Pancreatic cancer is diagnosed late and patient outcome is further diminished due to dense desmoplasmic tumor microenvironment resulting from the proliferation of fibroblasts and increase in fibrosis within the stroma Citation[1], limiting the chance for complete excision of these tumors. Furthermore, prolonged treatment with chemotherapy results in multidrug resistance. In recent years, scientists and physicians have expanded significant efforts to explain the underlying mechanism of chemoresistance for improving both duration of survival and quality of life. The highly complex nature of drug resistance renders current single agent or multiple drug combinations ineffective, leading to poor patient survival making pancreatic cancer one of the deadliest malignancies. To date, a variety of distinct and disparate molecular mechanisms have been suggested including the role of ATP binding cassette (ABC)-transporters, increased DNA repair and dysregulation of the apoptotic machinery, enhanced cytoplasmic inactivation of drug metabolites and overexpression of oncogenes or downregulation of tumor suppressor genes. Despite these advances, our understanding of chemoresistance in pancreatic cancer is fragmentary and incomplete. Recent studies in our lab and by other groups Citation[2-4] have pointed to the important role played by microRNAs (miRNAs) and cancer stem cells (CSCs) in etiology and progression of various cancers, including pancreatic, marking their importance not only for diagnostics but also as potential targets for reversing drug resistance.
Before discussing the role of miRNAs and CSCs, it is important to stress that pancreatic cancer chemotherapy may fail due to de novo (intrinsic) or acquired drug resistance Citation[5]. For patients exhibiting intrinsic drug resistance, therapy is ineffective from the onset of treatment. In contrast, acquired resistance occurs following subsequent exposure of tumor to drug. In this case, tumors show some initial response to therapy and subsequently become irresponsive, recur and metastasize. This distinction is important as the type of drug resistance may influence treatment approach. Regardless of the processes leading to drug resistance, pancreatic cancer chemoresistant cells are characterized by epithelial–mesenchymal transition (EMT) phenotype, CSCs and distinct oncogenic miRNA signatures Citation[2,3]. Hence, insight into cells exhibiting these mechanisms and the interrelationship could potentially result in new potent therapeutic strategies.
One reason some chemoresistant pancreatic tumors are difficult to treat is their increased invasiveness possibly due to EMT during which epithelial cells assume a mesenchymal phenotype. As a result of EMT, epithelial cell–cell junctions are lost, actin cytoskeleton reorganized and epithelial cadherin (commonly known as E-cadherin, cadherin-1 or CDH1) protein expression diminished. Additionally, expression of mesenchymal markers (e.g., vimentin and α-smooth muscle actin) and matrix metalloproteinases (MMPs) are enhanced. Recently, a positive correlation has been suggested between the acquisition of EMT phenotype and chemoresistance in pancreatic cancer cells Citation[6]. Specifically, numerous pancreatic cell lines exhibiting EMT features such as high Zinc finger E-box-binding homeobox 1 (ZEB1) levels and low cadherin 1 expression were found to be resistant to gemcitabine, cisplatin and 5 fluorouracil (5-FU) Citation[7]. In contrast, pancreatic cell lines with low mesenchymal and high epithelial marker expression were sensitive to these anticancer agents. More importantly, reports in the literature suggest EMT characteristics are acquired as pancreatic cancer cells increasingly become resistant to gemcitabine. Furthermore, inhibiting the EMT regulatory gene LOXL2 sensitized pancreatic cancer cells to gemcitabine Citation[8]. Similarly, repressing notch signaling leads to a partial reversal of the EMT phenotype due to mesenchymal–epithelial transition (MET). Although these studies link EMT phenotype in pancreatic cancer to chemoresistance, additional research is required to fully understand the EMT phenotype drug resistance paradigm and how it can be exploited for treating pancreatic cancer.
