Abstract
Studies in animal models have demonstrated that the protease ADAMTS7 plays a role in neointima formation after arterial mechanical injury, by facilitating vascular smooth muscle cell (VSMC) migration. Furthermore, recent human genetic studies have revealed an association between DNA polymorphisms at the ADAMTS7 gene locus and risk of coronary artery disease (CAD). Functional studies have shown that a CAD-associated polymorphism in the coding region of the ADAMTS7 gene affects ADAMTS7 maturation and VSMC migration. This editorial highlights these findings and discusses targeted ADAMTS7 inhibition as a possible novel approach to treat CAD.
1. Introduction
The ADAMTS (‘a disintegrin and metalloproteinase with thrombospondin motifs’) family is a group of 19 proteases belonging to the class of zinc metalloproteinases, first identified in 1997. They play a key role in connective tissue organization, coagulation, inflammation, angiogenesis and organ development Citation[1]. Their effects on proteolysis and degradation of extracellular matrix and cartilage are widely recognised and for this reason have attracted interest for potential therapeutic targeting in arthritis. While different members have also been implicated in a wide range of other diseases, coronary artery disease (CAD) and atherosclerosis has not featured prominently until recently Citation[1]. Emerging experimental data along with independent and hypothesis-free findings from genome-wide association studies (GWAS) have spurred interest in one particular member, ADAMTS7, and its role in atherosclerosis. This article will briefly examine the evidence behind its association with atherosclerosis and discuss its potential for application as a therapeutic target for CAD.
2. Structure and function of ADAMTS7
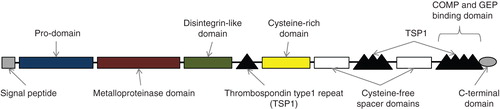
The ADAMTS proteases are initially synthesised as pre-proenzymes which include a signal peptide, a pro-domain, a catalytic metalloproteinase domain, a disintegrin domain, a central thrombospondin-type-like repeat, a cysteine-rich domain, a cysteine deplete spacer domain and a thrombospondin type 1 (TS) motif. Individual members of the family differ in the number of C-terminal TS motifs, and some have unique C-terminal domains ().
Figure 1. Schematic representation of the ADAMTS7 protein (see text). The terminal TS motif domain is the site for cartilage oligomeric matrix protein (COMP) and granulin-epithelin precursor (GEP) binding.
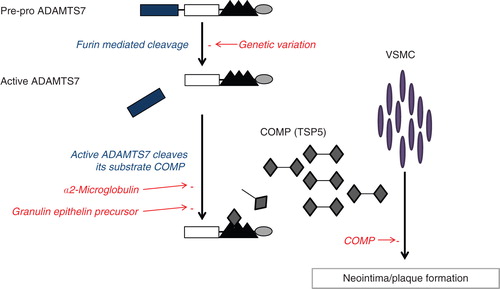
ADAMTS7 is similar in structure to ADAMTS12 and contains four C-terminal TS motifs Citation[2]. It is present in various tissues and its production is up-regulated by inflammatory mediators and free radicals Citation[1]. The active form of ADAMTS7 is derived by cleavage of the pro-domain by furin-mediated processing Citation[3]. ADAMTS7 can degrade cartilage oligomeric matrix protein (COMP) which is also known as thrombospondin-5, a non-collagenous extracellular matrix protein required for functional cartilage in joints Citation[4,5]. α2-Macroglobulin (α2M) inhibits ADAMTS7-mediated COMP degradation while granulin-epithelin precursor, a chondrogenic growth factor, also disturbs COMP degradation by ADAMTS7 by forming complex protein–protein networks () Citation[5,6]. To date, most research on ADAMTS7 has focused on its role in bone and joint disease. However, recent animal and human genetic studies have revealed an important role of ADAMTS7 in vascular disease and CAD.
3. Neointima formation
Neointima formation is regarded as an aggressive response to vessel injury such as that seen following coronary stent implantation. An animal model of this process is created, for example, by denudation of the carotid artery. Using this approach, Wang et al. quantified and demonstrated an initial decrease of ADAMTS7 protein level in the vessel wall within the first 24 h, followed by a subsequent increase up to 2 weeks after injury with maximal expression after 1 week Citation[7]. Importantly, ADAMTS7 was found to co-localise with vascular smooth muscle cells (VSMCs) in the newly formed neointima which developed after vessel injury. The authors also demonstrated that augmented expression of ADAMTS7 by an adenovirus infection method enhanced VSMC proliferation and migration in in-vitro experiments and increased neointima formation in vivo in the rat model, while suppression of ADAMTS7 expression by small interfering RNA (siRNA) had the opposite effect Citation[7]. In agreement, more recently Aherrahrou et al. showed in a mouse knockout model that loss of ADAMTS7 led to reduced neointima information following carotid artery injury induced by ligation Citation[8]. Thus, ADAMTS7 appears to be pivotal for developing intimal hyperplasia post vascular injury which is likely achieved by enhancing VSMC migration.
