Abstract
Introduction: The 5-grass pollen tablet (Oralair®, Stallergenes, Antony, France) is a once-daily preseasonal and coseasonal sublingual immunotherapy (SLIT) that is effective in controlling the symptoms of allergic rhinoconjunctivitis and in reducing the need for symptomatic medication.
Areas covered: The body of safety data gathered from the 5-grass pollen tablet clinical development program, post-approval studies, and more than 6 years of real-life experience demonstrates the safety and tolerability profile of the 5-grass pollen tablet across all age groups. Adverse events (AEs) are generally mild or moderate in severity, and rarely lead to treatment discontinuation. AEs also tend to decline in frequency and severity over time and with repeated treatment. The most frequent treatment-emergent AEs are local-site oropharyngeal reactions (e.g., oral pruritus, throat irritation, tongue pruritus, mouth edema, ear pruritus), which are consistent with the sublingual route of administration.
Expert opinion: The first dose of the 5-grass pollen tablet should be administered under the supervision of an experienced physician, to allow for optimal monitoring and timely management of AEs, should they occur. The 5-grass pollen tablet can be administered at home after the first dose, and patients and carers should be educated on how to manage adverse reactions, unplanned treatment interruptions and situations in which SLIT should be withheld.
1. Introduction
Allergic rhinitis (AR), often in combination with conjunctivitis Citation1, Citation2, affects ∼ 500 million people worldwide Citation[3], including an estimated 113 million in Europe Citation[4] and 30 – 60 million in the US Citation[5]. AR places a heavy burden on affected individuals, who often experience an impaired quality of life Citation[6]. AR can cause a range of physical symptoms, including sleep disturbance, daytime fatigue and somnolence, decreased cognitive functioning, impaired performance at school and work Citation[7] and may also affect mood and social functioning Citation[7].
Guidelines on the management of AR recommend an approach that combines patient education with specific allergen avoidance, symptomatic pharmacotherapy and allergen-specific immunotherapy (AIT) Citation5, Citation8, Citation9. Although drug treatment can help reduce the incidence and severity of symptoms, AIT is the only therapy currently available that can modify the course of the disease Citation8, Citation9.
AIT administered subcutaneously (subcutaneous immunotherapy [SCIT]) has been used to treat allergic rhinoconjunctivitis (ARC) for over 100 years and is effective in reducing symptoms and the use of symptomatic pharmacotherapy in patients with AR and ARC Citation10, Citation11. However, the use of SCIT is limited by safety concerns and the discomfort and inconvenience of frequent injections. Despite the established efficacy of SCIT, very few allergy sufferers take advantage of the treatment – it is estimated that only < 5% of the US population with ARC or asthma receive SCIT, which involves monthly or bimonthly injections for 3 – 5 years.
Sublingual immunotherapy (SLIT), involving local absorption of allergens under the tongue, may overcome many of the limitations of SCIT. SLIT is easy to administer at home and does not require injections. SLIT has also demonstrated efficacy at reducing the symptoms of AR and ARC and appears to have a better safety profile than SCIT Citation12, Citation13. Although there is insufficient evidence from head-to-head comparisons of SCIT with SLIT to draw firm conclusions regarding the superiority of one mode of delivery over the other, results from studies published after 2007 suggest that SLIT may be more effective than SCIT Citation14, Citation15.
It is timely to review the safety of the 300 index of reactivity (IR) 5-grass pollen tablet (Oralair®; Stallergenes, Antony, France), and present safety data available from the clinical development program (n = 1514), post-approval safety studies (n = 1728) and the > 6 years of post-marketing experience since its first approval in 2008.
2. Overview of the 5-grass pollen tablet
As recently described, the 5-grass pollen tablet is a once-daily preseasonal and coseasonal SLIT that is effective in controlling the symptoms of ARC and in reducing the need for symptomatic medication Citation[16]. Its key characteristics are shown in the Drug summary box (Box 1) Citation17, Citation18, and its mechanism of action and pharmacodynamic properties have been recently reviewed and published in detail elsewhere Citation[16].
Box 1. Drug summary.
2.1 Posology
For patients aged > 5 years in the EU, and aged 10 – 17 years in the US, the 5-grass pollen tablet should be administered as a 100 IR dose on day 1, a 2 × 100 IR dose on day 2 and then a 300 IR/day dose thereafter Citation17, Citation18. In the US, uptitration is not necessary for patients aged 18 – 65 years, who can begin treatment with the 300 IR/day dose Citation[18].