Most malignant tumors including pancreatic cancers are heterogeneous and harbor a small population of cells (approximately 1% of the tumor) known as CSCs, which are chemoresistant and responsible for tumor initiation, propagation, resistance to chemotherapy and recurrence Citation[9]. Cancer stem cells are a major reason why current therapies fail in patient care as traditional anticancer agents are designed to primarily target bulk tumor cells and very few are effective against CSCs. One reason CSCs are extremely resistant to chemotherapy is because they express at least one ABC efflux transporter Citation[10,11]. For instance, drug efflux transporters have been identified in leukemic stem cells accounting for their resistance to daunorubicin. There is growing evidence that pancreatic CSCs play a role in the chemoresistance of pancreatic cancer. High doses of gemcitabine, a commonly utilized chemotherapeutic agent, were unable to eliminate CSCs although the majority of pancreatic cancer cells were killed in cell culture. In humans, pancreatic CSC populations CD44+/CD24+ are more resistant to gemcitabine and correlate with poor prognosis. Therefore, it is clear that pancreatic CSCs not only contribute to tumorigenesis and metastasis, but are also resistant to commonly utilized chemotherapeutic agents. Although the underlying mechanisms governing drug resistance in CSCs remain to be fully elucidated, therapeutic strategies targeting CSCs can be a promising way to treat pancreatic cancer.
miRNAs are single-stranded 18 – 25 nucleotides long, highly conserved, noncoding RNA products. First, primary miRNA transcripts are encoded by RNA polymerase II or III gene transcription and subsequently cleaved and modified into mature miRNA Citation[12,13]. These mature miRNAs are then integrated into the RNA-induced silencing complex (RISC) chaperoning the complex to bind a specific target. Once bound to a target mRNA, miRNAs either degrade the target mRNA or repress translation Citation[12,13]. The mediatory role of miRNAs in drug resistance stems primarily from their intrinsic tumor suppressor or oncogenic activities that originate via posttranscriptional inhibition of gene expression. Examples of miRNAs with tumor suppressor activity include miR-22, miR-34, miR-141, miR-200 and let7 whereas miRNAs such as miR-21, miR-155, miR-221, miR-203 and miR-520 display oncogenic activity Citation[3]. It is common for upregulation of oncogenic miRNAs to occur in cancer patients. These oncomirs influence tumor pathology through numerous mechanisms including repressing translation of tumor suppressor proteins. Recent evidence suggests specific miRNA signatures may explain chemoresistance in pancreatic cancer cells. It has been shown that downregulation of miR-200a, miR-200b and miR-200c is synonymous with gemcitabine-resistant pancreatic cancer cells. Another study also showed increased oncogenic miR-155 levels in pancreatic cancer cells following gemcitabine treatment whereas patients with low levels of the oncogenic miR-21 were responsive to pancreatic cancer treatment.
Recent investigations also suggest miRNAs may regulate the EMT process Citation[14]. For instance, miRNAs have been shown to regulate EMT by regulating Cadherin 1 and additional EMT-related molecules. In a study where miR-200a, miR-200b and miR-200c were downregulated in gemcitabine-resistant pancreatic cancer cells, an EMT phenotype was noted. It was also observed that re-expression of the mir-200 family of miRNAs upregulated cadherin 1 and downregulated ZEB1 and vimentin. Additionally, EMT-type cells resistant to gemcitabine have downregulated let7 members.
The pivotal regulatory role of miRNA in drug resistance is also observed in their contribution to the generation of CSCs and their chemoresistance. Emerging evidence suggests dysregulation of certain miRNAs contributes to the generation of CSCs. Recent findings indicate an interrelationship between EMT phenotype, CSCs, chemoresistance and miRNAs () Citation[15]. Drug resistance positively correlates with the presence of CSCs, which in turn is linked to EMT phenotype acquisition. However, aberrant expression of miRNAs occurs in all cases. Clearly, miRNAs play significant roles in EMT and CSCs. Nonetheless, additional studies of miRNA expression profiles in pancreatic CSCs and their function in EMT-like cells are needed to improve our understanding required for developing novel therapeutic approaches for targeting CSCs and addressing chemoresistance.
