Abstract
Objectives: To demonstrate, using human factors engineering (HFE), that a redesigned, pre-filled, ready-to-use, pre-asembled follitropin alfa pen can be used to administer prescribed follitropin alfa doses safely and accurately.
Methods: A failure modes and effects analysis identified hazards and harms potentially caused by use errors; risk-control measures were implemented to ensure acceptable device use risk management. Participants were women with infertility, their significant others, and fertility nurse (FN) professionals. Preliminary testing included ‘Instructions for Use’ (IFU) and pre-validation studies. Validation studies used simulated injections in a representative use environment; participants received prior training on pen use.
Results: User performance in preliminary testing led to IFU revisions and a change to outer needle cap design to mitigate needle stick potential. In the first validation study (49 users, 343 simulated injections), in the FN group, one observed critical use error resulted in a device design modification and another in an IFU change. A second validation study tested the mitigation strategies; previously reported use errors were not repeated.
Conclusions: Through an iterative process involving a series of studies, modifications were made to the pen design and IFU. Simulated-use testing demonstrated that the redesigned pen can be used to administer follitropin alfa effectively and safely.
1. Introduction
Ovulation induction and assisted reproductive technology (ART) (including in vitro fertilization [IVF] or intracytoplasmic sperm injection [ICSI]) are two important fertility treatments that require multiple days of injections. For both, tailoring of the daily injected follicle-stimulating hormone (FSH) treatment needed is dependent upon each individual patient’s response; therefore, dose modifications may be required. With the need for multiple days of dosing with adjusted doses, a multiple use, variable dosing pen device offers a practical approach to self-administered treatment. An example of how pen devices have been used successfully is in the treatment of diabetes, where such devices have been utilized for > 20 years Citation[1]; insulin pens were well accepted by patients because they offer easy, safe, accurate, and discreet injection Citation[2]. Pen devices for the delivery of recombinant human FSH (r-hFSH) have been available in Europe for use in ovulation induction and ART since 2002 Citation[3].
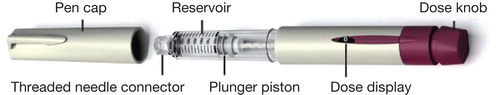
The redesigned follitropin alfa (r-hFSH) pen injector (GONAL-f®; pre-filled pen; Merck Serono, Geneva, Switzerland, a subsidiary of Merck KGaA, Darmstadt, Germany) was approved by the European Commission in May 2011 Citation[4]. It was since approved by the US FDA in October 2013 (GONAL-f RFF® Redi-ject™; EMD Serono, Inc., Rockland, MA, USA, a subsidiary of Merck KGaA, Darmstadt, Germany) Citation[5], and the pen has now been launched in 65 countries worldwide; in countries other than the USA, the pen is called GONAL-f Prefilled Pen. After attaching the needle and setting the dose, the redesigned pen () is a disposable, pre-filled, ready-to-use, pre-assembled pen that includes a number of feature modifications to the previous pen. These include the ability to adjust the dose by small incremental steps within approved doses; set the dose in one step; adjust a bi-directional dose dial that allows dialed doses to be increased or decreased; confirm the dose delivered and, if necessary, the dose amount required from a second pen to complete the prescribed dose; and see the approximate amount of medication remaining in the pen through a graduated scale on the clear cartridge reservoir Citation[6]. The pen is intended for subcutaneous self-injection and is available in three dosing preparations: 300, 450, and 900 IU. It has received the 2014 GOOD DESIGN Award for Design Excellence. The annual award program recognizes the most innovative and cutting-edge industrial, product, and graphic designs produced around the world.
To minimize risks to users, it is important that devices, such as pen injectors, are evaluated for use in the countries in which they will be approved. In the USA, such testing is required for manufacturers to provide the FDA with validation of control and prevention of use-related risks for new or modified devices for their intended use Citation[7]. Human factors engineering (HFE) is an interdisciplinary approach to evaluating and improving use safety, efficiency, and robustness of work systems. HFE addresses multiple aspects of work and task analysis, user-interface, and instruction design; product evaluation and usability assessment; communication, collaboration, and teamwork; training; and systems resilience, adaptation, and failure Citation[8]. Understanding and controlling use risk requires human factors methods that identify how the medical device is used, under what conditions, and in what environments Citation[9]. A risk-based analysis and design approach focuses on the most risk-critical user–device interactions and assumes that, whereas device optimization should be the primary focus for mitigation, labeling and training may also be considered two ways to eliminate or reduce potential harm to the user Citation[9].