ADAMTS7 may exert its effects on VSMC migration by degradation of its primary substrate COMP Citation[4]. Experiments, also by Wang et al., revealed a parallel increase in COMP fragments and ADAMTS7 levels following carotid injury in rats Citation[7]. Similarly, overexpression of ADAMTS7 in VSMC led to a dose-dependent decrease in full-length COMP and increases in COMP fragments Citation[7]. If COMP was replenished by adenovirus infection, it attenuated ADAMTS7-mediated VSMC migration Citation[7]. While neointima formation is a specific response to vessel injury, VSMC mobilization is a very relevant process for plaque formation and stability thereby implicating ADAMTS7 in the biology of atherosclerosis.
4. Plaque development
GWAS have revolutionised our approach to discovery of novel pathways predisposing to risk of complex diseases by virtue of their hypothesis-free nature Citation[9]. The first wave of GWAS for CAD identified multiple novel loci, including the now prominent 9p21 region Citation[9]. The nature of these early studies necessitated unprecedented sample sizes which meant compromising phenotypic resolution and combining patients with stable and unstable CAD. Hypothesising that stable CAD is primarily a gradual atherosclerosis-driven process while unstable CAD, which can lead to myocardial infarction (MI), is more dependent on mechanisms predisposing to plaque rupture, Reilly et al. dissected these phenotypes using cohorts with coronary angiography data Citation[10]. With this approach they identified a novel locus, ADAMTS7 (rs1994016) associating with risk of atherosclerosis (but not MI) and postulated that ADAMTS7 may thus play a role in coronary plaque development. Subsequently, two independent GWAS also reported association between variants in and near ADAMTS7 and CAD. In one of these studies, the C4D consortium, with a two-stage GWAS of almost 70,000 Caucasian and South Asian subjects, reported five novel loci including ADAMTS7 (rs4380028) Citation[11]. In the second study, the CardioGRAM consortium performed GWAS and meta-analysis on almost 114,000 cases and controls, and reported 13 novel loci, again including ADAMTS7 Citation[12]. At the ADAMTS7 locus, the major allele (A) of the most significant SNP identified, rs3825807 was associated with an 8% increase in odds of CAD. Interestingly, in subgroup analysis of MI and angiographic CAD phenotypes, this variant showed a greater association with atherosclerosis than MI (OR 1.20 vs 1.08), supporting the earlier premise of Reilly et al.
Our group recently reported that ADAMTS7 is present in human coronary and carotid atherosclerotic plaques in which VSMCs with accumulated ADAMTS7 are mostly located near the intima-media border and the fibrous cap Citation[13]. Furthermore, in a general population cohort study (n = 787), we detected an association between the presence of carotid atherosclerosis and the minor allele (G) of the ADAMTS7 SNP rs3825807, with the G/G genotype having a protective effect Citation[13].
This SNP is a non-synonymous polymorphism, with the adenine (A) to guanine (G) change resulting in a serine (Ser)-to-proline (Pro) substitution in the pro-domain of ADAMTS7 (). In a series of in vitro experiments with VSMCs isolated from different individuals, we demonstrated that VSMCs of the protective G/G genotype had decreased migratory activity compared to those with the A/A genotype. Genotype appeared to influence levels of cleaved COMP in the media of the respective cells rather than levels of ADAMTS7 per se. Additionally we noted a fivefold lower concentration of cleaved pro-domain of ADAMTS7 in the media conditioned by VSMCs of the G/G genotype compared to A/A Citation[13]. These results suggest that the rs3825807 polymorphism leading to a Ser-to-Pro substitution in the prodomain, influences ADAMTS7 maturation, COMP degradation and VSMC migration and is associated with subclinical atherosclerosis, thereby linking genetic association findings with mechanistic pathways identified from animal models ().
5. Calcification
Recent data have also suggested a role for ADAMTS7 in vessel and plaque calcification. COMP appears to inhibit VSMC calcification by inhibiting bone morphogenetic protein 2 expression and preventing osteogenic changes of VSMC Citation[14]. Indeed ADAMTS7 is up-regulated in calcified VSMCs from renal patients while repression of miR-29a/b (an inhibitor of ADAMTS7) in turn facilitates VSMC calcification by targeting the 3′ un-translated region (UTR) of ADAMTS-7 Citation[14]. Further supporting evidence has also emerged from a recent genetic association study of aortic root and coronary calcification, a phenotype closely related to atherosclerotic plaque development. Although there was no association at genome-wide level, the ADAMTS7 locus was identified along with 9p21 as significantly associating with coronary artery calcification in a follow-up analysis of high-risk variants Citation[15].