2.2 Contraindications
In the EU and US, the 5-grass pollen tablet is contraindicated in patients with severe, unstable and/or uncontrolled asthma and/or hypersensitivity to any inactive ingredient in the product. Additional contraindications in the EU only include concomitant use of β-blockers and the presence of severe immunodeficiency, autoimmune disease or malignant disease; and in the US only, contraindications include history of any severe local or systemic allergic reaction to SLIT Citation17, Citation18.
2.3 Use in special populations
5-grass pollen tablet therapy should be withheld in patients experiencing acute asthma exacerbations, and in patients with oral inflammation or wounds (including those following oral surgery or tooth extraction/loss); treatment should be stopped until complete healing has occurred. The 5-grass pollen tablet should only be administered during pregnancy if there is a clear need and be used with caution in women who are breast-feeding Citation17, Citation18.
3. Safety of the 5-grass pollen tablet in clinical trials
The overall clinical safety experience with the 5-grass pollen tablet is based on pooled data from all patients enrolled in eight clinical studies, regardless of the dose or treatment regimen, treatment duration, patient age or study duration () Citation19, Citation20, Citation21, Citation22, Citation23, Citation24, Citation25, Citation26.
Table 1. Summary of the 5-grass pollen tablet clinical trials included in the pooled safety analysis.
3.1 Characteristics of the safety population
Of the 2512 participants across the clinical development program, 1514 were actively treated and 998 received placebo.
Eligible patients were aged 5 – 65 years with a clinical history of grass pollen-associated ARC for ≥ 2 years, confirmed by positive skin-prick test and allergen-specific serum IgE titer ≥ 0.7 kU/L. Polysensitized patients were included if they did not display significant clinical symptoms of allergy because of allergens other than grass pollen, during the grass-pollen season. Patients with asthma requiring treatment other than with inhaled β-2 agonists, and those receiving continuous therapy with corticosteroids or β-blockers, were excluded.
At baseline, randomized patients had ARC for at least 13.5 years and 17.0% had intermittent mild asthma. The majority of patients were polysensitized as judged by a positive skin-prick test to grass pollen and at least one other allergen.
Demographics and baseline characteristics were similar between the 5-grass pollen tablet and placebo groups ().
Table 2. Demographics and baseline characteristics of the safety population (n = 2512).
3.2 Exposure to treatment in the safety population
In the 5-grass pollen tablet clinical development program, different treatment regimens were tested in natural-field studies, including both 2- and 4-month preseasonal periods preceding the coseasonal treatment period. In the long-term study in adults, patients had discontinuous preseasonal and coseasonal treatment for three consecutive grass-pollen seasons Citation21, Citation22.
Overall, patients were treated for ∼ 3 – 6 months. Those who completed the treatment component of the long-term study received therapy for ∼ 8 – 14 months. The majority (91%) of patients were treated for at least 3 months, with > 40% being treated for > 6 months. Across the 5-grass pollen tablet clinical development program, 87 – 100% of patients were adherent to therapy (defined as taking ≥ 80% of their prescribed number of tablets) ().
Table 3. Extent of exposure to study treatment in the safety population (n = 2512), overall and in children and adolescents.
3.3 Analysis of safety data from clinical trials
As observed for each clinical study, at least one treatment-emergent adverse event (TEAE) was reported in 76.9% (n = 1164) of patients receiving active treatment and in 69.8% (n = 697) of placebo-treated patients. Study drug-related TEAEs were reported more frequently in the active treatment group (58.1%; n = 880), compared to the placebo group (20.0%; n = 200).
The TEAEs reported more frequently in the active-treatment group were consistent with application-site reactions, including oral pruritus, throat irritation, tongue pruritus, mouth edema and ear pruritus (). All these findings were in keeping with the safety profile of the sublingual route of administration.
Figure 1. Treatment-emergent adverse events occurring in ≥ 5% of patients in either treatment group (safety population: n = 2512) are shown.
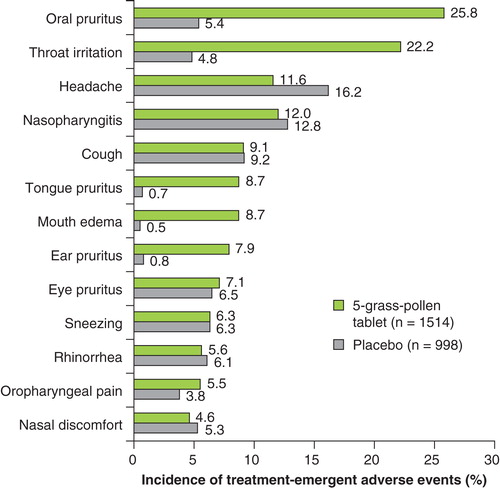
Of note, in the overall safety population (n = 2512), asthma was reported as a TEAE at a similar incidence in patients using the 5-grass pollen tablet (3.6%, n = 55) or placebo (4.7%, n = 47). The vast majority of TEAEs resolved rapidly and without treatment.
