Abstract
Despite significant scientific advances toward the development of a safe, nontoxic and effective radiation countermeasure for acute radiation syndrome (ARS) over the past six decades, no drug has been approved by the US FDA. Several biologics are currently under development as radiation countermeasures for ARS, of which three have received FDA Investigational New Drug (IND) status for clinical investigation. Presently, two of these agents, entolimod (CBLB502) and HemaMax (recombinant human IL-12) are progressing with large animal studies and clinical trials. Neupogen (G-CSF, filgrastim) has recently been recommended for approval by an FDA Advisory Committee. Filgrastim, GM-CSF (Leukine, sargramostim), and PEGylated G-CSF (Neulasta) have high potential and well-documented therapeutic effects in countering myelosuppression and may receive full licensing approval by the FDA in the future. The former two biologics are available in the US Strategic National Stockpile (SNS) for use in the event of nuclear or radiological emergency. The Emergency Use Authorization (EAU) application for entolimod may be filed soon with the FDA. Biologics are attractive agents that are progressing along the path for FDA approval, to fill the unmet need for ARS countermeasures.
1. Introduction
Human acute radiation syndrome (ARS) follows intense, acute whole-body or significant partial-body radiation, of doses > 1 Gy, delivered at relatively high rates. Clinical components of ARS include the hematopoietic sub-syndrome (H-ARS, 2 – 6 Gy), gastrointestinal sub-syndrome (GIS; 6 – 8 Gy) and the cerebrovascular (> 8 Gy) sub-syndrome Citation[1]. Dividing ARS into these ‘sub-syndromes’ oversimplifies the clinical reality of ARS as it often involves complex, concurrent and multi-organ dysfunctions. Cerebrovascular sub-syndrome is considered incurable, whereas H-ARS alone or in combination with GIS, are more likely to be amenable to countermeasures; therefore, the later two sub-syndromes are specific targets for the development of novel medical countermeasures (MCM). There are several biologics at different developmental stages to be considered as MCM for ARS (, and ) Citation[2]. A brief description and current status of promising biologics are provided in this article.
Figure 1. Schematic representation of the biological agents as radiation countermeasures under development. Currently, there are three agents with FDA IND status: entolimod, HemaMax and Neupogen. Neupogen and Leukine have been procured for SNS availability and are expected to obtain FDA EUA in the near future. PEGylated G-CSF is not currently stocked in the SNS but may also obtain FDA EUA approval once filgrastim is approved. Additional countermeasure candidates, at various developmental stages, are presented.
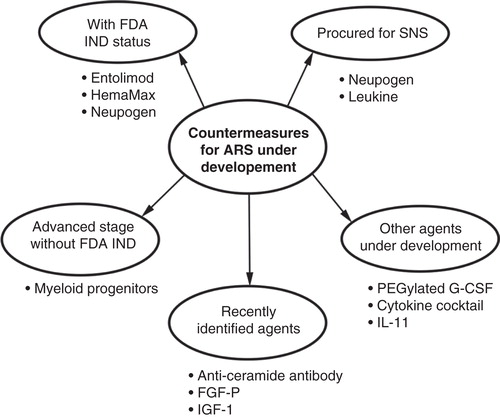
Table 1. Biologics with US FDA IND/procured for SNS/close to FDA approval.
Table 2. Promising biologics under development as radiation countermeasures for ARS.
2. Regulatory issues of biologics approval for use as ARS countermeasures
Biologics are evaluated for marketing by the FDA through the filing of a Biologic License Application (BLA), the equivalent to New Drug Application for other agents. The Public Health Services Act authorizes the FDA to regulate biologics. The agents discussed here qualify for consideration by the FDA under BLA for approval.
Since conventional human clinical efficacy trials for ARS MCM are not possible due to ethical reasons, these trials are substituted with Animal Efficacy Rule, a very stringent and possibly more difficult FDA approval pathway. The criteria required to move through this approval process include: well-characterized animal model(s) that is predictive of human response, a good understanding of the mechanism of action of radiation injury and that of the MCM, study end point focused on prevention of mortality or major morbidity, as well as the good understanding of pharmacodynamics so that the effective human MCM doses can be determined. This pathway relies heavily on a large animal model for preclinical safety and efficacy studies Citation[3].