In summary, we have described emerging evidence from independent GWAS, animal models, and cell and molecular studies implicating ADAMTS7 in the pathogenesis of atherosclerosis. The totality of evidence indicates that ADAMTS7 can facilitate VSMC migration in the arterial intima and promote vascular calcification through degradation of COMP, thereby leading to atherosclerotic plaque development. This raises the intriguing possibility that therapeutic blockade of ADAMTS7 directly or indirectly by interfering with its substrates may protect against atherosclerosis.
6. Expert opinion
ADAMTS7 represents a potentially exciting and novel therapeutic target for atherosclerosis. We have seen how multiple lines of evidence using different strategies have aligned to identify this pathway as having direct relevance for atherosclerosis. Importantly, human and animal models of genetic variation with loss of function in this gene appear to show protective phenotypes without undue harm or developmental abnormalities. However, one must exercise caution against over-interpretation of the available evidence especially as the data are both sparse and importantly require further independent validation. Additional experiments are necessary and will help consolidate these findings especially those that examine the effects of rare genetic loss or gain of function in human and animal models.
As a potential target, as with other family members, ADAMTS7 is an attractive option due to its tight substrate specificity, because the C-terminal regions of the enzymes, which influence protein recognition and matrix localization, reduce the risk of off-target effects. This is in contrast to matrix metalloproteinases (MMP), a related family of proteases whose substrates are of much more broad-spectrum and non-selective MMP inhibitors had substantial side effects in early trials. It is possible, however, that off-target effects may still occur given the close relationship between ADAMTS7 and ADAMTS12. Furthermore, despite COMP being considered the primary substrate for ADAMTS7, there is uncertainty in the available data regarding relevant enzyme kinetics; cleavage rates or the relationship between COMP levels and disease measures in animals and humans. All of these questions remain to be addressed but if confirmed synthetic inhibitors competing with specific COMP binding sites may prove to be the most suitable therapeutic option, although other elements within the pathway could also be explored for therapeutic potential, for example, by utilising α2M, a powerful endogenous inhibitor of ADAMTS7, or by supplementation with COMP.
The long history of arthritis research in this field has already created a substantial body of underpinning research into the chemical structures of the ADAMTS enzymes and putative compounds that could be used to inhibit them. For example, compounds are already in development for other ADAMTS members such as ADAMTS4 and 5 to inhibit degradation of aggrecan in joints Citation[16]. This may facilitate development of an ADAMTS7 inhibitor, but it should be noted that none of these other agents are yet in clinical practice despite a decade of research.
In conclusion, while the data on the role of ADAMTS7 in atherosclerosis are still maturing, if validated it represents an exciting and unique treatment target for CAD.
Declaration of interest
The authors state no conflict of interest and have received no payment for preparation of this manuscript.
Bibliography
- Wang L, Wang X, Kong W. ADAMTS-7, a novel proteolytic culprit in vascular remodeling. Sheng Li Xue Bao 2010;62:285-94
- Hurskainen TL, Hirohata S, Seldin MF, et al. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem 1999;274:25555-63
- Somerville RP, Longpre JM, Apel ED, et al. ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain. J Biol Chem 2004;279:35159-75
- Liu CJ, Kong W, Ilalov K, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J 2006;20:988-90
- Luan Y, Kong L, Howell DR, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage 2008;16:1413-20
- Guo F, Lai Y, Tian Q, et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum 2010;62:2023-36
- Wang L, Zheng J, Bai X, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res 2009;104:688-98
- Aherrahrou Z, Kessler T, Schmidt K, et al. Abstract 15199: knockout of the Coronary Artery Disease Risk Gene Adamts-7 Inhibits Neointima Formation and Stenosis of Arteries. Circulation 2011;124:A15199
- Patel RS and Ye S. Genetic determinants of coronary heart disease: new discoveries and insights from genome-wide association studies. Heart 2011;97:1463-73
- Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 2011;377:383-92
- Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet 2011;43:339-44
- Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011;43:333-8
- Pu X, Xiao Q, Kiechl S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet 2013;92:366-74
- Du Y, Gao C, Liu Z, et al. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler Thromb Vasc Biol 2012;32:2580-8
- van Setten J, Isgum I, Smolonska J, et al. Genome-wide association study of coronary and aortic calcification implicates risk loci for coronary artery disease and myocardial infarction. Atherosclerosis 2013;228(2):400-5
- Shieh HS, Tomasselli AG, Mathis KJ, et al. Structure analysis reveals the flexibility of the ADAMTS-5 active site. Protein Sci 2011;20:735-44