ABSTRACT
Introduction: The conditioning regimen used in cord blood transplantation (CBT) may significantly impact the outcomes. Variable pharmacokinetics (PK) of drugs used may further influence outcome. Individualized dosing takes inter-patient differences in PK into account, tailoring drug dose for each individual patient in order to reach optimal exposure. Dose individualization may result in a better predictable regimen in terms of safety and efficacy, including timely T cell reconstitution, which may result in improved survival chances.
Areas Covered: Conditioning regimens used in CBT varies significantly between and within centres. For busulfan, individualized dosing with therapeutic drug monitoring has resulted in better outcomes. Anti-thymocyte globulin (ATG), used to prevent rejection and GvHD, significantly hampers early T-cell reconstitution (IR). Timely IR is crucial in preventing viral reactivations and relapse. By individudalizing ATG, IR is better predicted and may prevent morbidity and mortality.
Expert Opinion: Individualization of agents used in the conditioning regimen in CBT has proven its added value. Further fine-tuning, including new drugs and/or comprehensive models for all drugs, may result in better predictable conditioning regimens. A predictable conditioning regimen is also of interest/importance when studying adjuvant therapies, including immunotherapies (e.g. cellular vaccines or engineered T-cell) in a harmonized clinical trial design setting.
1. Introduction
Allogeneic hematopoietic cell transplantation (HCT) can be a potentially curative treatment option for a variety of diseases including leukemia, bone marrow failure (BMF), inborn errors of metabolism (IEM), and primary immune deficiencies (PID). Its success is limited by complications like graft-versus-host-disease (GvHD), rejection, relapse, and (viral) infections. These complications can be serious threats to patients undergoing HCT, hampering survival chances. Fortunately, over the last decades, many steps have been made toward reducing mortality and morbidity; better HLA-typing, centralized care, monitoring for infectious complications, and new agents in conditioning regimens and GvHD-prevention became available. Furthermore, the use of alternative stem-cell sources such as unrelated (mismatched) donors, haplo-identical donors, and cord blood (CB) donors made HCT available for almost all patients. As all these sources contain different number and phenotypes of cells the optimal conditioning regiment may be different to enable the best effect for each source of cells. Over the last decade, individualized dosing and therapeutic drug monitoring (TDM) has been introduced in the field of HCT aiming to optimize the outcomes.
Differences in pharmacokinetics (PK) of agents, which are part of the conditioning, can be associated with variable myeloablation and immune suppression before and after CBT resulting in different outcomes. By relating the exposure of these agent to clinical outcomes, the most optimal exposures of each medications can be determined. Individualized dosing takes inter-patient differences into account and tailors the drug dose for each individual patient to optimal exposures. This may mediate a predictable regimen in terms of safety and efficacy. Also, immune reconstitution (IR), crucial in preventing relapse and viral reactivation may be better predictable. Such a better predictable regimen may subsequently result in improved survival chances. In the current paper, we reviewed literature on individualized conditioning regimens in umbilical cord blood transplantation (CBT) aiming for better predictable regimens in terms of safety and efficacy, including IR, to optimize the survival chances after CBT.
2. Umbilical CBT
Umbilical CB was first introduced as an alternative stem-cell source in allogeneic HCT by Gluckman et al. treating a patient with Fanconi anemia.[Citation1] In the following decades, the use of CB has significantly increased and is currently considered a good alternative cell source when suitable HLA-identical related and unrelated donors are lacking.
Compared to the more traditional cell sources such as bone marrow (BM) or peripheral blood stem cells (PBSC), the advantages of using CBT include the reduced incidence of GvHD,[Citation2] while maintaining antileukemic effect.[Citation3–Citation6] Due to banking and HLA-matching before storage, CB is promptly available donor, and less stringent HLA-matching criteria apply, resulting in suitable donors for most patients.[Citation7] Disadvantages include the lower nucleated cell- and CD34+ cell dose/kg, which may lead to prolonged neutropenia (and associated problems, including higher probability on graft failure), and the higher costs of a CB unit compared to other stem-cell sources. Moreover, early T-cell reconstitution (i.e. within 3–12 months after transplantation) is suggested to be slower in UCB when compared to BM or PBSC,[Citation8–Citation11] leading to an increased incidence of viral reactivations associated with increased transplant-related mortality. Despite this higher TRM in some reports, interestingly the probability on overall and leukemia-free survival was similar to BM and PBSC, suggesting a stronger graft-versus-leukemia effect.[Citation12–Citation14]
3. Clinical results: CBT versus other stem-cell sources
Several studies have compared CBT to other donor cell sources for patients with hematological malignancies and benign disorders. Unfortunately, well-designed randomized controlled studies are lacking. Despite this, there is growing evidence of comparable results for patients with malignant and benign disorders.
In benign disorders, the use of CBT is increasing, and in some diseases such as IEM and PID is even becoming the preferred HCT source. CBT allows for faster availability, which is of utmost importance for certain life-threatening rapidly progressive diseases (e.g. PID, metabolic disorders) and avoids invasive procedures for donors. In Hurler’s disease (MPS-1), Boelens et al. showed that patients receiving a CBT, matched sibling donor (MSD) or matched unrelated donor (MUD) transplant had similar event-free survival (EFS) probabilities.[Citation15] While EFS was lower in 4/6 HLA-matched CBT with low cell dose, those with high cell dose had similar EFS probability as either 5 or 6/6 matched units. Those with a mismatched MUD had inferior survival probability. Interestingly, full donor chimerism and normal enzyme levels after HCT were significantly more frequent in patients with CBT, despite similar busulfan (Bu)-based myeloablative conditioning. For PID (severe combined immune deficiency), Pai et al. reported on a cohort of children receiving a HCT.[Citation16] Survival was highest among those with a MSD, while other stem-cell sources including CBT perform comparably.
For patients with hematological malignancies both in a myeloablative and non-myeloablative setting, the use of CBT is a valuable alternative source of HCT when a suitable donor is lacking, as several studies found similar probabilities on overall and leukemia-free survival.[Citation2,Citation12,Citation14,Citation17] In studies by Rocha, Laughlin, and Eapen, all comparing donor sources in patients with leukemia, relapse rates in CBT were lower or comparable to BM and PBSC. Transplant-related mortality in the more historical studies was higher after CBT, which partly may be associated to lower cell dosed CB units as this appears to be a predictor for worse clinical outcomes.[Citation2,Citation18] An interesting and important observation in these cells source comparison studies are that lower chronic GvHD is reported in the CB group. This is important, as the complication is associated with substantial lower quality of life. Nowadays, some centers regard CB to be the preferred cell source for patients at high risk of relapse with or without minimal residual disease pretransplant.[Citation19] This is supported by the lower probability of relapse in majority of studies, even in very high-risk, MRD-positive patients.[Citation2,Citation17] The less stringent HLA-matching resulting in more HLA-disparity [Citation20] may explain this lower probability of relapse observed in CBT when compared to MUD and MMUD. Also, a recent report showed that CB T cells mediate stronger antileukemic activity compared to adult cells.[Citation13] In addition, novel donor CB selection strategies based on nonmaternal inherited antigen (NIMA) [Citation21] and/or numbers of predicted indirectly recognizable HLA epitopes (PIRCHES) [Citation22] may further reduce the risk of relapse after CBT. Over the last years haplo-identical transplantation has become a popular and cheaper alternative cell source for patients lacking identical donors. Especially, the post-HCT cyclophosphamide (Cy) strategy has become the alternative option of interest for some centers. The early outcomes look promising but longer term follow-up is needed as well as comparison of early and late outcomes to other cell sources including CB. This should also include chronic GvHD-free-EFS comparisons.
