Abstract
CXCL5, epithelial cell derived neutrophil attractant 78, is a CXC chemokine predominantly expressed on epithelial cells. It has specificity for CXCR2 receptors and is involved in the recruitment and activation of neutrophils. CXCL5 is considered a therapeutic target in liver cancer, since treatment with small-interfering RNAs or antibodies against CXCL5 can suppress tumor growth, proliferation, migration and invasion. Experimental evidence demonstrated that CXCL5 antibodies could reduce the tumor growth and synergistically increase the efficiency of the tyrosine kinase inhibitor, Gefitinib, without the addition of toxicity. A number of challenges are encountered and should be considered during the development and clinical application of CXCL5 target-specific drugs. The specificity of CXCL5 as a therapeutic target for certain types and duration of cancer should be more carefully clarified, since it seems that CXCL5 is involved in many molecular pathways and crosstalk between targeted chemokines/receptors. The concept that CXCL5 serves as the therapeutic target for liver cancer was evidenced by preclinical studies, and is the beginning of CXCL5-based drug discovery and development.
1. Introduction
Structure of CXCL5 was initially identified in stimulated epithelial cells since 1990s Citation[1], including a tripeptide motif Glu-Leu-Arg at the NH2 terminus related to neutrophil-activating peptide 2 and growth-related oncogene-α. CXCL5 shares structural homologic qualities with IL-8 and plays the similar role in processes of proliferation, metastasis, angiogenesis and neutrophils infiltration in variety of cancers in vivo and in vitro, as summarized in the Supplement Tables 1 and 2. CXCL5 was recently observed to be over-expressed in the highly metastatic liver cancer cells Citation[2] and in liver tumor tissues as compared with that in adjacent non-tumor tissues Citation[3]. CXCL5 could promote tumor proliferation, growth, migration, invasion, metastasis, and intratumoral neutrophil infiltration of liver cancers Citation[3,4].
CXCL5 has the specificity to CXCR2 receptors, although there are more receptors to be still discovered. CXCR2 is a member of the G protein–coupled receptor superfamily and allocated in endothelial cells through the activation of a tripeptide motif Glu-Leu-Arg and CXC chemokines, to promote cell proliferation, migration, and invasion and assist cancer cells in evading stress-induced apoptosis. CXCR2 were significantly over-expressed in liver cancer tissues compared with adjacent and normal liver tissues. mRNA and protein levels of CXCR2 were correlated with intrahepatic metastasis, portal cancer embolus and low differentiation of liver cancer in patients. Furthermore, CXCR2 was also relevant to stages of liver cancer classified by Tumor-Lymph Nodes-Metastasis Classification of Malignant Tumors Citation[5].
1.1 CXCL5-associated signal pathways in cancers
CXCL5 can function as the pro-inflammatory factor and angiogenic factor and interact with and without other cytokines to form CXCL5-associated and activated signal pathways (), which play important roles in the proliferation, invasion, metastasis, angiogenesis and survival in cancer. CXCL5-dependent and associated biological functions can be initiated and maintained by a variety of signaling pathways in the tumor microenvironment, including protein kinase B AKT, extracellular signal-regulated kinase (ERK), and signal transducer and activator of transcription (STAT) in cancers Citation[6]. The interaction between cancer cells within the microenvironment is a paradoxical cycle to exacerbate cancer progression and metastasis.
Figure 1. Regulators of CXCL5 and signal pathways activated by CXCL5. (1) Cytokines such as TNF-α, IL-17, IL-1β, KLF4 or Ron could activate the NF-κB signal pathways to induce CXCL5 production; (2) IL-27 could suppress the expression of CXCL5 though a STAT1-dominant pathway; (3) EGF could activate EGFR-downstream signal pathways e.g., PI3K or ERK or p38 MAPK to promote the production of CXCL5 in liver cancer; (4) CXCL5 activates Ras/MEK/ERK, PI3K/AKT, JAK/STAT, MSK1/Elk-1, Egr-1/cdk4 signal pathways and up-regulates transcript factors such as snail and NF-κB to promoteproliferation, migration, invasion, EMT, and neutrophils chemoattraction.