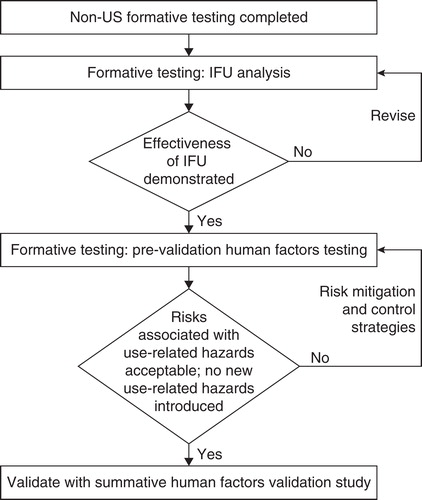
HFE includes multiple steps and follows an iterative process () that includes defining user populations and the use environment, a hierarchical analysis of tasks, development of a use error mitigation plan, and testing use error mitigation. An important first step includes the risk management plan and performing use-related risk analysis (failure modes and effects analysis [FMEA]). Following risk assessment, a vital part of the HFE process is simulated-use testing, which involves assessing the interactions between the users and the device in an environment that mimics the real-world experience of the user and culminates in simulated-use validation tests. A medical device is considered safe and effective if it is demonstrated that the control strategies are effective in reducing risk to as low as reasonably possible so that users may complete medication administration without critical or serious errors resulting in incorrect dosing or other patient or user harm.
Figure 2. The risk management process for addressing use-related risks with the redesigned follitropin alfa pen.
This paper summarizes results from an Instructions for Use (IFU) formative study and pre-validation and validation studies with the redesigned follitropin alfa pen, as well as modifications that were made to the IFU based on the study results and device design modifications to improve safety and accuracy of its use.
2. Patients and methods
Ethical approval for the study was provided by Chesapeake Research Review, Inc.
2.1 Institutional review board/ethics approval
All HFE studies were open-label, non-clinical, institutional review board-approved, simulated-use tests that were conducted by human factors engineers. Signed informed consent was obtained from each participant in the studies. Ethical pre-approval was obtained for the pre-validation and validation studies.
2.2 Failure modes and effects analysis
The FMEA was initiated using known problems with the follitropin alfa pen presentation identified using data from periodic safety update reports, product complaint summaries, and postmarketing surveillance information. Risk assessment was conducted on all steps required to administer follitropin alfa using the redesigned pen and by rating the risk level according to FMEA scores Citation[10]; this was derived by estimating a likelihood of occurrence on a scale of 1 – 10 (10 = very likely to occur) and a severity rating of 1 – 10 (10 = most severe). Then, a risk priority number (RPN) for use risk was calculated from the product of these two estimations. Based on this risk assessment, tasks within the injection process that were associated with the highest RPN were identified and included in the HFE test plan for participants.
2.3 Human factors testing
EMD Serono, Inc., retained Agilis Consulting Group LLC to conduct a series of human factors evaluations of the pen and the IFU, culminating with the simulated-use validation test.
Basic user-device interactions (essential tasks) were assessed, with particular focus on interactions that, if performed incorrectly or omitted, had the highest scored potential risks to the users (critical tasks). The tasks included setting the wrong dose, resulting in either an over- or underdose; mishandling air bubble removal and potentially forgetting to reset the pen to the prescribed dose, which could result in an underdose; not properly responding to delivery of an incomplete dose, which could result in an underdose; and improper handling of the pen, which could result in a needle stick injury.
Using this use error assessment, five different use scenarios representing situations with the potential for use errors were identified and formed the basis for simulated injections in our formative, pre-validation, and validation studies. The scenarios included tasks that required: selecting the correct pen (900, 450, or 300 IU) and administering a starting dose, adjusting a treatment dose, completing a partial dose using a second pen, resetting a dose on a pre-used pen, and recognizing that one pen fully dispensed will not complete the dose and that a second pen is needed. The use environment for performing the scenarios during the studies was to be a normal office or home environment with average lighting and noise and with some possible distractions, such as a ringing phone.
Participants were videotaped as they completed these study scenarios. The moderator observed, classified, and recorded the interactions. Assessment was based on human perceptual, cognitive, and physical action requirements for each step. Following the test, subjects were asked about the ease of use and any difficulties encountered, and a subjective assessment of ease of use was conducted.
2.4 Study populations
The FDA Human Factors guidance recommends that at least 15 participants per user group should be tested for devices intended for use by more than one group of users that have distinct abilities, training, experience, or use roles Citation[7]. User populations identified for simulated-use studies were: female patients aged ≥ 18 years currently seeking fertility treatment, with a diagnosis of female infertility or with a partner diagnosed with male infertility, whose indicated course of treatment was ovulation induction or ART, who were judged to be capable of safely and effectively performing self-injection, who may or may not have had experience using injectors, with various levels of education, and who were able to understand, speak, and read English; their significant others (male or female) who were typically individuals without medical qualifications who may deliver injections to patients with infertility, who may or may not have had experience using injection devices and with varying levels of education; and fertility nurse (FN) professionals (male or female) who were registered nurses or nurses with a nursing degree and who provide training to patients in administering injectable gonadotropin therapy for ART or ovulation induction and who may also administer such therapy on occasions. Different subjects were recruited for each study.