In the long-term study, the incidence and severity of TEAEs in patients treated with the 5-grass pollen tablet, administered 4 or 2 months before and during the grass-pollen season for 3 consecutive years, decreased with each consecutive season, and tolerability improved over the 3 years. In patients receiving active treatment, the incidence of application-site reactions decreased from > 57% in year 1 versus 41% in the placebo group to > 43% and > 36% in years 2 and 3 versus 17 and 18% in the placebo group, respectively Citation[22].
3.3.1 Serious adverse events
Across the clinical development program, no deaths or intensive care unit admissions were reported in any patient treated with the 5-grass pollen tablet. There were neither reports of ‘anaphylactic shock’ or ‘anaphylaxis’ nor any use of epinephrine with the 5-grass pollen tablet.
A similar proportion of patients receiving the 5-grass pollen tablet or placebo (1.5 and 1.1%, respectively) reported serious adverse events (SAEs), as defined in the supplementary material.
At least one SAE was reported by 22 patients in the active-treatment group, of which 3 were considered to be drug-related by the investigator. Two SAEs were application-site reactions and occurred on the first day of treatment, within 5 min of dosing. (Medical Dictionary for Regulatory Activities [MedDRA] preferred terms: angioedema and hypersensitivity). Treatment consisted of corticosteroid administration in one case, and combined corticosteroid, antihistamine and salbutamol therapy in the other. The third event, diarrhea, was concomitant with an infection. All drug-related SAEs resolved without sequelae.
3.3.2 Time to onset of TEAEs
Most of the commonly reported TEAEs (primarily application-site reactions) occurred during the first day, or within the first week, of treatment with 5-grass pollen tablet ().
Figure 2. Time to onset of most frequently reported treatment-emergent adverse events leading to premature study discontinuation in patients treated with the 5-grass pollen tablet.
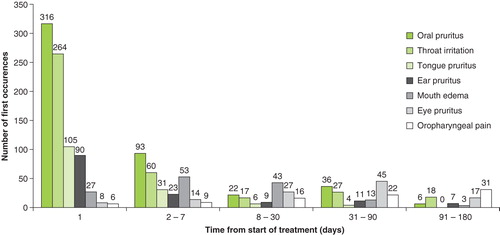
Of the 5.1% of patients who discontinued treatment because of TEAEs, 31.2 and 68.8% did so within the first week or first month, respectively, following commencement of therapy. Few children and adolescents (4.5%) discontinued treatment because of TEAEs, and all discontinuations occurred within the first 2 weeks following first intake.
The temporal pattern of first occurrence of the most frequently reported TEAEs leading to premature study discontinuation was assessed within the overall 5-grass pollen tablet clinical development program.
Two treatment-initiation schemes were assessed in adults, involving either direct administration of 300 IR 5-grass pollen tablet or a dose-escalation phase (100 IR on day 1, 2 × 100 IR on day 2 and 300 IR 5-grass pollen tablet thereafter); in pediatric patients, the latter regimen was always used.
As most TEAEs occurred soon after first intake, being mainly application-site reactions, the impact of the treatment-initiation scheme was analyzed in adults during the first 3 days of administration. Direct administration of 5-grass pollen tablet resulted in an increase in the percentage of patients reporting at least one TEAE, compared with those who underwent a dose-escalation phase (41.4 vs 23.2% on day 1, 16.5 vs 7.1% on day 2 and 6.4 vs 3.2% on day 3, respectively).
Over the entire study, the incidence of TEAEs was higher with the direct-administration scheme than with dose escalation (5-grass pollen tablet: 79.9 vs 62.6%, placebo: 70.5 vs 48.7%, respectively). All of the most frequently reported individual TEAEs (i.e., application-site reactions) had a greater incidence with the direct-administration scheme.
3.4 Safety of the 5-grass pollen tablet in subpopulations of interest
3.4.1 Safety by age group
The incidence, nature and severity of adverse events (AEs) reported during treatment with 5-grass pollen tablet in the clinical studies were similar between the adult and pediatric study populations (). The most commonly reported TEAEs across both 5-grass pollen tablet-treated subgroups were application-site reactions (e.g., oral pruritus, throat irritation, mouth edema), occurring at similar incidences.
Table 4. TEAEs occurring in the safety population (n = 2512), overall and by age and treatment groups.