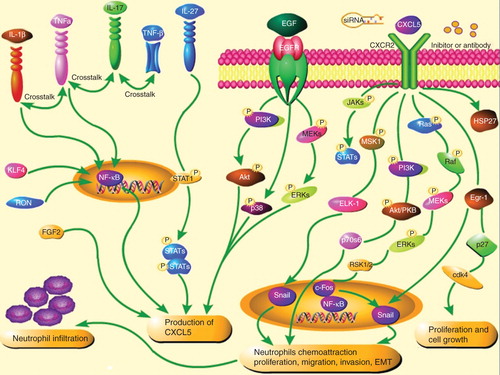
In liver cancer, CXCL5 could activate ERK1/2, p38 MAPK and phosphoinositide3-kinase (PI3K)-AKT pathways to promote migration and invasion of liver cancer cells Citation[2]. Downregulation of CXCL5 could reduce cancer cell proliferation and migration through PI3K-AKT and ERK1/2 signaling pathways. AKT and NF-κB activation are crucial for the chemotaxis and migration of neutrophils to the microenvironment of liver cancer. Neutrophils can potentiate cancer cell migration, invasion, and dissemination by secreting immunoreactive molecules such as hepatocyte growth factor, oncostatin M, β2-integrins or neutrophil elastase. Activation of the JAK/STAT pathway stimulates cell proliferation, differentiation, migration and apoptosis. STAT is also involved in vascular endothelial growth factor signaling and plays a pivotal role in angiogenesis.
Transcription factor Snail was proposed to regulate epithelial–mesenchymal transition (EMT) and cell migration in cancers. Snail transcriptionally suppresses the adherent junction protein E-cadherin, resulting in the occurrence of EMT and tumor progression, correlated with poor prognosis of patients with cancer. The association between CXCL5 and Snail signaling pathways was evidenced by the finding that the CXCL5-induced cancer progression was associated with the up-regulation of Snail through ERK/mitogen- and stress-activated protein kinase 1 (MSK1)/Elk-1/snail signaling pathway in breast cancer Citation[7] or Erg-1/Snail signaling pathway in prostate cancer Citation[8]. The over-expression of Snail could lead to elevated levels of CXCL5 in non-small cell lung cancer, suggesting that the inhibition of CXCL5-mediated Snail signaling may be an attractive therapeutic target for metastases of multiple cancers.
Breast tumor-associated osteoblasts-derived CXCL5 was associated with increased activation of Raf/MEK/ERK, MSK1, Elk-1 phosphorylation and Snail. The activation of Elk-1 facilitated the recruitment of phosphorylated MSK1, increasing histone H3 acetylation and phosphorylation of Snail promoter, or E-cadherin downregulation Citation[7]. Downregulation of CXCL5 increased the occurrence of cell apoptosis through the modulation of apoptosis-associated proteins Bcl2, Bax and cleaved-caspase3. Increased cleaved caspase-3 and decreased NF-κB1 can be a robust marker of highly aggressive tumor cells and involved in cytoskeleton remodeling Citation[9]. CXCL5 could increase the phosphorylation of heat shock protein 27, through which sustained AKT activity was stabilized by the direct interaction to enhance the formation of tumor spheroid Citation[10]. In addition, the combination of heparin-binding epidermal growth factor (EGF)-like growth factor and CXCL5 had a strong synergistic effect on cancer proliferation, EMT, migration or invasion.
1.2 CXCL5-associated factors in cancers
CXCL5 can be secreted by inflammatory cells and structure cells and induced by the challenge with cytokines like EGF, TNF-α, IL-1β or IL-17. EGFR play an important role in the development of cancer proliferation and metastasis and are proposed to serve as a ‘signaling hub’ for an increasing number of inflammatory mediators and possibly engage in extensive cross-talks with other signaling pathways. EGF directly and efficiently stimulated the overproduction CXCL5 in a dose- and time-dependent manner in liver cancer, which was inhibited by EGFR inhibitor Citation[11]. It suggests that the EGF–EGFR signaling pathway can play a crucial role in the mechanism of liver cancer-origin production of CXCL5. Furthermore, EGF could activate EGFR-downstream signaling pathways, for example, PI3K or ERK pathways, in a dose-dependent manner, to promote the production of CXCL5 and mediate the inflammatory microenvironment, cell proliferation, apoptosis or migration in liver cancer. The downregulation of PI3K and ERK pathway by PI3K and ERK inhibitors could inhibit the production of CXCL5 from liver cancer cells and inhibit the cell proliferation and migration in liver cancer cells Citation[11].
Cytokines, for example, TNF-α, IL-17A or IL-1β, could activate the NF-κB signaling and induce CXCL5 production through similar transcriptional and posttranscriptional mechanisms, suggesting the existence of cross-talking between receptors of chemokines and cytokines Citation[12]. IL-17 can amplify the immune response by triggering the production of chemokines, cytokines and cell-surface markers, and cause leukocyte chemotaxis and inflammation. Recent studies showed that IL-17 and TNF-α could cooperatively induce the production of CXCL5, through the activation of new gene transcription rather than NF-κB-dependent signaling pathway Citation[12]. Recently, Ma et al. found that IL-17A produced mainly by Vγ4 γδ T cells induced CXCL5 production by tumor cells to enhance the infiltration of myeloid-derived suppressor cells to tumor sites in a CXCL5/CXCR2-dependent manner in liver cancer Citation[13]. The pro-inflammatory cytokines like IL-1β could cooperate with TNF-α to induce the over-expression of CXCL5 via activation of NF-κB Citation[14]. IL-27 is a member of the IL-12 cytokine family with pro-inflammatory and anti-inflammatory functions, as well as anti-tumor properties through the inhibition of tumor proliferation and angiogenesis. Experimental study demonstrated that the stimulation with IL-27 could suppress the expression of CXCL5 though a STAT1-dominant pathway Citation[15], probably responsible for the regulation of IL-27-dominated antitumor effects.