In summary, these landmark papers reviewed above showed that CBT can be considered as an alternative cell source with similar estimated survival chances as HLA-identical related and unrelated donors, for benign disorders and malignant indications for HCT. Important to consider is that most studies included historical patients (treated >10 years ago), which may have influenced the outcomes. Cell-dose selection criteria have improved outcome and HLA-typing on high resolution, as well as selection for preferred mismatches (e.g. NIMA, PIRCHES), may improve the outcomes of CBT as well. Moreover, further improvement may be achieved by improving lymphocyte reconstitution, in particular T-cell reconstitution.
4. Unmet needs in CBT
The unmet needs in CBT, and likewise in HCT in general, include reduction of the short- (e.g. viral disease, GvHD) and long-term toxicity (including chronic GvHD) of the procedure as well as getting better disease control (efficacy). Most safety and efficacy problems are associated with absence of timely and balanced IR (neutrophils and T-cell reconstitution), which may even be more hampered after CBT; therefore, strategies aiming for predictable T-cell IR may result in better survival chances. This may be achieved by individualization of treatment, making a transformation from one-size-fits-all to tailored, individualized transplantation.
4.1. Neutrophil engraftment
Historically, engraftment was a major drawback of using CB as a stem-cell source. With lower numbers of infused cells, both total nucleated cells (TNC) as well as CD34+ cells, neutrophil engraftment was slower compared to BM or PBSC.[Citation2,Citation12,Citation14,Citation17] Advantages have been made in improving engraftment, including a larger inventory of stored CB units. Nowadays, the stored units contain higher numbers of TNC (and CD34+). Additionally, stimulation of neutrophil proliferation with granulocyte colony-stimulating factor is routinely used following CBT. These interventions have improved neutrophil engraftment following CBT when compared to BM or PBSC. In more recent studies, where a lower limit of acceptable cell dose/kg was taken into account, CBT performs comparably to other sources in terms of engraftment, especially in children.[Citation15,Citation16]
If a single unit is not containing sufficient number of cells, a strategy to overcome the limiting cell dose is infusion of 2 CB units or combining a single CB unit with other cell source graft: e.g. CD34+ selected haplo-identical cells (haplo-CB transplantation). These strategies are mainly applied in the adult transplantation setting, where body weights are generally higher compared to children. Both strategies rely on a higher number of infused TNC and/or CD34+ to ensure early engraftment from one winning units (double cord) or initially from the haplo-donor (haplo-cord), followed by gradually increasing single cord-blood donor chimerism. In pediatrics and young adults, the use of double CB transplant gave equal survival chances between single (with sufficiently high cell dose) and double-cord transplants with a higher probability on acute GvHD 3–4 and chronic GvHD in the double cord group.[Citation23] Using these strategies, steps have been made to make CBT safer, aiming for similar survival chances as after related or unrelated donor transplantation.[Citation23–Citation30] More recently, strategies such as the use of ex-vivo-expanded CD34+ cells have led to a significant improvement in time to neutrophil engraftment.[Citation31,Citation32] Various strategies have been used, such as Notch, StemRegenin-1, and nicotinamide.[Citation33–Citation35] Currently, various clinical trials are running and phase III randomized controlled studies are planned.
4.2. T-cell IR
With temporary absent or delayed reconstitution of the humoral immunity not being a major obstacle in current clinical practice of CBT (also because this can be easily substituted), restoration of cellular immunity is a significant hurdle. Delayed reconstitution of lymphoid lineages, most importantly T cells, has been associated with inferior survival chances. Viral reactivations [Citation36–Citation38] and relapse of malignancy [Citation9,Citation39–Citation41] both depend on adequate T-reconstitution and influences survival chances.[Citation8,Citation9,Citation42]
In most literature, CBT has been associated with delayed or very poor T-cell IR.[Citation9–Citation11,Citation43,Citation44] Important to realize is that in these studies, antithymocyte globulin (ATG) was used frequently, which is suggested to be the main predictor influencing early T-cell reconstitution. Many reports are available showing a relationship between dosage and timing of ATG and early T-cell IR.[Citation9,Citation37,Citation42,Citation45–Citation47] Relatively low exposure of ATG to the graft already leads to a significant delay in T-cell IR while acute-GvHD was not influenced by exposure after CBT. This suggests that exposure after graft infusion should be avoided in CBT.[Citation9] If a patient lacks early T-cell IR (no ability of peripheral expansion of the infused T cells), the IR needs to come from thymic output which can take at least 6–9 months, but more likely up to several years after transplant.[Citation48] This leaves the patients vulnerable for viral reactivations and relapse.
More recently, several centers have omitted ATG (or other serotherapy) from the conditioning regimen in CBT. This strategy has been shown to be feasible in malignancies and is occasionally used in primary immune deficiencies. Results from these studies show excellent early T-cell IR, possibly even superior to BM or PBSC.[Citation10,Citation11,Citation45,Citation46] Hiwarkar et al. compared viral reactivations between cell sources: patients receiving CB without ATG in the conditioning showed lowest incidence of viral reactivation (cytomegalovirus, Epstein–Barr virus, adenovirus), even lower than BM without ATG.[Citation36] Additionally, CB-derived T cells mediate a more powerful antileukemic effect [Citation13] and tumor responses compared to adult T cells.[Citation2,Citation6,Citation19] A drawback of this strategy may be an increased incidence of GvHD. This was described in other manuscript from same group, but this GvHD was mainly acute and not chronic and did not influence TRM.[Citation46] Although ATG exposure after transplantation did not influence the probability of aGvHD (and significantly hampers CD4+ IR), sufficient exposure before transplantation was associated with lower probability on aGvHD.[Citation9] Furthermore, not using in vivo T-cell depletion of the host may also be a problem in immune-competent recipients (e.g. BMF or IEM and hemoglobinopathies) in preventing rejection. Thus, by individualizing the dosing and timing of ATG before infusion of donor graft, the chances on aGvHD and rejection may be reduced, while timely T-cell IR is promoted resulting in better survival chances.
5. Currently used conditioning regimens and outcomes
In current daily practice, many conditioning regimens are used in allogeneic HCT, including CBT-setting. Most of the regimens used in CBT were simple copies from regimens used in transplantation using the conventional sources: related and unrelated BM or PBSC. The huge variety of regimens makes comparisons between centers, and even between stem-cell sources, difficult as the conditioning regimen used can influence the outcomes. Furthermore, a conditioning regimen used for BM may not be optimal in CBT as the number of cells and the phenotype of cells is different. The currently used conditioning regimens and GvHD-prophylaxes in centers performing a substantial number of CBT’s on a yearly basis are shown in .[Citation9,Citation11,Citation15,Citation37,Citation41,Citation49–Citation59]
Table 1. Currently used conditioning regimens in HCT centers performing large numbers of cord blood transplantation.
In adults, where the main indication is leukemia, most centers use a total body irradiation-based conditioning, mostly combined with Cy and/or fludarabine (Flu). Other centers use Bu-based conditioning.[Citation60] In children, indications for CBT are more diverse; we focused on conditioning regimens used in CBT for leukemia, PID and IEM. In general, most centers use a chemotherapy-based conditioning for all indications, mostly Bu- or Flu-based. No center uses the same conditioning in all pediatric indications, nor were the same conditioning regimens for adults and children with the same indication. Historically, decision-making was mainly based on expert opinion not on clear evidence.
Historically, most patients received ATG containing regimens, while nowadays most centers choose not to use ATG or other serotherapy, such as alemtuzumab in CBT, neither in adults nor children. The main reason for this is that exposure to the graft of ATG (and alemtuzumab) significantly depletes the T cells in the CB-graft. Early T-cell reconstitution relies in the first months mainly on peripheral expansion of the infused T cells. Therefore, over-exposure to serotherapy may lead to delayed or absent T-cell reconstitution during the first 6–9 months after CBT, making a patient at risk for viral reactivations and relapse. When serotherapy is not part of the conditioning regimen, IR following CBT can result in a restoration of adaptive immunity within 2 months after transplantation.[Citation46,Citation61] A drawback, however, of omitting ATG is the higher incidence of acute GvHD as discussed above. Developing better balanced individualized conditioning regimens is a current excitement in the field of HCT.
