab and pembrolizumab) promote antitumor immunity, reactivating T-cell proliferation and activity. This efficient strategy currently represents one of the major oncological breakthroughs, with impressive clinical durable responses observed in cancer patients Citation[6,7], particularly in melanoma Citation[8-10], RCC Citation[11,12], NSCLC Citation[13] and more recently in bladder cancer patients Citation[14].
However, many questions remain unanswered. Reply will mean expanding the rational experimentation and use of immunotherapy. For example, is immunotherapy really the best weapon to fight cancer? And if it is, is it suitable for all cancer types and patients?
Cancers harboring a high rate of somatic mutations seem to have greater chance of responding to checkpoint blockade. The reason may lie in the predominant role, in tumor immunity, of neo-antigens – product of passenger mutations – that are highly represented in these malignancies Citation[15,16]. A particular case is related to classic Hodgkin’s lymphoma, in which we can observe alterations in chromosome 9p24.1 that increase the abundance of the PD-1 ligands, PD-L1 and PD-L2 and promote their induction through Janus kinase signal transducer and activator of transcription signaling. For these reasons, in a recent ongoing study, nivolumab demonstrated a substantial therapeutic activity and an acceptable safety profile in patients with previously heavily treated relapsed or refractory Hodgkin’s lymphoma Citation[17]. Therefore, the current narrow spectrum of cancers in which PD-1 pathway blockade has significant clinical activity might be expanded in the next future horizons beyond melanoma, lung, renal and urothelial cancers.
Interestingly, tumor-specific mutant antigens can function as important targets of checkpoint inhibitors, but can also be used to develop personalized cancer-specific vaccines, rekindling the interest towards a therapeutic option almost abandoned for the disappointing results reported until now. Therefore, should we really drop out the stimulation of immune response by using antigen-specific vaccines? Is active immunotherapy really over? Maybe we will respond to these questions in the next 15 years.
Moreover, is it possible to identify a cohort of patients most likely to benefit from checkpoint blockade? And which biomarkers may help us in predicting treatment response and guiding treatment decisions? Among all possible tumor features investigated, the expression of PD-L1 on cancer cell seems to be the single characteristic that most closely correlates with worse clinical outcome and with tumor response to PD-1 pathway inhibitors Citation[18-20]. However, conflicting results compromise the prognostic significance of tumor PD-L1 expression and its role as a predictor of therapeutic response Citation[21-23]. More recently, cancer research has focused on the association between the expression of PD-L1 on tumor-infiltrating immune cells and response to treatment Citation[14,24,25]. Tumeh et al. showed that an elevated density of pre-existing CD8+ T cells at the invasive tumor margin, together with the presence of immune cells expressing PD-1 and PD-L1, enabled PD-1 blockade to mediate tumor regression. The accumulation of CD8+ T cells suggest that tumors that have already been recognized by the immune system, and therefore containing infiltrating immune cells, show a higher sensibility to checkpoint inhibitors.
As previously said, immunotherapy seems to be more effective in highly mutagenized tumors, as a result of a constant – albeit ineffective – stimulation of the immune system from neo-antigens arising from genetic mutations. To further support this hypothesis, data from a genomic analysis of melanoma patients treated with the anti-CTLA-4 antibody ipilimumab have been recently presented Citation[26]. The higher was the tumor mutational load, the greater were the clinical benefit of checkpoint blockade and the overall survival. Moreover, using genome-wide somatic neo-epitope analysis and patient-specific HLA typing, the authors identified a neo-antigen landscape specifically present in tumors with a strong response to CTLA-4 blockade, providing a rationale for examining exomes of patients treated with anti-CTLA-4 agents. Therefore, genetic biomarkers may help in predicting the probability that tumors contain antigens recognized by T cells able to mount a relevant anticancer immune response Citation[27].
Despite considerable progresses, the way to select patients whose cancer is sufficiently immunogenic and whose immune system is sufficiently reactive to mediate an effective anti-tumor response is still far from over. Efforts should be aimed at delineating a predictive model of treatment response that integrates features of the tumor (with its mutational load), of the host’s immune system, and of their mutual interactions.