A large number of factors are involved in the activation, signaling and interaction of CXCL5 (). Kruppel-like factor 4 regulates genes involved in cell-cycle progression and epithelial differentiation and can activate CXCL5 during the development of cancer in an NF-κB-dependent manner Citation[16]. The up-regulation of the proto-oncogene Ron, a human receptor for the macrophage-stimulating protein, was associated with CXCL5-induced angiogenesis, since Ron-negative cancer cells had low capacities of producing CXCL5 and generating angiogenesis, which could be prevented by exogenous Ron Citation[17]. Fibroblast growth factor 2 plays an important role in the growth, differentiation, migration, and survival of cancer and can increase the expression and production of CXCL5 in endothelial cells to promote the mobilization of hematopoietic stem cells from endosteal osteoblasts or bone marrow to peripheral blood in a CXCL5/CXCR2-associated mechanism Citation[18].
1.3 CXCL5 in liver cancer
Data from gene microarray profiling of inflammatory cytokines and receptors showed that CXCL5 was over-expressed in liver cancer cells with high metastatic potential when compared with those with low metastatic potential Citation[11]. CXCL5 was found to be over-expressed in both hepatocellular carcinoma and intrahepatic cholangiocarcinoma tissue and associated with poor prognosis of patients with liver cancer Citation[3]. It strongly implicates that CXCL5 may play an important and critical role in the formation of liver cancer. Our previous studies demonstrated that both liver cancer cells per se and hepatocytes adjacent to liver cancer could produce CXCL5 and promote cell proliferation, migration, invasion and angiogenesis in an autocrine and paracrine mechanism Citation[2,3]. We also found the over-expression and over-production of CXCL5 mainly in highly metastatic liver cancer cells through the activation of PI3K-AKT, ERK1/2, p38 MAPK and JNK signaling pathways to accelerate cell proliferation, migration and invasion Citation[2]. Liver cancer cells with the low capacity of metastasis became more sensitive to the stimulation of CXCL5 after the pretreatment with EGF. CXCL5 has directly chemoattractant effects on the recruitment of neutrophils from the circulation into the tissue. Over-expression of CXCL5 in tumor tissues harvested from patients was found to be correlated with the intratumoral infiltration of neutrophils, poor survival and high recurrence. CXCL5 plays a critical role tumorigenesis, metastasis and autoimmune inflammatory diseases by regulating angiogenesis and neutrophil infiltration Citation[3]. It needs to furthermore validate whether CXCL5 can be applied as the monitoring biomarkers to indicate the severity, duration, sensitivity to drugs and prognosis of liver cancer. Serum levels of CXCL5 gathered with clinical phenotypes were suggested as risk factors to predict the disease progression and prognosis of patients with nasopharyngeal carcinoma Citation[19].
1.4 Therapeutic potentials of CXCL5
CXCL5 can be considered as a therapeutic target in liver cancer, evidenced by the fact that treatment with small-interfering RNA or antibody against CXCL5 was found to suppress tumor growth, proliferation, migration and invasion. Experimental evidence demonstrated that CXCL5 antibody could reduce the tumor growth and synergistically increase the efficiency of the tyrosine kinase inhibitor gefitinib without the addition of toxicity in lung cancer Citation[10]. It seems that CXCL5 acts as a limiting factor in multiple cancers. Targeting CXCL5 may provide therapeutic benefits for cancer chemotherapy or immunotherapy as a critical adjunct antiangiogenic therapy against cancer.
However, a number of challenges are encountered and considered during the development and clinical application of CXCL5 target-specific drugs. For example, it is important for such drugs to reach the maximal specificity, efficacy, and low toxicity. The specificity of CXCL5 as the therapeutic target to the type and duration of cancer should be more carefully clarified since it seems that CXCL5 is involved in the complexity of molecular pathways and crosstalk between targeted chemokines/receptors. There is still controversy that CXCL5 in some cancers may not act as the protective factors, so the efficacy of CXCL5 needs to be repeatedly demonstrated. Broad biological functions and involvements of CXCL5 and the receptor may lead to the unexpected toxicities or side-effects of CXCL5-targeted therapies for liver cancer. It is possible that local deliveries of CXCL5 inhibitors, for example, through the portal vein or hepatic artery, may increase therapeutic effects within the liver and minimize the potential toxicity optimally. It should be further defined whether the sensitivity of cancer to CXCL5 inhibitors may be dependent upon the severity of the disease and what monitoring measurements should be selected to reflect the efficacy of CXCL5 therapy. Even though there are a large number of questions and challenges to be solved, it is right to consider CXCL5 as the therapeutic target for liver cancer.