Interestingly, as mentioned above the choice of conditioning regimen over the last decades was mostly expert opinion based. No solid evidence existed showing that one of conditioning regimens was superior to others. Most centers balance the necessity for intense conditioning, which may be needed for disease control and myeloablation, with toxic effects, both short term (e.g. GvHD, veno-occlusive disease, idiopathic pneumonia syndrome) and long term (growth in children, endocrinopathies, fertility, chronic GvHD including bronchitis obliterans). In this decision-making, possible inter-patient variability of PK is not taken into account. As PK measures may also influence PD endpoints, the outcomes may be optimized by studying the PK and PD in cohorts of patients to determine the optimal exposure of each component of the conditioning regimen. Over the last decades, there has been an increasing interest in pharmacological research in agents used in HCT (e.g. cyclosporine, tacrolimus, ATG, Busulfan). With these findings, a more evidence-based decision can be made on how to design the most optimal conditioning regimens (in term of exposure and combination of agents), which may result in the best predictable outcomes.
6. Toward individualized conditioning
As mentioned above, it is well recognized that differences in conditioning regimen may contribute to differences in outcome. Even within patients receiving the same conditioning regimen with comparable doses, outcomes may not be the same, due to variability in PK and PD of agents used in the conditioning (). Variables such age, body size, organ function, and concomitant medications can influence the PK profile resulting in variable PD outcomes.[Citation62–Citation65]
Figure 1. Schematic representation of fixed dosing (top row) and individualized dosing (bottom row). In fixed dosing, most variability is found in concentration/exposure and drug effects, leading to under- and overdosing in a part of the population. In individualized dosing, the variability is found in the dose, thereby accounting for differences in PK, leading to more on-target exposure and drug effects. Adapted with permission from: Steeghs N, Best Practice: TDM in oncology. Where there is evidence. Presented at the International Association for Therapeutic Drug Monitoring and Clinical Toxicology 2015 in Rotterdam, the Netherlands.
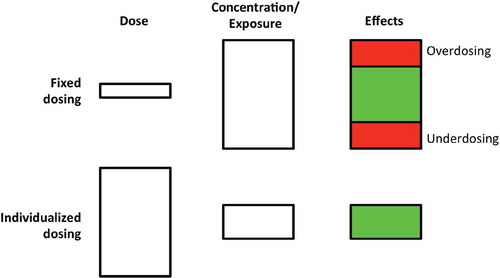
From a pharmacological perspective, drug exposure (i.e. drug concentration over time), rather than the drug dosing, is driving the effects. Many drugs used in the conditioning regimens are dosed in mg/kg or mg/m2, assuming a linear increase in clearance with increasing weight or body surface area, while this most often is untrue.[Citation66–Citation68] Also, other factors potentially influencing PK such as renal function, obesity, or age are not taken into account. As a consequence, using the same dose (expressed in mg/kg or mg/m2) for all patients, a vast variability in drug exposure is introduced, which may lead to under- or overdosing of certain patients ()). It would be preferable to adjust dosing, taking into account all factors influencing PK and PD (i.e. individualized dosing), resulting in comparable drug exposure and drug effects for all patients ()). These individualized dosing regimens may contribute to better predictable regimens in terms of safety and efficacy and in the context of HCT also predictable IR which reflects safety and efficacy.[Citation62,Citation69,Citation70]
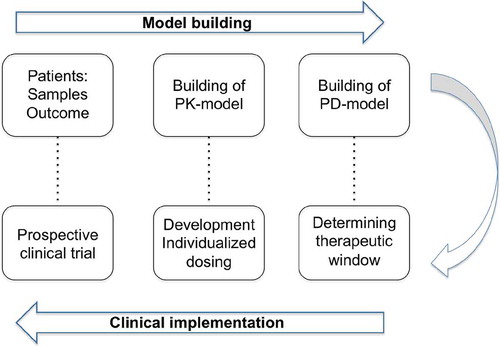
To develop individualized dosing regimens, the PK and PD of a drug of interest needs to be described in a population PK/PD model. With the PK and PD described, dose can be calculated to reach optimal effects, taking into account all factors influencing PK and PD. To finalize, a proposed individualized dosing regimen should be prospectively validated to assess its performance both in reaching optimal exposure and effects (). Ultimately, PK and PD of all components of the conditioning can be modeled in a multi-agent PKPD model. As the individual components of the conditioning regimen can each have their own optimal effect and can influence each other, modeling in a multi-agents model may even further optimize drug efficacy and safety. Such a multi-agent PKPD modeling has so far not been studied in the context of HCT.
Figure 2. The development of individualized dosing. First, in the model-building phase, samples are collected from the population of interest. Next, the PK and PD are described in this population. In the clinical implementation phase, using the PD-model, the therapeutic window is determined. Knowing the target exposure, the optimal dosing is calculated using the PK-model. This optimal dosing is evaluated in prospective clinical trial, potentially leading to a validated individualized dosing regimen.
TDM may further fine-tune the optimal drug exposure and is complementary to individualized dosing. This may be of value when significant variability in drug exposure remains following implementation of an individualized dosing regimen due to unpredictable PK. TDM can also be used without individualized dosing; however, substantial dosing adjustments and the additional costs are drawbacks. Additionally, PK and optimal exposure need to be described in order to perform TDM; therefore, the additional effort to design an individualized dosing regimen is minimal.
By individualizing the dose of all agents used in CB transplantation, the efficacy of the treatment may be improved while reducing unwanted toxicity, which may result in improved survival chances. A predictable conditioning is also of importance when harmonizing clinical trial design, where various novel therapies or interventions can be better compared as recently reviewed by an international consortium.[Citation71]
7. Examples of individualized dosing in HCT
Although PK/PD modeling has been around for decades,[Citation72] implementation of PK/PD-based individualized dosing regimens used prior to HCT including CBT, is scarce. This may have been due to lack of communication between physicians and pharmacometricians (PK/PD specialists), a difference in scope of research (i.e. more descriptive versus predictive models), and little confidence in the advantages of individualized dosing by physicians. However, the field of HCT has been a pioneer in the use of individualized dosing aiming to optimize the survival chances.[Citation63,Citation64,Citation67,Citation73,Citation74] Currently, most centers target to trough levels of cyclosporin A or tacrolimus in the context of GvHD prophylaxis and are using individualized Bu-dosing with TDM to aim for optimal exposure.[Citation75,Citation76] More recently, in some (inter)national studies a newly developed individualized ATG regimen aiming for improved and predictable T-cell IR was implemented.[Citation9,Citation37,Citation77]
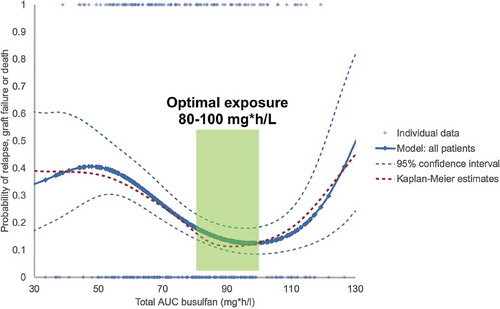
Busulfan PK has been studied by several groups (adult and pediatric), which has led to several PK-models, mainly developed in cohorts of infants and children but also in some adult cohorts.[Citation66,Citation67,Citation78–Citation80] The optimal therapeutic window has been established in multiple reports [Citation75,Citation76,Citation81,Citation82] (). This optimal exposure appears to be independent on cell source, match grade, indication, and concomitant conditioning agents.[Citation75,Citation76] Although the optimal exposure was similar among 1, 2, and 3 alkylators, patients receiving only Busulfan combined with Flu had lowest toxicity and superior survival chances (due to lower toxicity: e.g. VOD, GvHD, and IPS). The optimal cumulative target for Bu AUC0–4 days was found to be 90 mg h/L (equivalent to 5600 μmol min per day over 4 days), for all cell sources, including CB. Although conflicting data are present,[Citation83–Citation85] most (larger) studies including a meta-analyses suggest that the outcomes may be further optimized by combining Bu with Flu rather than with Cy and/or melphalan, mainly by reducing toxicity.[Citation53,Citation76,Citation86–Citation88] For a definite answer, best would be to study individualized dosing regimens in randomized controlled trials.