Figure 1. Interrelationship between EMT, cancer stem cells, miRNA and chemoresistance. Significant interrelationship exists between chemoresistance, epithelial to mesenchymal transition and metastasis, which adversely impacts treatment outcomes. Chemoresistance, a key feature of CSCs and cells undergoing EMT, is regulated by the dysregulation of miRNAs. Our proposed combination therapy with PTX and CYA simultaneously targets CSCs and bulk cancer cells, reverses EMT and restores expression of miRNAs altered during generation of chemoresistance and is a viable strategy for treating drug-resistant prostate cancer.
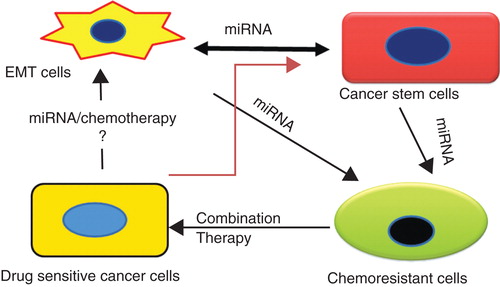
As miRNAs are the common thread connecting stemness and metastasis via regulation of EMT, targeting them along with CSCs and EMT phenotype can enhance the clinicians' armamentarium. Innovative ways of targeting or modulating miRNAs, CSCs and EMT phenotype individually or in combination could result in novel more potent ways for treating chemoresistance. As miRNAs appear to exert ubiquitous regulatory roles in EMT and CSCs, downregulating or re-expressing certain miRNAs could be a new strategy for overcoming chemoresistance. Accumulating evidence suggests some natural, non-toxic and well-studied compounds (e.g., curcumin, indole-3-carbinol and isoflavone) can regulate or target miRNAs, CSCs and EMT phenotype Citation[16-18]. Therefore, translation of these strategies from bench-to-bedside seems feasible and can easily be expedited to improve the quality of life of these patients.
As alluded to earlier, targeting miRNAs can potentially address chemoresistance in pancreatic cancer. Inhibiting miR-21 and 221, for example, was shown to sensitize gemcitabine-resistant pancreatic cancer cells. Similarly, introduction of miR-200 family improved sensitivity of resistant pancreatic cancer cells to gemcitabine. Small molecules such as isoflavone and curcumin are known to increase relative levels of miR-200 family in pancreatic cancer cells with EMT phenotype. This increase in miR-200 family expression levels enhanced the sensitivity of resistant pancreatic cancer cells to gemcitabine. Furthermore, curcumin also modulates expression of miR-21 and 22. As it may not be practical to modulate all miRNAs associated with resistant pancreatic cancer, the question that remains to be resolved is determining which miRNAs are crucial in developing CSCs and tumorigenesis to facilitate their inhibition or re-expression. Obviously, the assumption is that therapeutics employed for this purpose can be successfully delivered to disease target sites. However, the paucity of delivery platforms is a major hurdle, which also needs to be addressed.
Another plausible strategy for overcoming chemoresistance in pancreatic cancer involves directly eradicating cells with EMT phenotype and CSCs. Numerous anticancer agents capable of targeting EMT cells and CSCs have been investigated. For instance, combination of sorafenib (a multikinase inhibitor) and sulforaphane (an isothiocyanate broccoli) eliminated CSC features from pancreatic cancer cells. Additionally, quercetin targets EMT cells and pancreatic CSCs and elicits supra additive effects in pancreatic cancer cells containing CSC populations when combined with sulforaphane Citation[19].