2. Expert opinion
CXCL5 is selected as a candidate of diagnostic and therapeutic biomarkers from the mapping of genomics and proteomics of liver cancer tissues with potential clinical values. CXCL5 plays a central role in the formation of inflammatory microenvironment of liver cancer where CXCL5 can increase the growth and metastasis of liver cancer. The concept that CXCL5 serves as the therapeutic target for liver cancer was evidenced by preclinical studies, although it is a start of CXCL5-based drug discovery and development. It will be more valued if clinical bioinformatics can be applied to investigate CXCL5-specific and targeting dynamic network biomarkers in patients with liver cancer as performed in other studies Citation[20-23]. The inhibition of CXCL5 can be an alternative of new therapeutic strategies for liver cancer as either mono-therapy or combination with conventional chemotherapy or radiation.
IETT_A_993317_SM1869.doc
Download MS Word (72 KB)Acknowledgments
We would like to acknowledge M He for help with data collection.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
Bibliography
- Walz A, Burgener R, Car B, et al. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med 1991;174(6):1355-62
- Xu X, Huang P, Yang B, et al. Roles of CXCL5 on migration and invasion of liver cancer cells. J Transl Med 2014;12:193
- Zhou SL, Dai Z, Zhou ZJ, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 2012;56(6):2242-54
- Zhou SL, Dai Z, Zhou ZJ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis 2014;35(3):597-605
- Liu Z, Yang L, Xu J, et al. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res 2011;166(2):241-6
- Li A, King J, Moro A, et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol 2011;178(3):1340-9
- Hsu YL, Hou MF, Kuo PL, et al. Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/snail signaling pathway. Oncogene 2013;32(37):4436-47
- Kuo PL, Chen YH, Chen TC, et al. CXCL5/ENA78 increased cell migration and epithelial-to-mesenchymal transition of hormone-independent prostate cancer by early growth response-1/snail signaling pathway. J Cell Physiol 2011;226(5):1224-31
- Zheng J, Zhu X, Zhang J. CXCL5 knockdown expression inhibits human bladder cancer T24 cells proliferation and migration. Biochem Biophys Res Commun 2014;446(1):18-24
- Kuo PL, Huang MS, Hung JY, et al. Synergistic effect of lung tumor-associated dendritic cell-derived HB-EGF and CXCL5 on cancer progression. Int J Cancer 2014;135(1):96-108
- Huang P, Xu X, Wang L, et al. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med 2014;18(2):218-30
- Ruddy MJ, Shen F, Smith JB, et al. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol 2004;76(1):135-44
- Ma S, Cheng Q, Cai Y, et al. IL-17A produced by gamma delta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res 2014;74(7):1969-82
- Chandrasekar B, Melby PC, Sarau HM, et al. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem 2003;278(7):4675-86
- Kachroo P, Lee M H, Zhang L, et al. IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer. J Exp Clin Cancer Res 2013;32:97
- Tetreault M P, Wang M L, Yang Y, et al. Klf4 overexpression activates epithelial cytokines and inflammation-mediated esophageal squamous cell cancer in mice. Gastroenterology 2010;139(6):2124-34
- Thobe MN, Gurusamy D, Pathrose P, et al. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene 2010;29(2):214-26
- Yoon KA, Cho HS, Shin HI, et al. Differential regulation of CXCL5 by FGF2 in osteoblastic and endothelial niche cells supports hematopoietic stem cell migration. Stem Cells Dev 2012;21(18):3391-402
- Zhang H, Xia W, Lu X, et al. A novel statistical prognostic score model that includes serum CXCL5 levels and clinical classification predicts risk of disease progression and survival of nasopharyngeal carcinoma patients. PLoS One 2013;8(2):e57830
- Wang XD, Liotta L. Clinical bioinformatics: a new emerging science. J Clin Bioinforma 2011;1(1):1
- Wang XD. Role of clinical bioinformatics in the development of network-based Biomarkers. J Clin Bioinforma 2011;1(1):28
- Chen H, Wang X. Significance of bioinformatics in research of chronic obstructive pulmonary disease. J Clin Bioinforma 2011;1:35
- Chen H, Song Z, Qian M, et al. Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: a preliminary study. J Cell Mol Med 2012;16(6):1286-97
Supplementary material available online
Supplement Tables 1 and 2