The majority of agents at advanced stages of development have received FDA ‘fast track’ and ‘orphan drug’ statuses Citation[2]. The FDA fast track approval process is designed to facilitate development and expedite the review and approval processes for new treatments of serious or life-threatening conditions. Radiation MCM for ARS are considered emergency need drugs.
The US Emergency Use Authorization (EUA) program, established by Project Bioshield, is a critical tool for the medical and public health communities. It permits the FDA to approve the emergency, off-label use of products approved for other indications or the use of drugs, devices and medical products that currently have no prior approval, clearance or licensing by FDA. It is applicable to both civilian and military use, and it fills the need for timely medical treatment in emergency situations. The Strategic National Stockpile (SNS) program ensures that such agents are appropriately pre-positioned so that they are readily available and easily accessible for state and local public health agency distribution in the event of a national emergency.
3. Biologics at advanced stages of development as radiation countermeasures
As stated above, there are several biologics under development as radiation MCM for ARS ( and , ). Neupogen and two others, entolimod and HemaMax, have considerable efficacy and safety profiles and have received FDA Investigational New Drug status for clinical investigation. Mechanistic studies have suggested that the various countermeasures for ARS have different modes of action ().
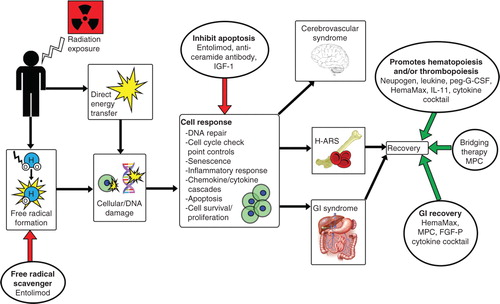
Figure 2. Simplified representation of systemic biological effects due to radiation exposure, with promising biologics intervening at various steps. Radiation induces free radical formation, DNA damage and apoptosis, which can then lead to ARS or death. Various biologics are able to minimize the damaging effects of irradiation through different mechanisms of action.
3.1 CSF
CSF have high potential and well-documented therapeutic efficacy in countering myelosuppression and may receive full licensing approval from the FDA in the future. Filgrastim, sargramostim and PEGylated filgrastim have already been used off-label for treating radiation accident victims Citation[4]. Currently, there are four recombinant leukocyte growth factors with BLA for related indications: BLA 103353 (Neupogen), BLA 125031 (Neulasta), BLA 103362 (Leukine) and BLA 125294 (TBO-filgrastim) Citation[4]. G-CSF/filgrastim has completed a good laboratory practice compliant study in a nonhuman primate (NHP) model. The efficacy of these agents in various animal models has been recently reviewed Citation[4]. The use of growth factors to treat radiation-exposed victims has been rationalized based on three facts: i) a large clinical database documenting consistent efficacy in mitigating chemotherapy-induced myelosuppression and that associated with stem-cell transplant conditioning regimens as well as consistent safety profile; ii) enhanced recovery from radiation-induced myelosuppression in four animal species and improved survival in sublethal and lethally irradiated animal models; and iii) demonstration of effective granulopoietic activity in a number of radiation-accident victims. Additionally, the American Society of Clinical Oncology extended their recommendation for use of recombinant human G-CSF (rhu G-CSF) and PEGylated rhu G-CSF to treat patients exposed to therapeutic doses of total-body radiation.
Radiation-accident reports show that CSFs have been used to treat the victims of 16 radiological and nuclear accidents with observed benefits Citation[4]. In three accidents CSFs were used within 48 h of accidents (Tokai-Mura, Soreq and Nesvizh), but in others CSF administration was initiated weeks after the incidence. The limited and anecdotal clinical data available regarding these growth factors validate their biological response; however, the variable and delayed manner in which these agents were administered makes the CSF’s role in recovery difficult to determine concisely.
