Abstract
Malignant brain tumors, which are the leading cause of cancer-related morbidity and mortality in children, span a wide spectrum of diseases with distinct clinical phenotypes but may share remarkably similar morphologic features. Until recently, few molecular markers of childhood brain tumors have been identified, which has limited therapeutic advances. Recent global genomic studies have enabled robust molecular classification of childhood brain tumors and the identification and consolidation of rare, seemingly disparate clinical entities. It is now increasingly evident that deregulation of epigenetic processes contributes substantially to heterogeneity in tumor phenotypes and comprise significant drivers of cancer initiation and progression. Specifically, DNA hypermethylation and silencing of critical tumor suppressor genes by DNA methyltransferases (DNMT) has emerged as an important and fundamental mechanism in brain tumor pathogenesis. These observations have been underscored by the recent discovery of TTYH1-C19MC gene fusions in an aggressive pediatric embryonal brain tumor, which results in deregulation and increased expression of a neural-specific DNMT3B isoform in C19MC-associated brain tumors. Our observations that pharmacological inhibitors of DNMTs and histone deacetylases significantly inhibit growth of cells derived from C19MC-associated tumors indicate targeting of epigenomic modifiers as a novel therapeutic approach for these highly treatment-resistant tumors.
1. Introduction
Brain tumors are the leading cause of childhood cancer-related death and disability and remain one of the most devastating and challenging cancers to treat. Surgical resection followed by intensive radio-chemotherapy remains the standard of care for the majority of malignant brain tumors arising in childhood. Although such intensive treatment approaches have substantially improved survival for some childhood tumors like medulloblastoma (MB), it has been largely ineffective for other brain tumors in children. Moreover, these multimodal approaches, even when successful, are associated with significant long-term neurocognitive and physiological deficits in survivors and underscore the need for more rational molecular-based therapies tailored to the biology of specific childhood brain tumors.
Intrinsic brain tumors arising in children encompass a heterogeneous group of diseases and are currently classified on the basis of morphological resemblance to major CNS cell lineages (glial, oligodendroglial, neuronal), histologic grading (grades I – IV) and clinical features including patient age and tumor location. Embryonal brain tumors, which are the largest group of malignant childhood brain tumors, comprise a spectrum of tumors with very similar primitive neuroectodermal histology and include MB and CNS-primitive neuroectodermal tumors (CNS-PNET) Citation[1], which are distinguished largely based on tumor location. However, in contrast to major advances in cure rates of localized MB in recent years with use of dose-intensive chemotherapy and neuro-axis irradiation (5-year OS rate ∼ 87%), survival for children with CNS-PNETs treated similarly remains poor Citation[2]. Until recently, the molecular nature and potential therapeutic pathways in CNS-PNETs remained unknown.
2. Amplification of C19MC, an ES-enriched miRNA cluster, marks a distinctly lethal brain tumor
Global profiling studies indicate CNS-PNETs comprise heterogeneous molecular entities. Notably, we and others have identified a subtype of CNS-PNETs (which we call Group 1 CNS-PNETs) distinguished by hallmark genomic amplification of the embryonic stem cell (ES)-enriched C19MC oncogenic miRNA cluster and histological features reminiscent of very early neural differentiation Citation[3,4]. Significantly in recent studies, we observed C19MC amplification in CNS-PNETs with different histologic diagnostic labels, including ETANTRs, ependymoblastoma and medulloepithelioma, originating in various CNS locations Citation[5,6]. These Group 1 tumors collectively comprise ∼ 25% of all CNS-PNETs arising in very young children (> 80% are < 4 years old), and are distinctly lethal tumors with characteristic rapid disease tempo. Despite the application of intensive multimodal therapy, projected 5-year OS for these young patients is < 10%, with only a very small number of survivors reported in the literature.
