Abstract
Adenosine receptors (ARs), the major targets of caffeine and theophylline, comprise four receptor subtypes designated as A1, A2A, A2B and A3. Over a dozen AR agonists are currently in clinical trials for various conditions, including cardiac arrhythmias, neuropathic pain, myocardial perfusion imaging, cardiac ischemia, inflammatory diseases and cancer. Adenosine (nonselective), regadenoson (A2A) and dipyridamole (act indirectly via ARs) have received regulatory approval for clinical use. The present editorial will give a brief update on the current status of AR agonists in clinical trials.
1. Introduction
Adenosine, a naturally occurring nucleoside, is the endogenous agonist of four members of G-protein-coupled receptors, A1, A2A, A2B and A3 adenosine receptors (ARs) Citation[1-3]. AR antagonism is the major mechanism of action at ingested doses of the alkylxanthines caffeine and theophylline, the most widely consumed drugs in the world.
Adenosine has a general cytoprotective function as a response to organ stress, such as hypoxia. There is growing evidence that AR agonists are attractive therapeutic targets for a number of conditions, including pain, cardiac arrhythmias, myocardial perfusion imaging, cardiac ischemia, inflammation and certain types of cancer Citation[4-9]. An increasing number of AR agonists are currently in clinical trials, and some have received regulatory approval.
The development of AR agonists as drugs has been limited in the past by the essential requirement of the retention of the ribose moiety of adenosine for agonist activity. However, non-nucleoside AR agonists are now also in development Citation[1-3]. The present article will briefly recapitulate the rationale behind the development and briefly review the current status of AR agonists in clinical trials for various conditions. This Editorial is an update of the review published 5 years ago in the same journal Citation[1] (Expert Opinion on Emerging Drugs, 12, 479, 2007).
2. Current status of AR agonists in clinical trials for various conditions
2.1 Cardiac arrhythmias
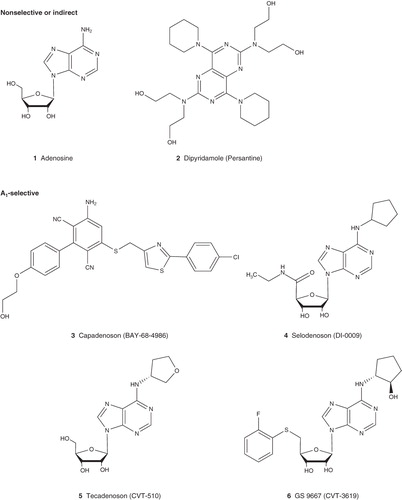
The first reported biological actions of adenosine are its depressant effect on heart rate and atrioventricular (AV) conduction () Citation[1-3]. This knowledge has continuously fueled considerable interest in pursuing AR agonists as drugs for cardiac arrhythmias, including atrial fibrillation (AF), supraventricular arrhythmias, paroxysmal supraventricular tachycardia (PSVT) and atrial flutter. Adenosine has been approved for PSVT under the name Adenocard, the effect of which is mediated via the A1AR, which is known to be abundant in the AV node of the heart. However, adenosine is a nonselective AR agonist, the adverse effects of which, such as flushing, dyspnea, chest discomfort and hypotension, are through the activation of other AR subtypes. Thus, there has been interest to develop more selective A1AR agonists having fewer side effects.
Table 1. Adenosine receptor (AR) agonists in clinical trials.
Tecadenoson (CVT-510), a full agonist for the A1AR, is in Phase III trial for patients with PSVT, AF and atrial flutter. It appears that low doses of tecadenoson have minimal effects on AV nodal conduction and blood pressure compared with adenosine. However, at high doses, like other full agonists for the A1AR, it may also display AV block. A Phase II clinical trial of another full A1AR agonist, Selodenoson, for AF has also been successfully completed. Both agonists showed promising therapeutic efficacy. However, their progress for further development will probably depend on potential side effects. A Phase II trial of BAY 68-4986 (capadenoson), a non-nucleoside A1AR agonist, in patients with persistent or permanent AF has been completed. This represents a novel chemotype among AR agonists for clinical development. A clinical trial of capadenoson for angina was withdrawn Citation[10].