During a recently conducted FDA meeting, members overwhelmingly voted (17:1) to support that filgrastim will produce clinical benefits to humans who have been exposed to radiation with doses capable of inducing myelosuppression Citation[5]. The one committee member, who voted ‘No’, concluded that those who survive a radiological or nuclear incident will most likely not have received a radiation dose high enough to produce myelosuppressive effects and therefore would not benefit G-CSF administration.
3.2 Entolimod
Entolimod is a truncated derivative of the Salmonella bacteria flagellin. Its pharmacological action is based on its binding to Toll-like receptor 5 and the activation of NF-κB signaling Citation[6]. Studies conducted with entolimod using rodent and NHP (good laboratory practice) models suggest that it will be a highly promising treatment for lethally irradiated humans, due to its ability to efficiently ameliorate H-ARS and GIS, as well as having an extended therapeutic time window after radiation exposure Citation[6-8]. Entolimod is currently in clinical development; a human safety study indicated that it was well tolerated and the biomarker results correspond to data from animal models. Cleveland BioLabs, Inc. (Buffalo, NY, USA) is preparing a pre-EUA application for entolimod.
3.3 HemaMax
HemaMax is recombinant human IL-12 (rhuIL-12) cytokine and has been shown to increase mice survival when a single dose was administered, either 24 h before or within 1 h after total-body irradiation. Currently, rhuIL-12 is being developed as a radiomitigator by Neumedicines Inc. (Pasadena, CA, USA) under the name HemaMax. Allometrically equivalent doses of mouse and human HemaMax had similar pharmacokinetics and significantly increased mouse and NHP survival, when administered 24 h post-irradiation, even when no antibiotics, fluids or blood products were administered Citation[9]. To demonstrate the safety of HemaMax, Neumedicines conducted a Phase Ib study where healthy volunteers were administered a single dose of HemaMax that is predicted to be the effective dose for treating H-ARS, based on NHP data; this trial suggests rhuIL-12 to be safe and well tolerated. Phase II equivalent data (randomized, double-blinded, good laboratory practice) showed that single administration of rhuIL-12 to NHPs significantly increased survival and reduced radiation-induced hematopoietic toxicity when administered 24 h post-irradiation. Administration of rhuIL-12 promotes multilineage hematopoietic recovery, immune functions and possibly, GI functions. Additionally, there is a report of successful interspecies dose conversion. Neumedicines is developing rhuIL-12 for the treatment of H-ARS for BLA submission to the FDA under the Animal Efficacy Rule Citation[10].
3.4 Additional potential biologics as countermeasures against ARS
Several additional biologics have been identified as potential countermeasures and have shown promise in murine and NHP models of ARS. Some of these agents have already been used off-label in radiological accident victims. Hopefully, in the future, they will be fully developed agents to combat radiation injury. Some of these potential agents are myeloid progenitors Citation[11,12], anti-ceramide antibody Citation[13], fibroblast growth factor-2 (FGF-2), its derived peptide (FGF-P) Citation[14], IGF-1 Citation[15] and various cytokines () Citation[16-18].
4. Expert opinion
Since no FDA-approved ARS MCM exits, there is an urgent need to develop such agents. Based on studies with large numbers of NHPs, entolimod appears to be a promising radiation countermeasure for H-ARS as well as for GIS Citation[7]. Entolimod’s existing efficacy, safety data and animal-to-human dose conversion are enough to proceed with a pre-EUA application to reduce the risk of death following radiation exposure Citation[7].
Independent of the FDA’s approval and licensing process, the US federal government has procured filgrastim and sargramostim to be stockpiled in the SNS under the Pandemic and All-Hazards Preparedness Reauthorization Act. Filgrastim and PEGylated filgrastim have demonstrated efficacy in recently conducted NHP studies, and these data have been submitted to the study sponsor for submission to the FDA for approval Citation[5,19,20]. In a recent study, G-CSF failed to demonstrate efficacy in the NHP model; however, this discrepancy may be due to the lack of supportive care Citation[10]. The Center for Disease Control currently holds both investigational new drug and EUA applications with the FDA for the use of Neupogen/G-CSF in the event of a nuclear or radiological incident.