Biological and therapeutic models of these tumors remain to be developed; however, recent molecular studies of primary tumors and patient-derived Group 1 tumor cell lines reveal promising therapeutic pathways for these highly lethal tumors. Notably, recent studies demonstrate C19MC-associated Group 1 tumors with various histologic labels and anatomic location exhibit a common transcriptional and epigenomic signature, distinct from that of other CNS-PNETs, thus indicating these tumors comprise a common molecular disease Citation[5,6]. Consistent with their distinctly primitive histology, transcriptional signatures of C19MC-associated tumors show most remarkable enrichment of pluripotency and early neural differentiation genes. Most notably, pluripotency gene LIN28, which is known to modulate cellular growth and differentiation via regulation of the highly conserved let-7 family of miRNAs and the PI3K-mTOR signaling pathway Citation[7], is one of the most highly expressed genes in Group 1 tumors. Indeed, in vitro studies demonstrate preservation of the LIN28-PI3K-mTOR axis and sensitivity to Rapamycin, an mTOR inhibitor, in two independent Group 1 cell lines Citation[5,8].
3. C19MC mediates deregulation of DNA methyltransferase in Group 1 CNS-PNETs
DNA methylation is a critical mechanism involved in epigenetic gene regulation during normal development and disease progression. In mammals, DNA methylation involves the transfer of a methyl group from the ubiquitous methyl donor D-adenosyl methionine to the 5-position cytosine (C-5) in CpG dinucleotides, a process catalyzed by three enzymatically active DNA methyltransferases (DNMT): DNMT1, DNMT3A and DNMT3B Citation[9]. DNMT1, which is the most highly expressed DNMT, functions as a maintenance methyltransferase following DNA replication to preferentially methylate hemi-methylated CpG sites on newly synthesize strands. In contrast, DNMT3A and DNMT3B function as de novo methyltransferases to methylate both hemi-methylated and un-methylated CpG sites.
CpG dinucleotides are mainly located in CpG islands proximal to gene promoters in repeated sequences and CpG island shores Citation[10]. Once promoter-CpG islands are methylated, the corresponding gene is silenced due to poor recognition by transcription factors and the recruitment of chromatin remodelers such as Methyl-CpG-binding domain (MBD) proteins. Other epigenomic modifiers, including histone deacetylases (HDAC) and histone methyltransferases (HMT), also contribute to heterochromatic states Citation[11]. Histone methylation at the promoter level varies based on extent of methylation and modified residues, which may result in activation or repression of the corresponding gene. For example, trimethylation of H3K4 is a marker for transcriptional activation, whereas trimethylation of H3K9 and H3K27 results in epigenetic silencing ().
Figure 1. Mechanisms of DNMT-mediated gene silencing. Activating methylation of H3K4 and acetylation of histone tails promotes a euchromatic state, enabling transcription factor binding, and activates transcription of corresponding gene. DNMT and HMT-mediated methylation of H3K9 and H3K27 results in recruitment of chromatin remodeler MBD proteins, which subsequently recruits HDAC complexes for the removal of acetyl groups from histone tails. This results in chromatin condensation, which prevents transcription factor binding and promotes gene silencing.
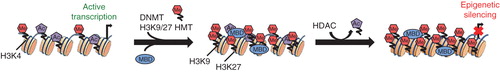
Epigenetic modifications, including DNA methylation and post-translational methylation, acetylation or deacetylation of histone tails, which together determine DNA accessibility and gene transcription states, are increasingly implicated in malignant transformation in different cancers. Gene mutations affecting post-translational modification of histones have been reported in a spectrum of pediatric embryonal and glial brain tumors and underscore epigenetic deregulation as important pathogenic mechanisms in childhood brain tumors. Mutations of H3K4 HMT (MLL2, MLL3), chromatin remodelers (SMARCA4, CHD7) and H3K27 demethylases (KDM6A, KDM6B) have been reported in MB Citation[12]. Remarkably, recurrent somatic mutations of histone gene H3F3A, leading to impaired methylation of histone tails, were identified in pediatric glioblastoma multiforme Citation[13]. Genetic alterations of DNMTs remain to be identified in brain tumors; however, substantial evidence suggests deregulation of DNMTs that function in maintaining and establishing de novo DNA methylation also contributes to pediatric brain tumor pathogenesis.
Subgroups of CNS-PNETs exhibit highly distinct DNA methylation patterns thus suggesting epigenomic mechanisms may also be important in these embryonal tumors Citation[5,6]. Indeed, recent RNA-sequencing studies revealed recurrent gene fusions of C19MC with TTYH1, a developmentally restricted chloride channel gene, associated with very high expression of some C19MC miRNAs and a neural-specific DNMT3B isoform in Group 1 tumors, due to C19MC-mediated targeting of RBL2, a transcriptional repressor of DNMT3B Citation[14]. These findings suggest DNMT3B as an important downstream effector of the C19MC oncogene and highlight DNMTs and cooperating epigenomic modifiers as potential novel therapeutic targets for these distinctly fatal tumors.
