Abstract
The Hedgehog (Hh) pathway is one of the central developmental signaling mechanisms, which have recently been shown to contribute to malignant progression of a variety of human cancers. Additionally, the role of Hh and other embryonic signaling pathways in the regulation and maintenance of the tumorigenic cancer stem / -initiating subpopulation underlines the importance of this pathway in human malignancies. The review ‘Targeting the Hedgehog signaling pathway for cancer therapy' by Li and coworkers has comprehensively described the potential of pharmacological targeting of Hh signaling. Here we provide an update on the current knowledge on i) the role of this pathway in human tumorigenesis and the rationale for therapeutic targeting, ii) the pharmacological approaches currently being investigated in preclinical and clinical studies, and iii) the outlook for future developments and efforts for establishing Hh antagonists as a valid approach in cancer treatment. Stratification of the tumor type according to Hh-specific expression patterns and clinicopathological characteristics, as well as investigation of possible tumor cell resistance against Hh antagonists, is the central area for further development.
1. Introduction
Within the last two decades, a lot of data were added to understand the process of tumor initiation and progression as summarized by Weinberg and Hanahan as the ‘hallmarks of cancer,' which has recently been extended by the model of cancer stem cells Citation[1]. Cancer stem (or -initiating) cells are defined by the unregulated expression of embryonic signaling mechanisms such as Hedgehog (Hh), Wnt, Notch, bone morphogenetic protein (BMP) or transforming growth factor-β (TGF-β) pathway, which play a central role during embryonic development and tissue maintenance as well as regeneration in adult organisms Citation[1,2].
As discussed by Li et al. in their review ‘Targeting the Hedgehog signaling pathway for cancer therapy' Citation[3], the Hh pathway cascade is activated upon ligand–receptor interaction at the cell surface (Hh ligands, the receptor Patched (Ptc) and the transmembrane protein Smoothened (Smo)), which initiates subsequent cytoplasmatic and nuclear signal transduction by Suppressor of fused (SuFu) and glioma-associated oncogene homologues (Gli) – for review see also Ingham et al. Citation[4]. Interestingly, the downstream gene targets of the Hh pathway regulate diverse cellular effects such as i) cell cycle progression by transcriptional activation of target genes such as cyclins and E2F1 as well as downregulation of the cell cycle inhibitors p21(CIP1/WAF-1) and p27. Furthermore, the Hh pathway regulates ii) apoptosis signaling by modulation of central anti-apoptotic proteins of the Bcl-2 family, and iii) promotes epithelial-to-mesenchymal transition (EMT) in many organs (for reviews see Citation[2,4]).
The oncogenic mechanisms of the Hh pathway can be characterized as either ligand independent or ligand dependent (reviewed in Citation[2,5,6]):
(i) The tumor-cell intrinsic, autonomous and ligand-independent type of Hh signaling is caused by mutations in Hh pathway components (e.g., inactivating mutations in Ptc1 or activating mutations in Smo).
(ii) Ligand-dependent oncogenic Hh signaling involve autocrine/juxtracrine/paracrine production of Hh ligands and signaling either in all tumor cells or in a small number of cancer stem cells and/or in surrounding stromal cells. Especially, paracrine mechanisms involving stromal cells functionally link different tumor compartments via production of factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), BMP, Notch and Wnt – to induce epithelial proliferation and differentiation.
Taken together, these diverse oncogenic activation mechanisms of Hh signaling could be susceptible toward pharmacological interference for novel drug development. This editorial comments the interesting and comprehensive review article ‘Targeting the Hedgehog signaling pathway in cancer therapy' by Li et al. Citation[3]. Based on this overview, we will critically evaluate i) the rationale and ii) therapeutic approaches and give iii) an expert opinion/outlook of therapeutic targeting the Hedgehog pathway in cancer therapies.
2. Rationale
In line with experimental data by our group (see Citation[2] for a recent review), the authors of the article ‘Targeting the Hedgehog signaling pathway for cancer therapy' Citation[3] provide a detailed overview on the specific roles of Hh signaling in human malignancies, which is here presented in a concise synopsis referring to the respective mode of Hh pathway activation ().