Figure 3. Weibull model of busulfan exposure in relation to EFS for all patients, showing the optimal exposure to be between 80-100 mg*h/L. Solid and dashed blue lines: Weibull model with 95% confidence intervals. Red dashed line: Kaplan Meier estimate. Reprinted from: Lalmohammed et al, Studying the Optimal Intravenous Busulfan Exposure in Pediatric Allogeneic Hematopoietic Cell Transplantation (alloHCT) to Improve Clinical Outcomes: A Multicenter Study, Biology of Blood and Marrow Transplantation 2015;21(2):S102-S103, with permission from Elsevier.
To better predict the T-cell IR, some groups have described the PK and PD of ATG (Thymoglobulin®).[Citation9,Citation42,Citation89–Citation93] From these PK models we have learned that dosing should be dependent on bodyweight as well as on the lymphocyte count prior to ATG, as these determine ATG clearance.[Citation89] Variations in levels of ATG or exposure to ATG before and after HCT were associated with various outcomes, such as CD4+ IR, TRM, relapse, and GvHD.[Citation9,Citation42,Citation91,Citation92] Most studies investigated concentrations at single time points as a predictor of outcome rather than total exposure (before and/or after HCT), making some reports hard to interpret. In the largest report in children, high exposure to ATG after transplantation was associated with lower chances on timely CD4+ IR (defined as twice over 50 × 106 per liter within 100 days after CBT: found to be the best predictor for outcomes), which was associated with lower survival and higher TRM and relapse rates [Citation9] (). Of note, in CBT even very low exposure of ATG after transplantation results in poor or even absent T-cell reconstitution during the first 6–9 months after transplantation (), while omitting ATG results in very fast CD4+ IR as described by various groups.[Citation30,Citation46,Citation94] Furthermore, the incidence of GvHD and rejection was mainly influenced by sufficient ATG exposure before infusion of the graft, not after. Taking these observations into account, an individualized dosing regimen was established, which is currently being investigated in a prospective study (PARACHUTE-trial; Dutch Trial Register identifier NTR4960).
Figure 4. Chance of successful reconstitution, incidence of acute graft-versus-host disease, and overall survival (A) Successful CD4+ T-cell reconstitution before day 100, defined as twice > 50 x 106/L (red 0’s) and grade 2–4 acute GvHD (blue I’s) versus AUC of active ATG after HCT. The logistic regression lines show the chance of successful reconstitution versus the AUC after HCT (red line) and the chance of developing acute GvHD of at least grade 2 versus the AUC after HCT (blue line). Every I or O represents a patient with their respective AUC after HCT (x axis) and whether they had an event (y axis, either yes [1; top] or no [0; bottom]). Therefore, the patient with an AUC after HCT of 480 AU × day/mL had no immune reconstitution and no GvHD. (B) Kaplan-Meier survival curve of overall survival according to successful CD4+ T-cell immune reconstitution. Reprinted from: Lancet Haematology, Volume 2, Issue 5, Admiraal et al, Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis, e194-e203. Copyright (2014), with permission from Elsevier.
![Figure 4. Chance of successful reconstitution, incidence of acute graft-versus-host disease, and overall survival (A) Successful CD4+ T-cell reconstitution before day 100, defined as twice > 50 x 106/L (red 0’s) and grade 2–4 acute GvHD (blue I’s) versus AUC of active ATG after HCT. The logistic regression lines show the chance of successful reconstitution versus the AUC after HCT (red line) and the chance of developing acute GvHD of at least grade 2 versus the AUC after HCT (blue line). Every I or O represents a patient with their respective AUC after HCT (x axis) and whether they had an event (y axis, either yes [1; top] or no [0; bottom]). Therefore, the patient with an AUC after HCT of 480 AU × day/mL had no immune reconstitution and no GvHD. (B) Kaplan-Meier survival curve of overall survival according to successful CD4+ T-cell immune reconstitution. Reprinted from: Lancet Haematology, Volume 2, Issue 5, Admiraal et al, Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis, e194-e203. Copyright (2014), with permission from Elsevier.](/cms/asset/b282b63c-036e-4ff7-8384-32c1fef3c70e/iebt_a_1164688_f0004_oc.jpg)
Figure 5. CD4+ T-cell reconstitution and overall survival according to area under the curve after haemopoietic stem cell transplantation by stem cell source. The effect of AUC of ATG after HCT on immune reconstitution in all patients (A), those who received cord blood transplants (B) and those who received bone marrow and peripheral blood stem cell transplants (C). Reprinted from: Lancet Haematology, Volume 2, Issue 5, Admiraal et al, Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis, e194-e203. Copyright (2014), with permission from Elsevier.
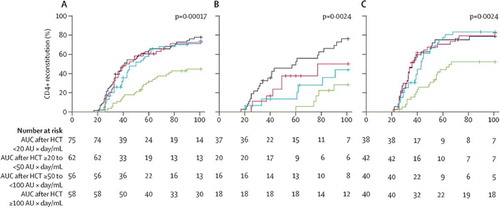
Figure 6. Summarizing figure showing the implications of individualized dosing in the conditioning of hematopoietic cell transplantation.
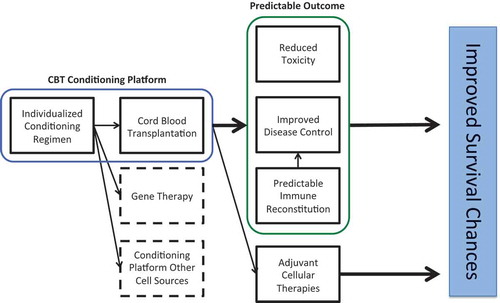
Taken together, the therapeutic windows of Busulfan and ATG are narrow and critical. Dose individualization appears to be essential for reaching optimal drug exposure. This may result in a better predictable conditioning regimen in terms of safety and efficacy associated with better survival chances.