3.4.2 Safety according to sensitization status
The incidence, nature and severity of AEs reported during treatment with the 5-grass pollen tablet were similar in monosensitized and polysensitized patients. The most frequently reported TEAEs with the 5-grass pollen tablet (i.e., application-site reactions, such as oral pruritus, throat irritation and mouth edema) occurred at similar incidences in monosensitized and polysensitized patients ().
Table 5. TEAEs occurring in the safety population (n = 2512), stratified by sensitization and asthma status.
3.4.3 Safety in patients with mild asthma
The incidence, nature and severity of AEs reported during treatment with the 5-grass pollen tablet were similar between patients with (n = 425) and without (n = 2085) intermittent mild asthma at baseline (). The most commonly reported TEAEs in patients with mild asthma, irrespective of treatment, were application-site reactions (e.g., oral, tongue, eye and ear pruritus, mouth edema, throat irritation), and these occurred at a higher incidence in patients on active treatment. No serious drug-related TEAEs occurred in patients with mild asthma at enrolment ().
3.5 AEs of special interest
Across the clinical development program, no deaths or intensive care unit admissions were reported in any patient treated with the 5-grass pollen tablet. There were neither reports of ‘anaphylactic shock’ or ‘anaphylaxis’ nor use of epinephrine.
3.5.1 Severe laryngopharyngeal disorders
Two patients using the 5-grass pollen tablet experienced laryngopharyngeal disorders (MedDRA preferred terms: angioedema, hypersensitivity), which occurred within 5 min after first intake and were considered to be drug-related. Treatment consisted of intravenous corticosteroid administration in the former case, and combined corticosteroid, antihistamine and salbutamol therapy in the latter. Symptoms resolved without sequelae.
3.5.2 Asthma-induced events
In the overall safety population (n = 2512), asthma was reported as a TEAE at a similar incidence in patients using the 5-grass pollen tablet (3.6%, n = 55) or placebo (4.7%, n = 47).
4. Safety of the 5-grass pollen tablet in post-marketing experience
Post-marketing surveillance or pharmacovigilance activities are conducted with the aim of confirming the safety profile of a product established during its clinical development and detecting any new potential AEs. The two methods of data collection used most frequently in pharmacovigilance are post-marketing safety studies and spontaneous reporting. When a new product is marketed, both solicited and unsolicited reports of AEs are collected using these two approaches. These two sources of safety information are complementary, because they contribute to signal detection and evaluation of the safety profile of the product under typical conditions of use.
4.1 Post-marketing authorization safety studies
Two multicenter, prospective, observational studies were conducted to assess the safety and tolerability of the 5-grass pollen tablet through the systematic collection of all AEs in the population exposed, whatever their causality with the 5-grass pollen tablet. These Post-marketing Authorization Safety Studies (PASS) were performed in Germany and enrolled 808 adults and 920 children and adolescents ().
Table 6. PASS conducted in Germany: main characteristics.
In total, safety data were systematically collected from 1728 patients in a real-life setting. Overall, adverse drug reactions (ADRs) led to discontinuation in 9.5% (n = 85) and 9.0% (n = 72) of treated patients in the 2008 and 2009 PASS, respectively. These withdrawals were mainly related to application-site reactions (primarily mouth edema and throat irritation). The presence of comorbid mild asthma did not appear to increase the risk of AEs nor their frequency or severity.
Nine serious ADRs possibly related to the 5-grass pollen tablet were reported during the two PASS: 4 in adults and 5 in children. In adults, one patient had a severe application-site reaction associated with chest and renal pain on the third day of 5-grass pollen tablet treatment, which resolved spontaneously. Another patient had oral pruritus on the first day of 5-grass pollen tablet treatment that resolved spontaneously; the patient also developed abdominal pain and diarrhea after another 15 days of treatment, related to aggravation of preexisting Crohn’s disease. One case of eye irritation occurred at month 6 of 5-grass pollen tablet treatment, which resolved after corticosteroid therapy. The remaining case concerned a female patient aged 54 years, who was hospitalized at 6 months after start of 5-grass pollen tablet therapy for a lumbar vertebral fracture caused by previously unsuspected stage III plasmacytoma, which was considered unlikely to have a chronologic causal relationship to treatment.
In children, three cases were considered as local allergic reactions, with first symptoms occurring a few minutes after 5-grass pollen tablet intake. One patient experienced throat pruritus with dyspnea on day 2 of administration, which was treated with corticosteroid injection and salbutamol inhalation. No laryngopharyngeal edema was reported, and the reaction resolved rapidly. In the other two cases, symptoms involved cutaneous reactions after 3 and 5 months of treatment in patients with underlying contributing factors (preexisting atopic dermatitis and neurodermatitis, respectively), requiring local treatments.