Table 1. Hedgehog pathway in human cancers and current clinical trials.
2.1 Hedgehog pathway activation by genetic alterations
The first evidence for the involvement of Hh signaling in human cancer by genetic alterations was observed in skin tumors (Gorlin syndrome (nevoid basal cell carcinoma or basal cell nevus syndrome) and the sporadic basal cell carcinoma (BCC)) as well as in medulloblastoma, which display either a loss-of-function mutation in Ptc-1 or a gain-of-function of Smo associated with constitutive Hh pathway activation (see , as well as reviewed in detail Citation[2]). Mutations in SuFu and Gli transcription factors have also been found, albeit to a lesser extent.
2.2 Hedgehog pathway activation by ectopic expression
As listed in , increased expression of members of the Hh pathway was found in tumors of various anatomic origin (gastrointestinal/aerodigestive/genitourinary tract, central nervous/hematopoietic system as well as skin and soft tissue) as well as in human cancer types of different histological origin (epithelial, mesenchymal, lymphatic and neuroectodermal) which, altogether, could not be linked to specific genetic alterations (such as amplifications, insertions or deletions). Similar to this, we characterized the expression pattern of members of the Hh pathway in different human cancer cell lines of the liver and pancreas in vitro and in vivo (using xenograft models) showing tumor-associated Hh signatures Citation[7,8]. One important aspect that is often neglected in the discussion of the relevance of tumorigenic Hh signaling is that overexpression is linked to the adjacent stroma: Hh components are ectopically expressed in tumor-surrounding stromal cells such as astrocytes, fibroblasts or endothelial cells (as described in, e.g., Citation[9]) and significantly influence tumor initiation and progression. Additionally, exaggerated inflammation could be linked to Hh pathway activation.
2.3 Link of Hedgehog pathway activity with molecular/pathological/clinical data
Interestingly, the ‘unregulated’ expression of Hh pathway members (as described above) correlates with molecular markers (such as p53, proliferation index Ki-67), the pathological status (such as histological tumor type, tumor initiation (precursors), differentiation and metastasis) and clinical data (such as clinical stage) in human breast, gastric, prostate and pancreatic cancer (e.g., Citation[10]) as well in hematological diseases such as myelodysplastic syndrome (unpublished data) demonstrating the complex cross talk of the ‘hallmarks of cancer' with Hh signaling in different stages in human tumorigenesis.
3. Therapeutic approaches
Currently, several attempts to develop and characterize pharmacological inhibitors of Hh pathway components are investigated in vitro and in vivo using different mouse models (for review see Citation[2,11]). These different approaches are discussed in deep by Li et al. Citation[3] focusing on clinical parameters of Phase I/II studies. Additionally, the authors include discussion of data on combinatory therapeutic treatments (such as Hh inhibitors and bevacizumab) as well as the interesting role of nutraceuticals such as soy isoflavone, curcumin, epigallocatechin-3-gallate, resveratrol and vitamin D in the regulation of the Hh signaling pathway.
Here, we summarize the quintessence of these small-molecule Hh inhibitors ( and ): Drugs that are currently investigated in Phase I – II clinical trials target the transmembrane receptor Smo – some of them are structurally analogous to the first Hh-targeting drug cyclopamine. At present, the most intensively studied drug is GDC-0449 (Vismodegib) showing promising data for treatment of BCC and medulloblastoma of which we exemplarily want to mention two papers: In 18 of 33 patients with metastatic or locally advanced BCC, an objective response accompanied primarily by mild to moderate side effects was observed and disease progression occurred in only four patients Citation[12]. These clinical observations of effectiveness of GDC-0449 as a single-agent HH-targeting approach must be critically evaluated with regard to a case report of a patient with chemorefractory metastatic medulloblastoma: though treatment of this patient resulted in a rapid regression of the tumor and reduction of symptoms, this effect was only transient and after 3 months followed by tumor regrowth via development of resistance toward GDC-0449 Citation[13].