8. Future of CBT
The two most important upcoming developments in the field of CBT are designing conditioning regimens that better predict early CD4+ IR and CB-based adjuvant cellular therapies. A predictable CD4+ IR is essential for an optimal effect of adjuvant cellular therapies, such as cell vaccines. In addition, new indications for CB therapy are being explored, including cerebral palsy and hypoxic ischemic brain injury: promising results have been reported for these new indications.[Citation95,Citation96]
8.1. Predictable early T-cell IR
As described above, there is apparently a delicate balance present between ATG (or other serotherapy: e.g. Campath) in CBT and prevention of GvHD and graft failure on one side, and T-cell IR on the other. Omitting ATG may be feasible in heavily chemotherapy pretreated or immune-compromised patients (leukemia’s, primary immune deficiencies) but is associated with higher probability on severe acute GvHD, but not chronic GvHD or TRM.[Citation37] Adding ATG to the conditioning may lead to delayed or absent early CD4+ IR by peripheral expansion, due to too high exposure of ATG after CBT.[Citation9]
Our group has worked on describing the PK and PD of ATG, the most commonly used drug for serotherapy in the Netherlands, in order to develop an evidence-based, individualized dosing regimen (see also Section 7). With this regimen, dosing and starting day can be chosen for each patient to ensure optimal ATG exposure before (and after) CBT. This individualized regimen has the advantages of sufficient recipient T-cell depletion and other immune cells with targets for antibodies in the ATG (e.g. antigen presenting cells) for the prevention of GvHD and graft failure, while exposure to ATG after infusion of cells is very low to prevent (too deep) T-cell depletion. When used in this individualized way, both early and late CD4+ IR following CBT will be at least similar (), but probably better than BM or PBSC transplants as suggested by various groups.[Citation9,Citation46,Citation94] The normal functionality of these cells is shown in some cell source comparison studies for probability on viral reactivation and relapse.[Citation2,Citation13,Citation36] Currently, a prospective clinical trial is recruiting in the Netherlands, investigating individualized ATG compared to historical fixed–dose ATG (see above). The primary endpoint is achieving CD4+ IR within 100 days.
More recently we initiated research into Flu, a purine analog used commonly next to Bu in CBT conditioning. Flu interacts with lymphocyte proliferation; therefore, exposure to Flu may play a role in T-cell IR.[Citation97,Citation98] The project is aimed at describing the population PK and PD of Flu in order to derive individualized dosing nomograms.
With the PK and PD of Bu, Flu and ATG described, a conditioning platform consisting of individualized drugs will be available. As a final step, a comprehensive model, integrating all agents, combined with patient and donor variables, will give a fully predictable and adjustable Bu–Flu–ATG backbone. Designing a platform that is predictable with regards to safety and toxicity (including CD4+ IR) is essential also in the context of harmonized clinical trial design to study effect of novel interventions such as adjuvant immunotherapies.[Citation71]
8.2. Targeted therapies, including adjuvant cellular therapies, after CBT
Targeted therapies, including adjuvant cellular therapies, given post-transplantation, are strategies being used and developed to get better disease control in patients receiving a HCT, including CBT for malignant disease as this remains an unmet need for certain indications. For a very selected group of patients, those with Philadelphia-positive ALL or CML, targeted small molecule therapies with tyrosine kinase inhibitors (TKI: e.g. imatinib, dasatinib) are given after HCT aiming to prevent disease relapse.[Citation99] More kinase inhibitors, when identified to have a role in the biology of leukemia and lymphoma, may be used post-HCT in future. Currently, no CB specific studies are known. Regarding cellular therapies, historically (and still in some protocols) unmanipulated lymphocyte infusions are given, while nowadays also more specific cell therapies are being developed, such as engineered T cells and cellular vaccines.
As relapse remains the main obstacle even after potentially curative HCT, novel combinational immunotherapeutic strategies are being developed aiming at preventing relapse after HCT. Currently, the most widely used type of additional immunotherapy combined with allogeneic HCT is donor lymphocyte infusion, where alloreactive T cells may help to eradicate residual tumor cells. Unfortunately, this ‘non-specific’ strategy suffers from severe toxic side effects, such as GvHD.[Citation100] Other approaches aim to increase innate or adaptive antitumor responses by transferring ex vivo-generated cells, such as chimeric antigen receptor (CAR)-modified tumor-specific cytotoxic T lymphocytes (CTL) or natural killer cells.[Citation101–Citation107] Although initial results seem promising, these procedures are often time-consuming (up to months) and may have limitations, such as HLA-restriction and uncertain functionality. Additionally, there is a highly variable induction of immunological memory upon transfer in the patient, which may restrict the broad eligibility of these treatments. Although CAR modified T cells looks promising, at least on the short term, several drawback, such as (life)-long B-cell lymphopenia in CAR T cells against CD19. Also, duration of effect, which may reflect the life span of these engineered T cells, remains unclear.
Another intriguing option is development of cell vaccines: increased antigen presentation provided by a DC-vaccine combined with the intrinsic increased proliferative capacity of the grafted CB cells may result in fast differentiation and proliferation of tumor-specific CTL early after CBT.[Citation108,Citation109] This early and mass expansion of tumor-specific CTL may subsequently result in clearance of minimal residual disease and prevention of relapses in cancer patients. Naïve CB-T cells display exceptional proliferative capacities, suggesting that efficient priming of these cells using a tumor-specific DC-vaccine will provide powerful antitumor activity. This may result in clearance of, and long-term immunological memory against, tumor cells. That CB T cells mediates a stronger antileukemic activity compared to adult cells was recently shown in a study by Hiwarkar et al.[Citation13]
For all these adjuvant immunotherapies, predictable T-cell IR is essential as the effect relies on absence of circulation ATG but also on presence of adequate number of T cells to mediate the desired antitumor effect.
9. Conclusion
CB is an emerging alternative cell source. In historical, mainly registry studies CB showed similar probabilities on survival compared to MUD, MMUD, and in some studies even compared to MSD transplantation. Some of the disadvantages such as prolonged neutrophil and platelet reconstitution have been overcome using higher cell-dosed units, double-cord transplants and more recently with expanded CB products. Furthermore, individualization of Bu has influenced donor-cell engraftment, relapse, and limited toxicity. More recently, the significant influence of exposure of ATG after CBT on T-reconstitution was recognized, while exposure before transplantation appears to be important in the prevention of rejection and GvHD. Naïve T cells from CB have been recognized to mediate powerful antiviral and antileukemia properties early after transplantation. To get the most potent effect, individualizing ATG dosing to improve T-cell IR after transplantation seems to be essential. Although a variety of regiments have been used, it seems that individualized ATG–Bu–Flu backbone used for CBT suggests being associated with the best predictable outcomes. Dose individualization is essential to optimize the effects. A predictable conditioning backbone is also essential for studying the effect of future adjuvant immune therapies or new agents to get better disease control, within a harmonized clinical trial setting ().
10. Expert opinion
Outcomes of CBT are reported to be highly variable. In addition to variables such as cell dose, underlying disease, comorbidities of the patients, the conditioning regimen used is recognized to impact the outcomes. Furthermore, differences in PK can be associated with variable myeloablation and immune suppression before and after CBT. Dose individualization of agents used in the conditioning regimen in CBT has proven its added value in terms of enhancing safety and efficacy. Further fine-tuning of individualized conditioning regimens, including all used agents and/or finding the optimal combination of agents, may result in better predictable conditioning regimens in terms of safety and efficacy including predictable T-cell IR. Furthermore, such a predictable conditioning regimen in CBT in the context of harmonized clinical-trial design is also of interest/importance to study the effect of adjuvant immunotherapies on CBT platforms, such as cellular vaccines, engineered T-cell therapies.
Currently, a large number of conditioning regimens are being used in allogeneic CBT. Besides differences in choice of drugs, the actual drug exposure varies due to variability in PK and PD between patients. The use of regular dosing regimens, with a linear increase in dose with body weight (mg/kg) or body surface area (mg/m2) leads to a highly variable exposure, with some patients being under- or overdosed. By using individualized dosing regimens, this variability in PK and PD is accounted for, resulting in more patients reaching optimal drug exposure and thereby drug effects.
While this may have major implications for patient care, research in comparing drugs or drug doses may also be impaired due to a skewed distribution in PK or PD.
An individualized dosing regimen is available and being used in clinical care for Bu.[Citation53] Additionally, exposure of ATG before and after HCT has shown to have impact on the outcomes. Individualized dosing regimen for ATG seems, therefore, crucial to influence the outcomes. To our best knowledge, these are the only drugs used in the conditioning regimen in allogeneic HCT for which individualized dosing regimens are available. However, efforts have been made to characterize the PK and PD of other drugs used including Flu,[Citation98,Citation110] treosulfan [Citation68] and Cy,[Citation111] which however did not yet result in practical guidelines or dosing recommendations.