The safety profile of the 5-grass pollen tablet was similar in adults and in children/adolescents in the two PASS. Safety data were consistent with those collected from the 5-grass pollen tablet clinical development program, regarding their nature, intensity and frequency.
In either PASS, there were no SAEs of asthma or asthma-related symptoms, no hospitalization related to the 5-grass pollen tablet, no epinephrine use, no cases of anaphylaxis and no severe laryngopharyngeal reactions. The definition of an SAE is available in the supplementary material.
4.2 Spontaneous reporting
Spontaneous reporting systems generate the largest amount of data for drug safety monitoring and have proven valuable in the early detection of patient safety issues related either to the drug itself or to its use. The market monitoring conducted by pharmaceutical companies on their spontaneous reporting system is mainly based on the calculation of reporting rates (number of spontaneous cases/number of patients exposed); because of underreporting, these ratios are not incidences but reporting rates. Together with the medical evaluation of each reported case, they allow the identification of potential signals. Details on spontaneous reporting are available in the supplementary material.
As documentation of cases remains an issue in a spontaneous reporting system, the product manufacturer has a dedicated pharmacovigilance team ensuring the accurate medical documentation of each case report, calculation of reporting rates and signal detection.
4.2.1 Reporting rates of ADRs
Since the launch of the 5-grass pollen tablet in 2008 and up to August 2014, it is estimated that 170,785 patients have been treated, including 55,056 patients aged < 18 years (representing approximately one-third of the total patient exposure). A total of 999 reports of ADRs have been collected internationally, of which 144 (14.4%) were considered to be serious. There were no reports of death or any long-term sequelae.
Cumulative reporting rates for AEs with the 5-grass pollen tablet are classed as uncommon (< 1%) to rare (< 0.1%), according to Council for International Organizations of Medical Sciences guidelines Citation[27]. Reporting rates are higher in patients aged < 18 years (0.72 per 100 patients) than in adults (0.45 per 100 patients). The same trend is observed for SAEs (0.11 vs 0.07 per 100 patients, respectively). One possible contributing factor to the higher reporting rate in children might be the closer monitoring given by parents and caregivers to this population Citation[28]. These ratios have remained stable since the launch of the 5-grass pollen tablet, and no safety signal has been detected throughout the 6 years of post-marketing monitoring.
4.2.2 Nature of spontaneous adverse reactions
The nature of ADRs reported during post-marketing surveillance is shown in ().
Figure 3. Nature of adverse drug reactions and serious adverse drug reactions occurring during 6 years of post-marketing experience with the 5-grass pollen tablet.
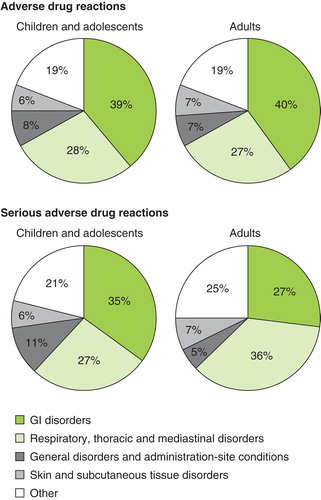
The most frequently reported ADRs are application-site reactions, specifically mouth and tongue edema and throat irritation. The types of ADRs reported are similar in both adults and children globally, reflecting the known safety profile of the 5-grass pollen tablet.
4.2.3 Reports of laryngopharyngeal reactions
In rare cases, patients experience severe local allergic reactions extending to the throat, with the sensation of a foreign body or throat tightness. These are symptoms of laryngeal or pharyngeal edema that can potentially lead to swallowing or respiratory difficulties. In order to detect and identify such cases, the following methodology has been applied: selection of appropriate medical terms, seriousness of ADRs, associated respiratory symptoms and defined treatment given (epinephrine, parenteral antihistamines and/or corticosteroids and/or β-2 agonists). Selected cases were medically reviewed and assessed.
Over the 6 years since its launch, 19 cases of severe laryngopharyngeal reactions have been reported to the product manufacturer in patients treated with the 5-grass pollen tablet, corresponding to a reporting rate of 0.011 per 100 patients. The rate of severe laryngopharyngeal reactions was similar in children and adults, with 7 (0.013 per 100 patients) and 12 (0.010 per 100 patients) cases reported, respectively. In more than half of cases (n = 10/19), symptoms occurred after the first 5-grass pollen tablet intake, and for most patients (n = 7/10), this was within < 30 min. Among these 19 cases, 2 patients received an adrenaline injection and 2 patients received oxygen therapy. For those patients for whom the outcome was stated (n = 18/19), all recovered fully, and within < 24 h for 13 patients.