2.2 Pain
The A1AR is abundantly expressed in spinal cord and other neuronal tissues. A1AR activation produced pain-relieving effects in a number of preclinical animal models Citation[4]. Adenosine is currently in Phase II trials for patients with perioperative pain and neuropathic pain. Selective A1AR agonists are being developed as analgesics.
However, it seems that several A1AR agonists, SDZ WAG 994, GR79236 and GW-493838, and one A1AR allosteric enhancer T62 Citation[11], have been withdrawn, possibly due to fact that these drugs may not penetrate the CNS sufficiently to cause a substantial effect. Also high doses may also produce adverse effects, as A1AR is abundant in a number of tissues including CNS, heart and adipose tissues. It has been suggested that partial agonists for the A1AR have the potential to produce a selective targeted response avoiding cardiovascular effects Citation[12]. Thus, partial A1AR agonists or biased A1AR agonists, that is, selective for certain signaling pathways, may be needed, in addition to those of increased A1AR selectivity, for further development.
A Phase II trial of the efficacy and tolerability of a novel A2AAR agonist BVT.115959 in the treatment of diabetic neuropathic pain has been completed in 2008, but there has not been an update since then.
2.3 Diabetes
Type 2 diabetes (T2D) patients have increased levels of nonesterified fatty acids (NEFA) in part due to β-adrenergic agonism, which decrease insulin sensitivity Citation[3,13]. Thus, lowering NEFA levels has an insulin-sensitizing effect. A full A1AR agonist, GR79236, has been in clinical trial for T2D but failed due to cardiovascular side effects (). Partial A1AR agonists might lower NEFA levels without cardiovascular or CNS side effects. Gilead Sciences initiated a Phase I trial of CVT-3619 (GS9667), a partial A1AR agonist, for T2D in 2008. This Phase I, single-blind, placebo-controlled, single ascending dose study evaluated the safety, tolerability and pharmacokinetics of oral CVT-3619, in 55 healthy and 23 obese volunteers. No clinically meaningful changes in heart rate or blood pressure were observed in the study, suggesting that CVT-3619 may increase insulin sensitivity and subsequently decrease blood glucose via lowering NEFA level without causing severe cardiovascular effects.
2.4 Myocardial perfusion imaging
The vasodilatory effect of the nonselective and short-acting agonist, adenosine, is mainly mediated via the A2AAR, which is abundant in the coronary blood vessels Citation[1-3]. Adenosine, under the name Adenoscan, is approved for myocardial perfusion imaging. In addition to its vasodilatory effect, adenosine may act at other ARs to induce side effects, for example, action on the A1AR may cause AV block and action on the A2BAR may induce bronchospasm especially in patients with asthma. Thus, selective A2AAR agonists are needed to replace adenosine.
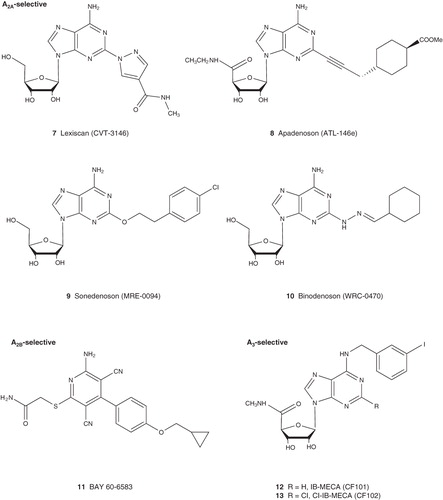
Three synthetic A2AAR agonists, regadenoson, binodenoson, apadenoson, are being applied to myocardial perfusion imaging, and regadenoson has received regulatory approval under the name of Lexiscan. The other two agonists are still waiting for approval. Dipyridamole (Persantine), which indirectly activates the A2AAR through increasing the concentration of adenosine as a nucleoside transporter inhibitor, is still being used for myocardial perfusion imaging.