There are however some hurdles in designing individualized drug dosing. As described above, the development of a dosing regimen includes describing drug PK and PD. In terms of PK, blood needs to be collected to determine drug concentration levels, finally resulting in a population PK model. Besides the possible logistical issues, collecting blood samples and accurate documentation of taking these samples, is a challenge in a pediatric setting, where observational studies are difficult due to ethical constraints. On the other hand, describing the PD and determination of the therapeutic window, that is the target exposure poses most challenges. This is especially true for drugs with effects for which no direct biomarker is available, or is also being influenced by concomitant medication (e.g. lymphocyte counts for ATG and Flu). Additionally, in drugs where the time between drug exposure and clinical effect is large (i.e. hysteresis), for instance incidence of relapse following clofarabine as conditioning, the exposure–effect relationship is hard to estimate.
Currently, cost effectiveness is playing an increasingly larger role in healthcare decision-making. In this perspective, we hypothesize that dose individualization may be quite cost-effective, especially in the field of HCT/CBT where all complications are very costly (e.g. treatment of GvHD, expensive anti-viral drugs, VOD, graft failure).[Citation53,Citation112] Additionally, the costs of the development of individualized dosing regimens are relatively low.
In the last years, dose individualization has proven its added value in terms of enhancing safety and efficacy. In the coming years, we expect to see more individualized dosing regimens emerging in the field of CBT, especially in pediatric transplantations, where differences in PK are major. In our view, we need fully individualized conditioning regimens including all drugs used. This way, outcome will be predictable and adjustable based on individual patients’ needs. Additionally, other drugs used in CBT may need individualization as well, including GvHD-prophylaxis and the treatment and prophylaxis for infectious diseases. Finally, the currently available models may be further sophisticated, describing not only PK or PD, but rather the complete spectrum of drug treatment, including dose, PK, biomarker response, clinical efficacy, and toxicity in one comprehensive model. We expect development and implementation of individualized dosing to take place in the next 10 years, thereby improving the knowledge and efficacy of clinical drug therapy, and improving clinical outcome following CBT. With individualized dosing, unwanted variability in drug exposure will be reduced, leading to predictable, adjustable, and improved outcome of CBT (). Such a predictable conditioning regimen can also be used as a transplantation platform in the context of harmonized clinical trial design to study the effects of adjuvant therapies: for example concomitant chemotherapy in conditioning or adjuvant immunotherapies.
Article highlights
While CB is a promising stem-cell source, predictable toxicity and efficacy will further enhance clinical outcomes, including T-cell IR.
Dose individualization, taking into account factors influencing PK and PD, leads to improved and better predictable outcomes.
Currently used conditioning regimens, including the drugs of choice and the dosage, are highly variable.
Individualized dosing is available for Bu and ATG, both having a major impact on transplant-related morbidity and mortality.
In future, we expect better predictable T-cell IR and posttransplant adjuvant therapies to further improve survival.
This box summarizes key points contained in the article.
Declaration of interest
R Admiraal’s salary is paid from a Rational Pharmacotherapy Program Grant (40-41500-98-11044) from the Dutch Organisation for Scientific Research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Gluckman E, Broxmeyer HE, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178.
- Eapen M, Rubinstein P, Zhang M-J, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi:10.1016/S0140-6736(07)60915-5.
- Willemze R, Rodrigues CA, Labopin M, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi:10.1038/leu.2008.365.
- Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi:10.1182/blood-2010-05-285304.
- Ponce DM, Zheng J, Gonzales AM, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1316–1326. doi:10.1016/j.bbmt.2011.01.011.
- Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi:10.1182/blood-2011-02-334870.
- Heemskerk MBA, Van Walraven SM, Cornelissen JJ, et al. How to improve the search for an unrelated haematopoietic stem cell donor. Faster is better than more! Bone Marrow Transplant. 2005;35:645–652. doi:10.1038/sj.bmt.1704865.
- Bartelink IH, Belitser SV, Knibbe CAJ, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. 2013;19:305–313. doi:10.1016/j.bbmt.2012.10.010.
- Admiraal R, Van Kesteren C, Jol-van Der Zijde CM, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haematopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2:e194–203. doi:10.1016/S2352-3026(15)00045-9.
- Jacobson CA, Turki A, McDonough S, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:565–574. doi:10.1016/j.bbmt.2012.02.002.
- Kanda J, Chiou L-W, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2012;18:1664–76 e1. doi:10.1016/j.bbmt.2012.06.005.
- Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi:10.1056/NEJMcp041956.
- Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced anti-tumor effects compared with adult peripheral blood T cells. Blood. 2015;126:2882–2891. doi:10.1182/blood-2015-06-654780.
- Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncol. 2010;11:653–660. doi:10.1016/S1470-2045(10)70127-3.
- Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using a various cell source in children with hurlers syndrome after Myelo-Ablative conditioning. An Eurocord-EBMT-CIBMTR collaborative study. Blood. 2013;121:3981–3987. doi:10.1182/blood-2012-09-455238.
- Pai S-Y, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–446. doi:10.1056/NEJMoa1410490.
- Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2972.
- Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol. 2014;5:68. doi:10.3389/fimmu.2014.00068.
- Milano F, Boelens JJ. Stem cell comparison : what can we learn clinically from unrelated cord blood transplantation as an alternative stem cell source ? Cytotherapy. 2015;17:695–701. doi:10.1016/j.jcyt.2015.03.003.
- Eapen M, Klein JP, Sanz GF, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: A retrospective analysis. Lancet Oncol. 2011;12:1214–1221. doi:10.1016/S1470-2045(11)70150-4.
- Rocha V, Spellman S, Zhang MJ, et al. Effect of HLA-matching recipients to donor noninherited maternal antigens on outcomes after mismatched umbilical cord blood transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2012;18:1890–1896. doi:10.1016/j.bbmt.2012.02.002.
- Thus KA, De Hoop TA, De Weger RA, et al. Predicted indirectly ReCognizable HLA epitopes Class I promote antileukemia responses after cord blood transplantation: indications for a potential novel donor selection tool. Biol Blood Marrow Transplant. 2016;22:170–173. doi:10.1016/j.bbmt.2015.08.014.
- Wagner JE, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685–1694. doi:10.1056/NEJMoa1410490.
- Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. doi:10.1182/blood-2011-10-388512.
- Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–758. doi:10.1182/blood-2012-08-449108.
- Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi:10.1182/blood-2009-04-215525.
- Martín-Donaire T, Rico M, Bautista G, et al. Immune reconstitution after cord blood transplants supported by coinfusion of mobilized hematopoietic stem cells from a third party donor. Bone Marrow Transplant. 2009;44:213–225. doi:10.1038/bmt.2009.15.
- Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43:365–373. doi:10.1038/bmt.2008.329.
- Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi:10.1182/blood-2011-02-334870.
- Lindemans CA, Te Boome LCJ, Admiraal R, et al. Sufficient immunosuppression with thymoglobulin is essential for a successful haplo-myeloid bridge in haploidentical-cord blood transplantation. Biol Blood Marrow Transplant. 2015;21(10):1839–1845
- Lund TC, Boitano AE, Delaney CS, et al. Advances in umbilical cord blood manipulation—from niche to bedside. Nat Rev Clin Oncol. 2014;12:163–174. doi:10.1038/nrclinonc.2014.215.
- Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43:498–513. doi:10.1016/j.exphem.2015.04.011.
- Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi:10.1038/nm1110-1167.
- Wagner JE, Brunstein CG, Boitano AE, et al. Phase I/II trial of Stemregenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:1–12. doi:10.1016/j.stem.2015.10.004.
- Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124:3121–3128. doi:10.1172/JCI74556.
- Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48:803–808. doi:10.1038/bmt.2012.221.
- Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–132. doi:10.1182/blood-2013-05-502385.
- Admiraal R, De Koning C, Bierings M, et al. Early CD4 T cell reconstitution prevents viral reactivation and improves outcome after pediatric hematopoietic cell transplantation. In: ASBMT-CIBMTR Tandem Meeting., 2016.
- Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:919–927. doi:10.1016/j.bbmt.2006.05.008.
- Ishaqi MK, Afzal S, Dupuis A, et al. Early lymphocyte recovery post-allogeneic hematopoietic stem cell transplantation is associated with significant graft-versus-leukemia effect without increase in graft-versus-host disease in pediatric acute lymphoblastic leukemia. Bone Marrow Transplant. 2008;41:245–252. doi:10.1038/sj.bmt.1705891.
- Admiraal R, Chiesa R, Bierings M, et al. Early CD4+ immune reconstitution predicts probability of relapse in pediatric AML after unrelated cord blood transplantation: importance of preventing in vivo T-cell depletion using Thymoglobulin®. Biol Blood Marrow Transplant. 2015;21:S206. doi:10.1016/j.bbmt.2015.06.012.
- Willemsen L, Jol-Van Der Zijde CM, Admiraal R, et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant. 2015;21:473–482. doi:10.1016/j.bbmt.2015.06.012.
- Oshrine BR, Li Y, Teachey DT, et al. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. Biol Blood Marrow Transpl. 2013;19:1581–1589. doi:10.1016/j.bbmt.2013.08.003.
- Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi:10.1080/14653240701231014.
- Booth C, Veys P. T cell depletion in paediatric stem cell transplantation. Clin Exp Immunol. 2013;172:139–147. doi:10.1111/cei.2013.172.issue-2.
- Chiesa R, Gilmour K, Qasim W, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156:656–666. doi:10.1111/j.1365-2141.2011.08994.x.
- Bosch M, Dhadda M, Hoegh-Petersen M, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14:1258–1275. doi:10.3109/14653249.2012.715243.
- Williams K, Hakim FT, Gress RE. T-cell immune reconstitution following lymphodepletion. Semin Immunol. 2008;19:318–330. doi:10.1016/j.smim.2007.10.004.
- Shah GL, Shune L, Purtill D, et al. Robust vaccine responses in adult and pediatric cord blood transplantation recipients treated for hematologic malignancies. Biol Blood Marrow Transplant. 2015;21:2160–2166. doi:10.1016/j.bbmt.2015.06.012.
- Sanz J, Cano I, Gonzalez-Barbera EM, et al. Blood stream infections in adult patients undergoing cord blood transplantation from unrelated donors after myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2015;21:755–760. doi:10.1016/j.bbmt.2015.06.012.
- Lazaryan A, Weisdorf DJ, DeFor T, et al. Risk factors for acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation with umbilical cord blood and matched related donors. Biol Blood Marrow Transplant. 2015;22:134–140. doi:10.1016/j.bbmt.2015.09.008.
- Ostronoff F, Milano F, Gooley T, et al. Double umbilical cord blood transplantation in patients with hematologic malignancies using a reduced-intensity preparative regimen without antithymocyte globulin. Bone Marrow Transplant. 2013;48:782–786. doi:10.1038/bmt.2012.243.
- Bartelink IH, Van Reij EML, Gerhardt CE, et al. Fludarabine and exposure-targeted busulfan compares favorably with Busulfan/Cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2013;20:1–9. doi:10.1016/j.bbmt.2013.11.021.
- Parikh SH, Mendizabal A, Benjamin CL, et al. A novel reduced-intensity conditioning regimen for unrelated umbilical cord blood transplantation in children with nonmalignant diseases. Biol Blood Marrow Transplant. 2014;20:326–336. doi:10.1016/j.bbmt.2013.11.021.
- Wall DA, Carter SL, Kernan NA, et al. Busulfan/melphalan/antithymocyte globulin followed by unrelated donor cord blood transplantation for treatment of infant leukemia and leukemia in young children: the cord blood transplantation study (COBLT) experience. Biol Blood Marrow Transplant. 2005;11:637–646. doi:10.1016/j.bbmt.2005.05.003.
- Martin PL, Carter SL, Kernan NA, et al. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184–194. doi:10.1016/j.bbmt.2005.09.016.
- Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective cord blood transplantation (COBLT). Biol Blood Marrow Transplant. 2005;11:149–160. doi:10.1016/j.bbmt.2004.11.020.
- Saliba RM, Rezvani K, Leen A, et al. General and virus-specific immune cell reconstitution following double cord blood transplantation. Biol Blood Marrow Transplant. 2015;21:1284–1290. doi:10.1016/j.bbmt.2015.06.012.
- Kurtzberg J, Prasad VK, Carter SL, et al. Results of the cord blood transplantation study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi:10.1182/blood-2008-03-140830.
- Sanz J, Wagner JE, Sanz MA, et al. Myeloablative cord blood transplantation in adults with acute leukemia: comparison of two different transplant platforms. Biol Blood Marrow Transplant. 2013;19:1725–1730. doi:10.1016/j.bbmt.2013.09.015.
- Flinsenberg TWH, Spel L, Jansen M, et al. Cognate CD4 T-cell licensing of dendritic cells heralds anti-cytomegalovirus CD8 T-cell immunity after human allogeneic umbilical cord blood transplantation. J Virol. 2015;89:1058–1069. doi:10.1128/JVI.01850-14.
- Admiraal R, Van Kesteren C, Boelens JJ, et al. Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child. 2014;99:267–272. doi:10.1136/archdischild-2013-303721.
- McCune J, Bemer M. Pharmacokinetics, pharmacodynamics and pharmacogenomics of immunosuppressants in allogeneic haematopoietic cell transplantation: part I. Clin Pharmacokinet. 2015 Nov 30. [Epub ahead of print]
- McCune J, Bemer M, Long-Boyle J. Pharmacokinetics, pharmacodynamics, and pharmacogenomics of immunosuppressants in allogeneic hematopoietic cell transplantation: part II. Clin Pharmacokinet. 2015 Nov 13. [Epub ahead of print]
- Brill MJE, Diepstraten J, Rongen AV, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51:277–304. doi:10.2165/11599410-000000000-00000.
- Savic RM, Cowan MJ, Dvorak CC, et al. Effect of weight and maturation on busulfan clearance in infants and small children undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1608–1614. doi:10.1016/j.bbmt.2013.08.014.
- Bartelink IH, Boelens JJ, Bredius RGM, et al. Body weight-dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clin Pharmacokinet. 2012;51:331–345. doi:10.2165/11598180-000000000-00000.
- Brink MH, Ackaert O, Zwaveling J, et al. Pharmacokinetics of treosulfan in pediatric patients undergoing hematopoietic stem cell transplantation. Ther Drug Monit. 2014;36:465–472. doi:10.1097/FTD.0000000000000047.
- Knibbe CAJ, Danhof M. Individualized dosing regimens in children based on population PKPD modelling: are we ready for it? Int J Pharm. 2011;415:9–14. doi:10.1016/j.ijpharm.2011.02.056.
- De Cock RFW, Piana C, Krekels EHJ, et al. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67 Suppl 1:5–16. doi:10.1007/s00228-009-0782-9.
- Nierkens S, Lankester AC, Egeler RM, et al. Challenges in the harmonization of immune monitoring studies and trial design for cell-based therapies in the context of hematopoietic cell transplantation for pediatric cancer patients. Cytotherapy. 2015;17:1667–1674. doi:10.1016/j.jcyt.2015.09.008.