Laryngopharyngeal reactions are well-identified risks of SLIT Citation[13] and can be easily recognized by the patient and rapidly identified, allowing them to seek immediate medical assistance.
4.2.4 Reports of anaphylaxis
Systemic allergic reactions, including anaphylactic shock, are a concern with AIT, particularly with SCIT regimens Citation[29]. Identification of cases of anaphylaxis from spontaneous reports in the product manufacturer’s database involves the selection of appropriate SAEs; use of clinical criteria for diagnosing anaphylaxis Citation[30]; the selection of patients treated with interventions proven effective in the acute management of anaphylaxis, for example, epinephrine, intravenous fluids, parenteral corticosteroids or antihistamines, inhaled β-2 agonists and medical review and assessment.
Over 6 years of 5-grass pollen tablet use, 12 cases of severe anaphylactic reactions considered by physicians as possibly related to treatment have been reported, corresponding to a reporting rate of 0.007 per 100 patients in both adults (n = 8) and children (n = 4). In more than half of cases (n = 8/12), the event occurred < 30 min after first 5-grass pollen tablet intake. All patients recovered fully within < 48 h. In four patients, time to onset varied from 1 week to several months. Of note, in two cases (time to onset of 30 days and 5 months, respectively) another etiological cause was suspected. Epinephrine was administered in two pediatric patients aged 9 and 11 years, in response to first symptoms suspected to be anaphylaxis related to 5-grass pollen tablet use. Both patients recovered rapidly (one within minutes and the other within 24 h) with no sequelae.
No case of anaphylactic shock was reported to the product manufacturer’s international pharmacovigilance department over these 6 years.
Post-marketing experience supports the favorable safety profile of the 5-grass pollen tablet, with the majority of spontaneous safety reports being consistent with local-site reactions, particularly mouth edema and throat irritation. No deaths or accounts of long-term sequelae have been reported.
As expected with the broader use of any SLIT, rare cases of severe laryngopharyngeal reactions and anaphylaxis have been reported in the wider post-marketing setting, occurring at rates of 0.011 and 0.007 per 100 patients, respectively, which can be considered as rare and very rare reporting rates. Typically, such ADRs occur within minutes after dosing; thus, monitoring of patients during the first 30 min after 5-grass pollen tablet intake is required according to current prescribing information Citation17, Citation18.
For those rare events that do not occur immediately after first intake, healthcare professionals (HCPs) should provide thorough counseling to patients, ensuring that they can recognize the signs and symptoms of anaphylaxis and severe laryngopharyngeal reactions, so that appropriate and timely management can be provided should such severe events occur Citation17, Citation18.
In the 6 years of post-marketing experience since the launch of the 5-grass pollen tablet, neither signal nor specific risk associated with the 5-grass pollen tablet has been noted, confirming its known favorable safety profile. The post-marketing safety findings are comparable to those seen in the two PASS and during clinical development and are in keeping with the literature on the safety of SLIT Citation[13].
4.2.5 Review of eosinophilic esophagitis
Two cases of eosinophilic esophagitis (EoE) have been reported, both occurring in adult patients. Both cases did not result in hospitalization or emergency treatment and no formal causal relationship with the 5-grass pollen tablet has been established for either case. No case of EoE has been reported in the pediatric population exposed to the 5-grass pollen tablet.
4.2.6 Review of misuse
The 6 years of post-marketing monitoring show that the 5-grass pollen tablet is largely prescribed in accordance with product recommendations.
One recent report of misuse with a patient treated in-season with the 5-grass pollen tablet has been published and described a case of suspected anaphylaxis Citation[31]. It is important that the recommended precautions are followed, including administering the first dose under the supervision of a clinician with experience in the diagnosis and treatment of severe allergic reactions. Patients should be monitored during a mandatory office-waiting period of at least 30 min, so that appropriate and timely management can be provided should such severe events occur.
5. Conclusion
The favorable safety profile of the 5-grass pollen tablet has been demonstrated during the clinical development program (n = 1514), post-approval safety studies (n = 1728) and over 6 years of real-world post-marketing experience. The positive findings during the 6-year post-marketing monitoring period also highlight that the 5-grass pollen tablet is generally prescribed in accordance with recommendations Citation13, Citation17, Citation18.
6. Expert opinion
In clinical trials, the 5-grass pollen tablet shows good safety and tolerability in all age groups, and can be conveniently administered at home after the first dose, if well tolerated. Treatment should be initiated 4 months before the expected onset of the pollen season and maintained until the season ends. AEs are generally mild or moderate in severity, and rarely lead to treatment discontinuation. Furthermore, AEs tend to decline in frequency and severity over time and with repeated treatment.