4. Targeting DNMTs and related proteins in high-risk embryonal brain tumors
DNMTs and modifiers of histone/histone tail methylation and acetylation, which are functionally linked, are frequently deregulated by tumor-specific events and act together to effect transcriptional changes that promote tumor development. Nucleoside analogues of cytidine modified at the C-5 position that can be incorporated into replicating DNA and impair methylation of de novo DNA are potent DNMT inhibitors. Pharmacologic DNMT inhibitors such as 5-azacytidine (5-AzaC) and 5-aza-2′-deoxycytidine (5-AzadCyD), which have shown promising effects in model systems and human clinical trials, are thought to act in part via progressive DNA hypomethylation and reactivation of critical tumor suppressor loci.
Hypermethylator tumor phenotypes associated with enrichment of CpG island methylation has been described in a spectrum of adult and pediatric brain tumors and has also been observed in the C19MC-associated Group 1 CNS-PNETs. Mechanisms that drive and underlie hypermethylator phenotype in specific brain tumors remain to be elucidated; however, studies in malignant glioma cells show 5-AzadCyD treatment promotes differentiation, reduces global DNA methylation and results in reactivation of the p53 tumor suppressor pathway Citation[15]. Significantly, we have observed that cell lines derived from C19MC-associated Group 1 CNS-PNETs, which express high levels of DNMT3B, are also remarkably sensitive to treatment with 5-AzaC, as well as Vorinostat, an HDAC inhibitor Citation[5]. These data provide the first functional evidence that targeting epigenomic modifiers represent promising, novel therapeutic approaches for these recalcitrant tumors (). DNMT inhibitors, 5-AzaC and Vorinostat are known to permeate the blood−brain barrier Citation[16,17], and have established safety profiles, thus making them very attractive candidate drugs to take forward rapidly into clinical trials for these high-risk brain tumors.
Figure 2. Targeting DNMTs as novel therapeutics in high-risk CNS-PNETs. C19MC amplification or TTYH1-C19MC gene fusions result in overexpression of C19MC miRNAs. C19MC-mediated post-translational downregulation of RBL2 leads to de-repression of DNMT3B and high expression of an embryonic, brain-specific DNMT3B isoform that promotes a highly primitive neural transcriptional and epigenomic program in CNS-PNETs. Treatment with DNMT or HDAC inhibitors, 5-AzaC and Vorinostat, inhibits growth of cell lines from C19MC-associated lethal embryonal brain tumors by promoting cell death or differentiation.
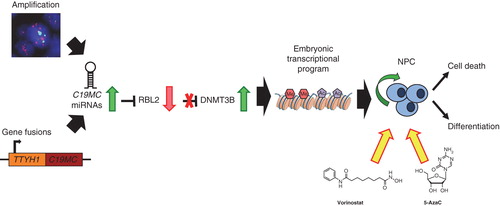
Although the specific cellular effects and predominant molecular targets of 5-AzaC and Vorinostat in Group 1 CNS-PNET tumor cells need to be further studied in preclinical models, it is interesting to note that synergistic effects without additional toxicities have been reported with combined DNMT and HDAC inhibitor therapy in other cancers Citation[18]. In addition to mTOR inhibitors, our prior studies suggest that inhibitors of the WNT pathway may be additional promising therapeutics for tumors driven by C19MC Citation[3]. Current approaches with conventional and high-dose chemotherapy regimens have been associated with frequent early disease progression and underscore a need to incorporate or substitute biologic agents early in disease treatment. There is an urgent need to undertake preclinical studies to evaluate efficacy and toxicities of sequencing single or multiple candidate biologic agents identified to date, with or without conventional cytotoxic agents in order to define the most efficacious and least toxic regimens to take forward to clinical trials.
The identification of C19MC amplification as a hallmark feature of a distinct class of infantile embryonal brain tumors has greatly facilitated studies of tumor biology and therapies for this previously poorly recognized ‘orphan’ entity. Our studies demonstrate C19MC mediates oncogenesis in part via modulation of the tumor epigenome and suggest promising new therapeutic avenues for this presently incurable tumor. In addition to preclinical studies, future functional investigations to uncover other effectors downstream of C19MC and DNMT3B will be important for informing robust therapeutic models.