- Sheiner L, Beal S. Evaluation of methods for estimating population pharmacokinetic parameters. I. Michaelis–Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8:553–571. doi:10.1007/BF01060053.
- Willemze AJ, Cremers SC, Schoemaker RC, et al. Ciclosporin kinetics in children after stem cell transplantation. Br J Clin Pharmacol. 2008;66(4):539–545.
- Wallin JE, Friberg LE, Fasth A, et al. Population pharmacokinetics of tacrolimus in pediatric hematopoietic stem cell transplant recipients : new initial dosage suggestions and a model-based dosage adjustment tool. Ther Drug Monit. 2009;31:457–466. doi:10.1097/FTD.0b013e3181aab02b.
- Bartelink IH, Bredius RGM, Belitser SV, et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:231–241. doi:10.1016/j.bbmt.2008.11.022.
- Lalmohamed A, Bartelink I, Van Reij L, et al. Studying the optimal intravenous busulfan exposure in pediatric allogeneic hematopoietic cell transplantation (alloHCT) to improve clinical outcomes: a multicenter study. Biol Blood Marrow Transpl. 2015;21:S102–3. doi:10.1016/j.bbmt.2014.11.124.
- Pascal L, Tucunduva L, Ruggeri A, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126:1027–1033. doi:10.1182/blood-2014-09-599241.
- Bartelink IH, Van Kesteren C, Boelens JJ, et al. Predictive performance of a busulfan pharmacokinetic model in children and young adults. Ther Drug Monit. 2012;34:574–583. doi:10.1097/FTD.0b013e31826051bb.
- Long-Boyle J, Savic R, Yan S, et al. Population pharmacokinetics of busulfan in pediatric and young adult patients undergoing hematopoietic cell transplant: a model-based dosing algorithm for personalized therapy and implementation into routine clinical use. Ther Drug Monit. 2015;37:236–245. doi:10.1097/FTD.0000000000000131.
- McCune JS, Bemer MJ, Barrett JS, et al. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res. 2014;20:754–763. doi:10.1158/1078-0432.CCR-13-3045.
- Geddes M, Kangarloo SB, Naveed F, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–228. doi:10.1016/j.bbmt.2008.07.006.
- Russell JA, Kangarloo SB, Williamson T, et al. Establishing a target exposure for once-daily intravenous busulfan given with fludarabine and thymoglobulin before allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19:1381–1386. doi:10.1016/j.bbmt.2013.07.002.
- Raj R, Dozeman L, Button A, et al. Myeloablative busulfan with cyclophosphamide (BuCy) versus busulfan with fludarabine (BuFlu) in myeloid neoplasms. J Clin Oncol. 2014;32:7041. doi:10.1200/JCO.2013.54.6911.
- Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–709. doi:10.1200/JCO.2013.49.0219.
- Baron F, Labopin M, Peniket A, et al. Reduced-intensity conditioning with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: A report from the acute leukemia working party of the European group for blood and marrow transplantation. Cancer. 2015;121:1048–1055. doi:10.1002/cncr.29530.
- Rambaldi A, Grassi A, Masciulli A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–1536. doi:10.1016/S1470-2045(15)00200-4.
- Ben-Barouch S, Cohen O, Vidal L, et al. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transpl. 2016;51:232–240. doi:10.1038/bmt.2015.238.
- Damlaj M, Alkhateeb H, Partain D, et al. Fludarabine busulfan compared to fludarabine melphalan is associated with increased relapse risk in reduced intensity conditioning transplant despite pharmacokinetic dosing. In: ASH., 2015.
- Admiraal R, Van Kesteren C, Jol-Van Der Zijde CM, et al. Population pharmacokinetic modeling of Thymoglobulin® in children receiving allogeneic-hematopoietic cell transplantation (HCT): towards improved survival through individualized dosing. Clin Pharmacokinet. 2015;54:435–446. doi:10.1007/s40262-014-0214-6.
- Kakhniashvili I, Filicko J, Kraft WK, et al. Heterogeneous clearance of antithymocyte globulin after CD34+-selected allogeneic hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:609–618. doi:10.1016/j.bbmt.2005.05.001.
- Podgorny PJ, Ugarte-Torres A, Liu Y, et al. High rabbit-antihuman thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant. 2010;16:915–926. doi:10.1016/j.bbmt.2010.02.027.
- Remberger M, Persson M, Mattsson J, et al. Effects of different serum-levels of ATG after unrelated donor umbilical cord blood transplantation. Transpl Immunol. 2012;27:59–62. doi:10.1016/j.trim.2012.06.003.
- Call SK, Kasow KA, Barfield R, et al. Total and active rabbit antithymocyte globulin (rATG;Thymoglobulin) pharmacokinetics in pediatric patients undergoing unrelated donor bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:274–278. doi:10.1016/j.bbmt.2008.11.027.
- Admiraal R, Van Kesteren C, Lacna A, et al. Individualized dosing and therapeutic drug monitoring for anti-thymocyte globulin to improve outcome following cord blood transplantation: proof of concept. In: ASBMT-CIBMTR Tandem Meeting., 2016. 10.1016/j.bbmt.2015.11.438
- Park D-H, Borlongan CV, Willing AE, et al. Human umbilical cord blood cell grafts for brain ischemia. Cell Transplant. 2009;18:985–998.
- Carroll JE, Mays RW. Update on stem cell therapy for cerebral palsy. Expert Opin Biol Ther. 2011;11:463–471. doi:10.1517/14712598.2011.557060.
- McCune JS, Mager DE, Bemer MJ, et al. Association of fludarabine pharmacokinetic/dynamic biomarkers with donor chimerism in nonmyeloablative HCT recipients. Cancer Chemother Pharmacol. 2015;76:85–96. doi:10.1007/s00280-015-2768-x.
- McCune JS, Vicini P, Salinger DH, et al. Population pharmacokinetic/dynamic model of lymphosuppression after fludarabine administration. Cancer Chemother Pharmacol. 2014;75:67–75. doi:10.1007/s00280-014-2618-2.
- Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27:1254–1262. doi:10.1038/leu.2012.352.
- Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010;36:528–538. doi:10.1016/j.ctrv.2010.03.004.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi:10.1016/S0140-6736(14)61403-3.
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi:10.1056/NEJMoa1215134.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi:10.1056/NEJMoa1410490.
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38–177ra38. doi:10.1126/scitranslmed.3005930.
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi:10.1056/NEJMoa0910383.
- Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor – positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi:10.1182/blood-2011-02-334870.
- Zhou X, Di Stasi A, Tey S-K, et al. Long-term outcome and immune reconstitution after haploidentical stem cell transplant in recipients of allodepleted-T-cells expressing the inducible caspase-9 safety transgene. Blood. 2014;123:blood – 2014–01 – 551671. doi:10.1182/blood-2014-01-551671.
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi:10.1016/j.immuni.2013.05.019.
- De Haar C, Plantinga M, Blokland NJ, et al. Generation of a cord blood-derived Wilms Tumor 1 dendritic cell vaccine for AML patients treated with allogeneic cord blood transplantation. Oncoimmunology. 2015;4:e1023973. doi:10.1080/2162402X.2015.1008371.
- Long-Boyle JR, Green KG, Brunstein CG, et al. High fludarabine exposure and relationship with treatment-related mortality after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:20–26. doi:10.1038/bmt.2010.53.
- Laínez JM, Orcun S, Pekny JF, et al. Comparison of an assumption-free Bayesian approach with optimal sampling schedule to a maximum a posteriori approach for personalizing cyclophosphamide dosing. Pharmacotherapy. 2014;34:330–335. doi:10.1002/phar.1346.
- Corbacioglu S, Cesaro S, Faraci M, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–1309. doi:10.1016/S0140-6736(11)61938-7.