Real-life experience with the 5-grass pollen tablet mirrors that of the clinical development program, further supporting its favorable safety and tolerability profile. The spontaneous reporting process implemented by the product manufacturer is robust, ensuring the early detection of any patient safety issues. The majority of spontaneous safety reports are local-site oropharyngeal reactions, consistent with the sublingual route of administration, and no deaths or AEs with long-term sequelae have been reported.
Treatment-related SAEs typically occur soon after dosing. For this reason, the first dose of the 5-grass pollen tablet is taken in the office under the medical supervision of a clinician experienced in the diagnosis and treatment of severe allergic reactions, and the patient is subsequently monitored for ≥ 30 min Citation[32]. This also provides an opportunity for education of patients and carers, which can increase recognition of future AEs, should they occur, enabling rapid intervention. Patients should be provided with specific instructions on how to manage adverse reactions or unplanned treatment interruptions, when and what to report to their clinician and any situations in which SLIT should be withheld (e.g., oropharyngeal infection, oral abrasion, acute gastroenteritis and/or asthma exacerbation). The clinician should also consider carefully if the patient or their carer are able to follow these instructions and adhere with the treatment regimen Citation[13]. To date, neither the literature nor post-marketing surveillance has highlighted a causal relationship between SLIT systemic reactions and the presence of oropharyngeal infections or lesions (e.g., aphthous ulcers, gingivitis, EoE), but future research in this regard is warranted.
SCIT has been associated with near-fatal and fatal anaphylaxis; in contrast, no fatalities have occurred with SLIT Citation[33]. Although anaphylaxis and severe laryngopharyngeal reactions can occur rarely with the 5-grass pollen tablet, these adverse reactions have characteristics that lend themselves to mitigation through HCP and patient education. A common limitation of not only the 5-grass pollen tablet but also therapies in other conditions is that the detection of rare AEs, such as anaphylaxis, may be limited by the number of patients included in clinical trials and from post-marketing surveillance to date. A continuous-sentinel-event surveillance system was shown to provide an additional level of security to the safety of patients receiving SCIT Citation[34] and the ability to detect SAEs, and such a system is automatically put in place by the product manufacturer once any safety signal has been reported, depending on the impact.
Risk factors for the occurrence of SLIT SAEs have not been clearly established, although some of the factors identified are recognized risk factors for SCIT (e.g., height of season, history of previous systemic reactions, dose and accelerated dosing schedule). In addition, most patients with SLIT-related SAEs or anaphylaxis have asthma Citation[13]. AIT does not appear to be associated with an increased risk of autoimmune disease Citation35, Citation36. However, future research should address whether SLIT is appropriate and safe in patients with immune deficiency and autoimmune conditions, and whether it may induce autoimmune disease, and/or eosinophilic disorders.
7. Information resources
For more information about the 5-grass pollen tablet, please refer to the journal articles in the reference list marked as ‘of interest’ or ‘of considerable interest’ as well as the product manufacturer’s website (www.stallergenes.com/en.html).
IEDS_A_1017468_SM4233.doc
Download MS Word (23.5 KB)Declaration of interest
B Bons is an employee of Stallergenes. In the past 5 years, A Didier has received honoraria from Stallergenes for participating in expert advisory board meetings and for coordinating clinical studies on the 5-grass pollen tablet. Funding for this paper was received from Stallergenes, who funded medical writing assistance from James Reed of Newmed Publishing Services. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents, received or pending, or royalties.