Figure 1. Development of Hedgehog pathway-based antitumor therapies. A. The Hedgehog pathway and antagonists. In the absence of Hedgehog ligands (Hh), the transmembrane receptor patched (ptch) inhibits the smoothened (Smo) co-receptor. Subsequently, suppressor of fused (SuFu) deactivates Gli1, -2 transcription factors while activating the transcriptional repressor Gli3. Signaling mechanisms in the absence of ligands are indicated with dashed lines. In the presence of ligands such as sonic Hedgehog, Smo translocates to the primary cilium and releases the repression of Gli1, -2 via interaction with SuFu (the repressor Gli3 undergoes proteasome-mediated degradation). Pharmacological agents targeting Hh ligands, the Smo receptor or Gli transcription factors are shown in red. Those currently investigated in clinical trials (Phase I–II) are marked with an asterisk (see also ). B. Operational procedure for the establishment of Hedgehog-based personalized antitumor therapies. Establishment of individualized tumor treatment includes comprehensive molecular characterization (mRNA, microRNA, protein expression and functional testing of candidate drugs in primary cell cultures – culminating in rational patient stratification including clinicopathological characteristics).
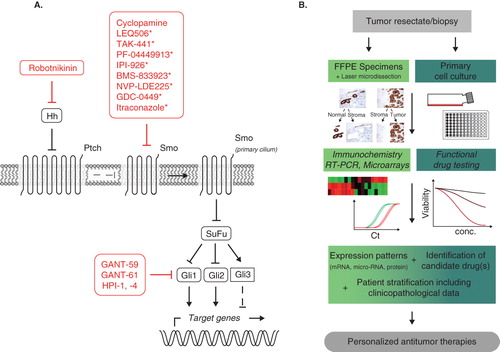
Subsequent mutation analysis of progressive lesions after GDC-0449 treatment revealed a heterozygous point mutation in the Smo locus, which was not detectable in the primary tumor tissue nor in normal skin. Interestingly, this mutation – not present in 64 archived medulloblastoma samples – inhibits the binding between GDC-0449 and Smo. This inhibition was reproducible in a subcutaneous allograft medulloblastoma derivative treated with GDC-0449 Citation[14]. A very recent pharmacokinetic dose-scheduling study of 63 adult patients revealed only few and fairly modest side effects, which is important for potential clinical use Citation[11,15]. It is interesting whether such resistance-conferring mutations also appear after treatment with the currently clinically tested alternative drugs that also target the Smo co-receptor – such as IPI-926, BMS-833923, NVP-LDE255 or itraconazole ( and ). Since the Hh pathway is still functional with this mutation, these data indicate the need for additional inhibitors acting downstream of Smo and Sufu in the pathway Citation[14].
Two such compounds were identified by Lauth et al. – named Gant-58 and Gant-61 Citation[16]. In a HEK293 cell model, Gant61 induced complete regression of human prostate cancer xenografts, whereas Gant58 only reduced tumor growth to an extent comparable with cyclopamine treatment Citation[16]. Two other inhibitors affecting Gli transcription factors (HPI1, 4) blocked proliferation of cerebellar granule neuron precursors, which express an oncogenic form of Smo that is resistant to cyclopamine Citation[17]. Such agents could be of special interest when acquired resistance to Smo inhibitors leads to recurrence after treatment with Smo antagonists Citation[18]. As discussed by Metcalfe et al., second-generation antagonists may be employed that retain their inhibitory function despite the presence of GDC0449-induced mutations Citation[19]. Taken together, results from the currently ongoing Phase I – II trials will evaluate the potential of the currently available drug and will allow a critical evaluation of the problem of drug resistance.