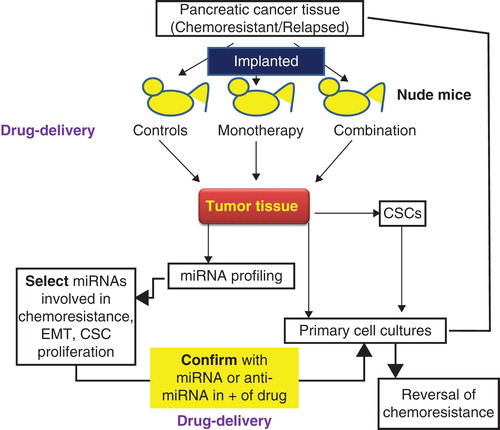
In view of the important role played by miRNAs in CSC proliferation, tumor formation and chemoresistance, it becomes imperative to study the interrelationship between miRNA and CSCs. There is also a pressing need to identify specific miRNAs that enhance cancer relapse and metastasis. This will not only provide a diagnostic and prognostic tool for monitoring cancer progression, but also identify novel therapeutic targets to suppress CSCs and treat pancreatic cancer using currently available combination therapy, or testing novel combinations of drugs. Targeting these miRNAs can potentially reverse chemoresistance and EMT phenotype, in addition to killing chemoresistant CSCs responsible for relapse and aggressive disease profile. is the summation of our ongoing efforts to devise a sustainable therapeutic strategy to treat advanced, chemoresistant pancreatic cancer. By implanting well-characterized human cancer tissue in immuno-compromised mice, we aim to create a personalized disease model that maintains original microenvironment substantially and is perfectly amenable to testing of novel small molecules or nucleic acid drugs and improved formulations and delivery vehicles. This experimental strategy allows identification of novel disease targets such as miRNAs that are involved in chemoresistance, CSC modulation and EMT phenotype. We have also tackled the challenge of ensuring maximal drug/nucleic acid delivery in vivo in a non-toxic fashion by focusing on improved biodegradable polymers that can be used to prepare micelles or as vehicles for conjugation of small molecule drugs and/or miRNA inhibitors of mimics targeting both bulk and CSCs. Further work in this direction will not only advance our understanding of chemoresistance, but also create a comprehensive strategy to treat aggressive pancreatic carcinoma using a multipronged approach.
Declaration of interest
RI Mahato receives funding from the Kosten Foundation.
Notes
Bibliography
- Bailey JM, Swanson BJ, Hamada T, Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 2008;14(19):5995-6004
- Hermann PC, Huber SL, Herrler T, Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1(3):313-23
- Sarkar FH, Li Y, Wang Z, Implication of micrornas in drug resistance for designing novel cancer therapy. Drug Resist Updat 2010;13(3):57-66
- Singh S, Chitkara D, Mehrazin R, Chemoresistance in prostate cancer cells is regulated by mirnas and hedgehog pathway. PLoS One 2012;7(6):e40021
- Szakacs G, Paterson JK, Ludwig JA, Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5(3):219-34
- Wang Z, Li Y, Kong D, Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 2009;69(6):2400-7
- Arumugam T, Ramachandran V, Fournier KF, Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 2009;69(14):5820-8
- Ruckert F, Joensson P, Saeger HD, Functional analysis of loxl2 in pancreatic carcinoma. Int J Colorectal Dis 2010;25(3):303-11
- Li C, Heidt DG, Dalerba P, Identification of pancreatic cancer stem cells. Cancer Res 2007;67(3):1030-7
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5(4):275-84
- Kim M, Turnquist H, Jackson J, The multidrug resistance transporter abcg2 (breast cancer resistance protein 1) effluxes hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res 2002;8(1):22-8
- Cheng G, Danquah M, Mahato RI. Micrornas as therapeutics targets for cancer. In: Lu Y, Mahato RI, editors. Pharmaceutical perspectives of cancer therapeutics. Springer; New York: 2009. p. 441-74
- Singh S, Narang AS, Mahato RI. Subcellular fate and off-target effects of sirna, shrna, and mirna. Pharm Res 2011;28(12):2996-3015
- Wang Z, Li Y, Ahmad A, Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol 2011;8(1):27-33
- Singh S, Chitkara D, Mehrazin R, Chemoresistance in prostate cancer cells is regulated by mirnas and hedgehog pathway. PLoS One 2012; In Press
- Li Y, Kong D, Wang Z, Sarkar FH. Regulation of micrornas by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res 2010;27(6):1027-41
- Li Y, Vandenboom TG II, Wang Z, Mir-146a suppresses invasion of pancreatic cancer cells. Cancer Res 2010;70(4):1486-95
- Sun M, Estrov Z, Ji Y, Curcumin (diferuloylmethane) alters the expression profiles of micrornas in human pancreatic cancer cells. Mol Cancer Ther 2008;7(3):464-73
- Zhou W, Kallifatidis G, Baumann B, Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol 2010;37(3):551-61