2.5 Cardioprotection
All four ARs have been reported to be cardioprotective via various mechanisms. Phase II clinical trial of adenosine preconditioning in myocardial protection has been completed. A clinical trial with adenosine for acute myocardial infarction is now in Phase III Citation[10]. In addition to adenosine, the nucleoside transporter inhibitor, dipyridamole, as an indirect AR agonist, has long been used in clinic for heart attack and stroke and is still in clinical trial for protection against ischemia-reperfusion injury Citation[10].
The selective, non-nucleoside A2BAR agonist, Bay60-6583, has been shown to provide protection from ischemia in animal models and it has been proposed for use in drug-eluting stents Citation[14,15].
The recent demonstration of the cardioprotective effect of the A3AR with a positive allosteric modulator, LUF6096, further highlighted the broad therapeutic potential of A3AR activation Citation[16,17].
2.6 Inflammatory diseases
Activation of A2A, A2B and A3ARs is known to have anti-inflammatory effects in part due to inhibition of the release of proinflammatory cytokines. Agonists for both A2A and A2B ARs might be useful treatment of intestinal inflammation Citation[18]. The anti-inflammatory role of the A2AAR has been extensively studied and confirmed in multiple animal models. However, the pro- or anti-inflammatory roles of both A2B and A3ARs are still controversial Citation[5-8,19].
A possible therapeutic role for adenosine during inflammation is being studied clinically Citation[10]. A clinical trial regarding the role of adenosine in the release of vascular endothelial growth factor and cytokines has been suspended (to be reevaluated). Adenosine has been in clinical trial for perioperative pain, presumably due to its anti-inflammatory properties that may contribute to pain relief in the peripheral setting of inflammation. A Phase IV trial of the effects of oral dipyridamole treatment on the innate immune response during human endotoxemia has been completed Citation[10], but clinical data are not available.
An A2AAR agonist, sonedenoson, is in clinical trial as an experimental topical drug for the treatment of chronic, neuropathic, diabetic foot ulcers due to its wound healing and anti-inflammatory effect. The beneficial effect of the A2AAR agonist BVT.115959 in treatment of diabetic neuropathic pain is also due to its anti-inflammatory effect. Regadenoson, the A2AAR agonist approved for myocardial perfusion imaging, is in trials in children and adults with sickle cell disease due to its anti-inflammatory effect Citation[10].
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease. Although the pathogenesis of RA is still largely unclear, it appears to be an autoimmune disease driven by activated T cells, with increased T-cell-derived cytokines. Although a number of cyclooxygenase inhibitors (aspirin, ibuprofen and diclofenac) are in clinical use, their therapeutic effects are often uncertain, and gastric and intestinal side effects are common. The A2AAR has long been proposed to be a target for the treatment of arthritis, but there have been no agonists reported in clinical trial principally for RA. Nevertheless, investigators are evaluating the effects of undisclosed A2AAR agonists on atherogenic parameters in the plasma of systemic lupus erythematosus and RA patients, based on the anti-inflammatory action of the A2AAR Citation[10]. Dipyridamole alone or in combination with prednisolone for patients with moderate to severe RA is in Phase II clinical trial. The A3AR agonist CF101 (IB-MECA) is currently in a Phase II trial for patients with RA via oral administration. Oral CF101 is also in trials for patients with psoriasis and dry eye disease, for which Phase II results showed significant improvement, and with glaucoma Citation[9].
2.7 Cancer
ARs are involved in cell proliferation, apoptosis, metastasis and angiogenesis, suggesting their role in cancer Citation[1-3,9]. AR agonists that induce apoptosis or inhibit proliferation, metastasis and angiogenesis may potentially have anticancer effect. Clinical trial of CF102, an A3AR agonist, in patients with advanced hepatocellular carcinoma is ongoing. A Phase I/II study of CF102 in patients with chronic hepatitis C genotype 1 has been completed. The indirect AR agonist dipyridamole is in trials together with other anticancer drugs for patients with stage II or stage III pancreatic cancer and advanced ovarian cancer Citation[10]. 8-Chloroadenosine, which is only weakly acting at ARs, is in a Phase I trial for patients with chronic lymphocytic leukemia.