4. Expert opinion
The experimental findings of Hh in vitro and in vivo are very exciting and are translated into clinical settings. The currently used drugs show promising results but their effectiveness is heterogeneous depending on the treated cancer types and the occurrence of drug resistance. These initial experiences raise two main questions: What are the optimal predictors for sustained therapeutic success and what are the key targets within the Hh pathway (see , especially part B):
i) As discussed in detail by Li and colleagues in their review ‘Targeting the Hedgehog signaling pathway for cancer therapy' (Citation[3], see also Citation[2]), the heterogeneous expression pattern of Hh in human cancers must be taken into account for predicting the efficacy and developing new Hh pathway antagonists. Therefore, the signature of the functionally most important Hh pathway members has to be evaluated on mRNA and protein levels with adequate reproducibility and reliability Citation[11]. To clarify the role of tumor precursors as well as of the adjacent stroma, these analyses have to be performed using selective micro-dissection of human tumor samples, which is time-intensive and technically challenging. This Hh-associated tumor signature should be further stratified by other molecular and genetic investigations as well as by the clinical staging. Additionally, the complex influence of epigenetics via DNA methylation and histone acetylation as well as the network of microRNA regulation should be included in establishment and validation of the tumor Hh signature.
ii) To enhance Hh-based targeted therapy, combinatory treatment of Hh pathway antagonists with established and already standardized tumor therapy as well as with alternative targeted drugs needs comprehensive evaluation in order to make use of potential additive or synergistic effects. Here, systematic analyses based on preclinical in vitro and in vivo models are necessary.
iii) Finally, it will be important to understand the drug resistance mechanisms as well as to develop approaches to overcome such resistance to Hh-small molecule inhibitors. Therefore, ongoing clinical trials have to monitor whether and under which conditions drug resistance occurs and to identify the underlying molecular alteration that could further be employed as a basis for alternative drug combinations.
Declaration of interest
T Kiesslich was supported by a research grant of the Jubiläumsfonds der Österreichischen Nationalbank (OeNB, grant No. 12677) and the research fund of the Paracelsus Medical University Salzburg (grant No. 08/07/037). The authors declare that they have no affiliation with any organization with a direct or indirect financial interest in the subject matter or materials discussed in the manuscript.
Acknowledgements
We thank S Stintzing (Department of Medicine III, University of Munich, Germany) for proofreading the manuscript.
Notes
Bibliography
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646-74.
- Kiesslich T, Berr F, Alinger B, Current status of therapeutic targeting of developmental signalling pathways in oncology. Curr Pharm Biotechnol 2011; In press.
- Li Y, Maitah MY, Ahmad A, Targeting the hedgehog signalling pathway in cancer therapy. Expert Opin Ther Targets 2012; In press.
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of hedgehog signalling across the metazoa. Nat Rev Genet 2011;12(6):393-406
- Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2010;1805(2):181-208.
- Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res 2006;12(20 Pt 1):5924-8
- Jabari S, Meissnitzer M, Quint K, Cellular plasticity of trans- and dedifferentiation markers in human hepatoma cells in vitro and in vivo. Int J Oncol 2009;35(1):69-80
- Neureiter D, Zopf S, Dimmler A, Different capabilities of morphological pattern formation and its association with the expression of differentiation markers in a xenograft model of human pancreatic cancer cell lines. Pancreatology 2005;5(4-5):387-97
- Desch P, Asslaber D, Kern D, Inhibition of GLI, but not smoothened, induces apoptosis in chronic lymphocytic leukemia cells. Oncogene 2010;29(35):4885-95
- Quint K, Stintzing S, Alinger B, The expression pattern of PDX-1, SHH, patched and Gli-1 is associated with pathological and clinical features in human pancreatic cancer. Pancreatology 2009;9(1-2):116-26
- Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer 2011;11(7):493-501.
- Von Hoff DD, LoRusso PM, Rudin CM, Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 2009;361(12):1164-72.
- Rudin CM, Hann CL, Laterra J, Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 2009;361(12):1173-8.
- Yauch RL, Dijkgraaf GJ, Alicke B, Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science 2009;326(5952):572-4
- Lorusso PM, Jimeno A, Dy G, Pharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res 2011;17(17):5774-82
- Lauth M, Bergstrom A, Shimokawa T, Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA 2007;104(20):8455-60
- Hyman JM, Firestone AJ, Heine VM, Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci USA 2009;106(33):14132-7
- Weiss GJ, Von Hoff DD. Hunting the hedgehog pathway. Clin Pharmacol Ther 2010;87(6):743-7
- Metcalfe C, de Sauvage FJ. Hedgehog fights back: mechanisms of acquired resistance against Smoothened antagonists. Cancer Res 2011;71(15):5057-61.