Notes
Bibliography
- RANathan, EOMeltzer, JDerebery, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc 2008;29:600-8
- NRosario, LBielory. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol 2011;11:471-6
- TOzdoganoglu, MSongu. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis 2012;6:11-23
- EFA Book on Respiratory Allergies. EValovirta. editor. 2011. Available from: http://www.theipcrg.org/download/attachments/689587/EFABookonRespiratoryAllergiesFINAL.pdf?version=1&modificationDate=1332965739000 [Last accessed 2 April 2014]
- DVWallace, MSDykewicz, DIBernstein, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008;122(2 Suppl):S1-84
- AUzzaman, RStory. Chapter 5: allergic rhinitis. Allergy Asthma Proc 2012;33(Suppl 1):S15-18
- WAO White Book on Allergy 2013 Update. RPawankar, STHolgate, GWCanonica, et al. editors. 2013. Available from: http://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf [Last accessed 23 February 2015]
- JBousquet, NKhaltaev, AACruz, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63(Suppl 86):8-160
- DKSur, SScandale. Treatment of allergic rhinitis. Am Fam Physician 2010;81:1440-6
- MACalderon, BAlves, MJacobson, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007(1):CD001936
- NErekosima, CSuarez-Cuervo, MRamanathan, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope 2014;124:616-27
- MACalderon, FESimons, HJMalling, et al. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy 2012;67:302-11
- GWCanonica, LCox, RPawankar, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J 2014;7:6
- JDretzke, AMeadows, NNovielli, et al. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol 2013;131:1361-6
- AMeadows, BKaambwa, NNovielli, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess 2013;17:1-322
- ADidier, UWahn, FHorak, et al. Five-grass-pollen sublingual immunotherapy tablet for the treatment of grass-pollen-induced allergic rhinoconjunctivitis: 5 years of experience. Expert Rev Clin Immunol 2014;10:1309-24
- Summary of Product Characteristics: Oralair® 100 IR & 300 IR sublingual tablets. Stallergènes SA, 2013. Available from: http://www.stallergenes.co.uk/fileadmin/images/corporate/gallery/Documents_pdf/2010-10–Oralair_fin_MRP_RCP.pdf [Last accessed 15 April 2014]
- US Prescribing Information: Oralair® (Sweet vernal, Orchard, Perennial rye, Timothy, and Kentucky blue grass mixed pollens allergen extract) Tablet for sublingual use. Stallergènes SA, 2014. Available from: http://oralair.com/docs/ORALAIR%20Prescribing%20Information-Med%20Guide.pdf [Last accessed 11 May 2014]
- LSCox, TBCasale, ASNayak, et al. Clinical efficacy of 300IR 5-grass pollen sublingual tablet in a US study: the importance of allergen-specific serum IgE. J Allergy Clin Immunol 2012;130:1327-34
- ADidier, HJMalling, MWorm, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol 2007;120:1338-45
- ADidier, HJMalling, MWorm, et al. Post-treatment efficacy of discontinuous treatment with 300IR 5-grass pollen sublingual tablet in adults with grass pollen-induced allergic rhinoconjunctivitis. Clin Exp Allergy 2013;43:568-77
- ADidier, MWorm, FHorak, et al. Sustained 3-year efficacy of pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol 2011;128:559-66
- FHorak, PZieglmayer, RZieglmayer, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol 2009;124:471-7
- THLarsen, LKPoulsen, MMelac, et al. Safety and tolerability of grass pollen tablets in sublingual immunotherapy–a Phase-1 study. Allergy 2006;61:1173-6
- Study NCT00803244. Safety and efficacy of Phase III study on 300 IR SLIT in patients suffering from grass pollen rhinoconjunctivitis (with or without asthma). Stallergènes SA, 2011. Available from: http://clinicaltrials.gov/show/NCT00803244 [Last accessed 20 April 2014]
- UWahn, ATabar, PKuna, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol 2009;123:160-6
- Council for International Organizations of Medical Sciences (CIOMS) Working Group III. Guidelines for preparing core clinical safety information on drugs. Geneva, Switzerland: 1995
- SMWallerstedt, GBrunlof, ASundstrom. Rates of spontaneous reports of adverse drug reactions for drugs reported in children: a cross-sectional study with data from the Swedish adverse drug reaction database and the Swedish Prescribed Drug Register. Drug Saf 2011;34:669-82
- TGEpstein, GMLiss, KMurphy-Berendts, et al. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008-2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract 2014;2:161-7
- HASampson, AMunoz-Furlong, RLCampbell, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117:391-7
- KCHsiao, JSmart. Anaphylaxis caused by in-season switchover of sublingual immunotherapy formulation. Pediatr Allergy Immunol 2014;25:714-5
- KALyseng-Williamson. Sublingual five-grass pollen tablets (Oralair®): a guide to their use as allergen immunotherapy for grass pollen-induced allergic rhinoconjunctivitis. Drugs Ther Perspect 2014;10.1007/s40267-40014-40131-40267
- MMakatsori, MACalderon. Anaphylaxis: still a ghost behind allergen immunotherapy. Curr Opin Allergy Clin Immunol 2014;14:316-22
- FMadsen, LFrolund, MChristensen, et al. Quality assurance of allergen-specific immunotherapy during a national outbreak of anaphylaxis: results of a continuous sentinel event surveillance system. J Investig Allergol Clin Immunol 2009;19:253-9
- ALinneberg, RKJacobsen, LJespersen, et al. Association of subcutaneous allergen-specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol 2012;129:413-9
- ALinneberg, FMadsen, TSkaaby. Allergen-specific immunotherapy and risk of autoimmune disease. Curr Opin Allergy Clin Immunol 2012;12:635-9
Supplementary material available online
Supplementary material
Notice of correction
Please note that changes were made to table 4, table 5 and section 4.2.3 after initial online publication of this article (4th March 2015).