PEGylated filgrastim has demonstrated better efficacy than filgrastim in NHPs Citation[19]. However, the stability, the implementation plans in the radiological nuclear incident scenario and potential side effects are not fully understood and are of concern Citation[4,21]. PEGylated filgrastim has been suggested as an alternative to filgrastim.
Recent developments with HemaMax indicate that this agent is ‘en route’ to regulatory approval Citation[9,10]. A single injection of HemaMax, without supportive care, significantly improved survival and promoted multilineage hematopoietic recovery in an NHP model of H-ARS. Initial published observations with myeloid progenitors appeared encouraging.
Each drugs’ sponsor have made clinical progress; these drugs are moving forward to fill the need for MCM that has increased over the past decade due to increased terrorist threats. In our opinion, the above biologics hold the most promise in the future due to their limited side effects and would be safe and effective agents when approved.
Acknowledgements
The authors are thankful to Col. L Andrew Huff and CAPT. David Lesser for helpful discussions. The opinions or assertions contained herein are the professional views of the authors and are not necessarily those of the Department of Defense, USA. The authors apologize to those having contributed substantially to the topics discussed herein that they were unable to cite due to space constraints.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents, received or pending, or royalties.
Notes
Bibliography
- Hall EJ, Giaccia AJ. Radiobiology for the Radiobiologist. 6th edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2006
- Singh VK, Newman VL, Romaine PL, et al. Radiation countermeasure agents: an update (2011 – 2014). Expert Opin Ther Pat 2014;24(11):1229-55
- U.S. Food and Drug Administration. Guidance for Industry: product Developoment Under the Animal Rule. 2014. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf [Last accessed 2014]
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine 2014;71(1):22-37
- U.S. Food and Drug Administration. FDA Advisory Committee Briefing Document: a Joint Meeting of the Medical Imaging Drugs Advisory Committee and the Oncologic Drugs Advisory Committee 2013. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM350151.pdf [Last accessed 5 February 2014]
- Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320(5873):226-30
- Cleveland BioLabs, Inc. 2014. Available from: http://www.cbiolabs.com/ [Last accessed 2014]
- Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther 2012;343(2):497-508
- Gluzman-Poltorak Z, Mendonca SR, Vainstein V, et al. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol 2014;7:31
- Neumedicines 2014. Available from: http:// www.neumedicines.com/ [Last accessed 27 October 2014]
- Singh VK, Christensen J, Fatanmi OO, et al. Myeloid Progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res 2012;177(6):781-91
- Cellerant Therapeutics. 2013. Available from: http://www.cellerant.com/ [Last] [accessed 26 September 2013]
- Rotolo J, Stancevic B, Zhang J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest 2012;122(5):1786-90
- Casey-Sawicki K, Zhang M, Kim S, et al. A basic fibroblast growth factor analog for protection and mitigation against acute radiation syndromes. Health Phys 2014;106(6):704-12
- Zhou D, Deoliveira D, Kang Y, et al. Insulin-like growth factor 1 mitigates hematopoietic toxicity after lethal total body irradiation. Int J Radiat Oncol Biol Phys 2013;85(4):1141-8
- Hao J, Sun L, Huang H, et al. Effects of recombinant human interleukin 11 on thrombocytopenia and neutropenia in irradiated rhesus monkeys. Radiat Res 2004;162(2):157-63
- Drouet M, Mourcin F, Grenier N, et al. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood 2004;103(3):878-85
- Drouet M, Grenier N, Herodin F. Revisiting emergency anti-apoptotic cytokinotherapy: erythropoietin synergizes with stem cell factor, FLT-3 ligand, trombopoietin and interleukin-3 to rescue lethally-irradiated mice. Eur Cytokine Netw 2012;23(2):56-63
- Hankey KG, Farese AM, Gibbs AM, et al. Pegfilgrastim administration significantly improves survival in an LD50/60 model of total body irradiation (TBI) in nonhuman primates (NHP). 59th Annual Meeting of the Radiatrion Research Society; New Orleans, LA; 2013. p. 318
- Farese AM, Cohen MV, Katz BP, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res 2013;179(1):89-100
- Dainiak N, Gent RN, Carr Z, et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep 2011;5(3):202-12