5. Expert opinion
Childhood embryonal tumors span many molecular entities. Reflecting their diverse biology, current pan-embryonal brain tumor chemo-radiotherapeutic regimens are highly successful for some but largely ineffective for other tumors. Embryonal brain tumors arising in younger children present particular therapeutic challenges due to the much greater susceptibility of these patients to acute and long-term toxicities of conventional chemo-radiotherapeutic treatments. Cumulative data indicate embryonal brain tumors with hallmark C19MC amplification represent a distinct molecular entity associated with a highly aggressive clinical phenotype that warrants a different and more specific treatment approach. Our studies suggest C19MC-mediated deregulation of DNMT3B is an important mechanism driving oncogenesis in these tumors and indicate that pharmacological antagonists of DNMTs and epigenomic modifiers with related functions represent important, novel therapeutics for this disease. Additional studies to identify genes and pathways affected by high expression of DNMTs are imperative for further delineating molecular and cellular mechanisms driving C19MC-associated tumorigenesis and designing more precise and less toxic therapies for these uncommon but deadly brain tumors.
Article highlights.
Therapies for malignant pediatric brain tumors are imprecise and highly toxic.
Epigenomic deregulation play important roles in pediatric brain tumor development and progression.
Tumor-specific alterations and deregulation of DNMTs and regulators of DNMTs suggest they are important cancer drivers.
C19MC amplification defines a distinct and highly lethal group of childhood embryonal/primitive neuroectodermal brain tumor.
C19MC promotes CNS-PNET oncogenesis by targeting tumor suppressor RBL2 and deregulating expression of DNMT3B.
Direct and indirect inhibitors of DNMTs represent promising novel therapies for C19MC-associated childhood brain tumors.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
This box summarizes key points contained in the article.
Bibliography
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114(2):97-109
- Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of children’s oncology group trial A9961. Neuro Oncol 2013;15(1):97-103
- Li M, Lee KF, Lu Y, et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell 2009;16(6):533-46
- Korshunov A, Ryzhova M, Jones DT, et al. LIN28A immunoreactivity is a potent diagnostic marker of embryonal tumor with multilayered rosettes (ETMR). Acta Neuropathol 2012;124(6):875-81
- Spence T, Sin-Chan P, Picard D, et al. CNS-PNETS with C19MC amplification and/or lin28 expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol 2014;128(2):291-303
- Korshunov A, Sturm D, Ryzhova M, et al. Embryonal tumor with abundant neuropil and true rosettes (etantr), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol 2014;128(2):279-89
- Zhu H, Shyh-Chang N, Segre AV, et al. The lin28/let-7 axis regulates glucose metabolism. Cell 2011;147(1):81-94
- Spence T, Perotti C, Sin-Chan P, et al. A novel c19mc amplified cell line links lin28/let-7 to mtor signaling in embryonal tumor with multilayered rosettes. Neuro Oncol 2014;16(1):62-71
- Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene 2001;20(24):3139-55
- Ioshikhes IP, Zhang MQ. Large-scale human promoter mapping using CpG islands. Nat Genet 2000;26(1):61-3
- Suganuma T, Workman JL. Crosstalk among histone modifications. Cell 2008;135(4):604-7
- Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet 2009;41(4):465-72
- Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012;482(7384):226-31
- Kleinman CL, Gerges N, Papillon-Cavanagh S, et al. Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet 2014;46(1):39-44
- Huang J, Chen K, Huang J, et al. Regulation of the leucocyte chemoattractant receptor fpr in glioblastoma cells by cell differentiation. Carcinogenesis 2009;30(2):348-55
- Chabot GG, Rivard GE, Momparler RL. Plasma and cerebrospinal fluid pharmacokinetics of 5-Aza-2′-deoxycytidine in rabbits and dogs. Cancer Res 1983;43(2):592-7
- Galanis E, Jaeckle KA, Maurer MJ, et al. Phase ІІ trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 2009;27(12):2052-8
- Braiteh F, Soriano AO, Garcia-Manero G, et al. Phase І study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res 2008;14(19):6296-301