In addition to the indications mentioned above, dipyridamole has been used clinically for other conditions related to the indirect activation of ARs and is currently still in extensive clinical trials for various conditions either alone or in combined therapy. For example, the therapeutic effect of dipyridamole is being compared with that of olanzapine in schizophrenia patients; dipyridamole is also in a Phase II clinical trial for anemia. If the positive effect is identified and the AR subtypes involved in dipyridamole's effect are defined, selective AR agonists could be further developed for those conditions.
3. Summary
Considerable efforts have been put in the development of AR agonists for various conditions, and one synthetic A2AAR agonist, regadenoson, has received approval for myocardial perfusion imaging. A number of full A1AR agonists have failed in the trials for some conditions in recent years due to their side effects. Partial agonists, biased agonists or allosteric enhancers for the A1AR, which may produce selective targeted response, could be further developed. The development of the selective A2BAR agonists for various conditions still lags behind the other subtypes. Two A3AR agonists are being developed for a number of conditions. The recently synthesized A3AR allosteric enhancers could be further explored for a number of conditions, such as inflammation, cancer and cardiac ischemia. With the availability of the agonist-bound AR crystal structure Citation[20], it is hoped that more selective or biased AR agonists will be rationally designed and developed. As mentioned earlier, caffeine and theophylline are AR antagonists; thus, limiting the intake of coffee and tea is necessary when AR agonists are administered. It is predicted that there will be more AR agonists receiving regulatory approval for various conditions in the near future.
Declaration of interest
The authors and this paper were supported by NIDDK Intramural Research, National Institutes of Health. The authors declare no other conflicts of interest.
Bibliography
- Gao ZG, Jacobson KA. Emerging adenosine receptor agonists. Expert Opin Emerg Drugs 2007;12(3):479-92
- Fredholm BB, IJzerman AP, Jacobson KA, International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev 2011;63(1):1-34
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 2006;5(3):247-64
- Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs 2008;17(12):1901-10
- Cristalli G, Muller CE, Volpini R. Recent developments in adenosine A2A receptor ligands. Handb Exp Pharmacol 2009(193):59-98
- Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 2008;7(9):759-70
- Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol 2011;61:145-86
- Feoktistov I, Biaggioni I. Role of adenosine A2B receptors in inflammation. Adv Pharmacol 2011;61:115-44
- Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today; In press
- Clinicaltrials.gov
- Romagnoli R, Baraldi PG, Tabrizi MA, Allosteric enhancers of A1 adenosine receptors: state of the art and new horizons for drug development. Curr Med Chem 2010;17(30):3488-502
- Dhalla AK, Santikul M, Smith M, Antilipolytic activity of a novel partial A1 adenosine receptor agonist devoid of cardiovascular effects: comparison with nicotinic acid. J Pharmacol Exp Ther 2007;321(1):327-33
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care 2007;10(2):142-8
- Eckle T, Krahn T, Grenz A, Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation 2007;115(12):1581-90
- Drug-eluting stents for adenosine receptor modulation. US20110189255; 2011
- Du L, Gao ZG, Nithipatikom K, Protection from myocardial ischemia/reperfusion injury by a positive allosteric modulator of the A3 adenosine receptor. J Pharmacol Exp Ther 2011; Epub ahead of print
- Gao ZG, Verzijl D, Zweemer A, Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol 2011;82(6):658-68
- Antonioli L, Fornai M, Colucci R, Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther 2008;120(3):233-53
- Gessi S, Merighi S, Varani K, The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 2008;117(1):123-40
- Xu F, Wu H, Katritch V, Structure of an agonist-bound human A2A adenosine receptor. Science 2011;332(6027):322-7