To date, several immunotherapeutic approaches to overcome tumor immune evasion are under investigation, including T-cell checkpoint inhibitors or agonists for T-cell-activating pathways, novel cytokines such as IL-12 and IL-15, therapeutic vaccines and elimination of immunosuppressive cells. But what is the potential efficacy and safety of combining different treatments including immunomodulatory molecules, cytotoxic drugs and molecular targeted agents? Combining immunotherapies with other established or investigational anticancer strategies may represent a novel interesting frontier to achieve greater therapeutic benefit in cancer patients Citation[28]. Immuno-oncology combinations include: combining or sequencing immunotherapies that target distinct immune pathways (additional agents aiming to reverse T-cell dysfunction such as other immune checkpoint inhibitors; vaccines, toll-like receptor agonists, type I IFN and oncolytic viruses that enhance antigen presentation; agents targeting other immune inhibitory mechanisms, such as inhibitors of indoleamine dioxygenase, Treg and myeloid-derived suppressor cells, that for example could be targeted by cyclosporine or G-CSF) or combining immunotherapies with established therapeutic modalities (chemotherapies, targeted agents or radiotherapy). The integration of immune- and radio/chemotherapy will allow not only to exploit the tumor shrinkage of cytotoxic/cytostatic agents, but also to potentiate the immune activity through radio/chemotherapy-induced modifications of the tumor microenvironment and release of novel tumor antigens. Similarly, targeted therapies may sensitize tumor cells to immune-mediated destruction, promoting effective maturation of dendritic cells, T-cell priming/activation/differentiation into long-lived memory T cells, increasing expression of death receptors or ‘distress’ ligands, reducing expression of pro-survival signals, abolishing the production of tumorigenic inflammation and inhibiting immunosuppressive cell types Citation[29]. Although the optimal dose, schedule, sequence and combination of a multimodality approach have yet to be delineated, combining immunotherapies may have the potential to improve patient outcomes due to the synergistic activity of different therapeutic approaches.
Another major focus in future years will be represented by the choice of the best setting for immunotherapy in RCC patients. Adjuvant? First or subsequent lines? Patients with low tumor burden? This last subgroup could represent a promising candidate to receive this approach. This hypothesis is supported by the positive results obtained by the use of IFN-α and IL-2 in patients with lung-only metastases Citation[30]. The results from ongoing Phase III trials of nivolumab will allow to clarify its efficacy and safety in first and advanced lines (NCT02231749, NCT01668784).
Fifteen years ago, we replaced the IL-2 and INF-α for molecular targeted therapies. Today, we believe that immune therapy will represent the future, or perhaps a combination of different therapeutic strategies that act synergistically in each tumor and individual patient. But expectations are really getting confirmed? Who would have thought that the earth revolves around the sun?
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Notes
Bibliography
- Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948;238:787-93
- Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014;515:572-6
- Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15:5323-37
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3(11):991-8
- Santoni M, Massari F, Amantini C, et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2013;62:1757-68
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2014. [Epub ahead of print]
- Massari F, Santoni M, Ciccarese C, et al. PD-1 blockade therapy in renal cell carcinoma: Current studies and future promises. Cancer Treat Rev 2015;41(2):114-21
- Zielinski C, Knapp S, Mascaux C, et al. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol 2013;24:1170-9
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62
- Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014;515:572-6
- Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014;515:577-81
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372(4):311-19
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014;20:5064-74
- Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2014;21(5):1071-7
- Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti- PD-1; BMS-936558; ONO-4538). J Clin Oncol 2013;31(Suppl):3016
- Fay AP, Callea M, Gray KP, et al. PD-L1 expression in non-clear cell renal cell carcinoma. J Clin Oncol 2014;32(Suppl 4):abstr 424
- Madore J, Vilain R, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 2014. [ Epub ahead of print]
- Shim HJ, Hwang JE, Jung SI, et al. Clinical impact of programmed cell death ligand 1 (PD-L1), programmed cell death 1 (PD-1), and CD8 expression in pancreatic cancer. J Clin Oncol 2014;32(Suppl):abstract e15258
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99
- Schumacher TN, Kesmir C, van Buuren MM. Biomarkers in cancer immunotherapy. Cancer Cell 2015;27:12-14
- Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res 2014;20:6258-68
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237-51
- Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001;345:1655-9