2.5 HFE procedures
2.5.1 Training
Training was given to all participants before the start of the study. All user groups received the same training in the use of the redesigned pen, regardless of knowledge and skill. EMD Serono, Inc., representatives trained a small group of nurse trainers (n = 3), who in turn trained the three user groups. In the real-world setting, these nurse trainers would be FNs, who would also be responsible for training additional FNs, as required. The training consisted of up to 1 h of step-by-step review of the IFU and instruction on how the redesigned pen was anticipated to be used once approved. The training covered basic information about the pen, the scope of intended use, explanation of the dosing regimen and how it may change over time, and the necessary operations to be performed, including performing simulated injections using a demonstration pen containing a placebo (i.e., sterile water-filled) cartridge into an injection pad. During training, a mock injection was conducted in which the pen emptied before the full dose had been given, as this may occur during normal use when a second pen would be required. To mimic the real-world setting, nurses who provided medical device training to patients trained infertile women and their significant others, either alone or more often together, or alternatively in small groups of up to three subjects. FNs were trained separately from patients and their significant others, either individually or in small groups. For FNs, there was a brief delay of about 1 h between training and testing, representing the real-world delays between familiarization and use. For women with infertility and their significant others in the pre-validation and validation studies, there was a delay of 7 – 10 days to reflect the anticipated time lag between pen device training and their first medication injection in the real world. In the mitigation validation study, there was minimal delay between training and testing, as it had been determined that the decay period was not the root cause of any observed use errors in the first validation study.
Instruction was also provided on general injection practices, such as injection site selection and preparation, needle recapping and disposal, and pen storage. The training materials included demonstration pens containing placebo, needles, mock injection pads, and the IFU. At no time were the users to inject themselves or others. Within the scheduled training session, infertility patients and their significant others were provided contact information for a nurse trainer in case they had questions during the decay period.
2.5.2 Formative studies
For the IFU formative study, participants (n = 17; six patients with infertility, six patients’ significant others, and five FNs) were familiarized with the pen device and instructed to follow the IFU throughout training. Participants performed nine basic user tasks – including simulated injections with placebo into an artificial skin injection pad – and were then interviewed using pre-defined questions about the adequacy of instructions from content and format standpoints. All failures and difficulties experienced during tasks were examined for their relationship to remaining IFU design attributes.
Participant responses regarding the usefulness of the IFU were generally positive, particularly with regard to the format, layout, and use of illustrations. Participants had some challenges with the IFU that required revisions. Most revisions fell into the following categories: general difficulty to perform tasks (e.g., redesign of the treatment diary to simplify use); general injection techniques (e.g., needle re-capping after use); multiple doses/multiple days (e.g., increased instructional clarity on completing an incomplete dose); and flow and order of the IFU (e.g., Table of Contents was refined). No issues in the IFU formative study were observed with the device that would preclude moving on to the pre-validation studies. Thus, the revsions were re-evaluated during the pre-validation study without repeating the IFU formative study.
2.5.3 Pre-validation studies
Two other pre-validation tests were performed sequentially, involving 12 patients with infertility, 10 patients’ significant others, and 15 FNs. All users were tested individually. For each task, step, and sub-step of simulated injections, participants were asked a set of questions to assess effectiveness of user interaction with the redesigned pen. The following observations occurred that resulted in serious use errors: needle stick while using a one-handed needle re-capping approach; retrieving outer needle cap from sharps container (two instances); and stating intent to remove exposed needle carefully and dispose of it in sharps container. As a result of these studies, a use-related hazard mitigation strategy was undertaken that involved outer needle cap design modification () and some re-wording of the IFU to improve alignment with the instructions on the use of ‘the one-handed needle recapping method’ from the FDA Medical Device website Citation[11]. These mitigations were evaluated and the hazards were deemed mitigated in the second formative pre-validation study.
2.5.4 Validation studies
The simulated-use validation studies were focused on confirming the control of risk related to one primary and two secondary use-related safety assertions about the redesigned pen. The primary safety assertion was that patients with indications for ovulation induction or ART (IVF/ICSI) can use the redesigned pen to administer their prescribed dose of follitropin alfa without errors in dosing. The secondary safety assertions were that the redesigned pen minimizes or eliminates actions that would result in needle exposures and needle stick injuries, and that the IFU effectively supports safe and accurate user performance.
The first validation study was an open-label study performed in five cities in standard market research facilities with average lighting and noise. This environment did not differ significantly from the anticipated environments of real-world use that could impact perceptual, cognitive, or physical action performance.
The HFE process may continue to require iterations, and the results observed in the first validation study required modification to the device design and IFU. Thus, a second validation (mitigation validation) study was performed to assess the effects of the risk control strategy and device modifications related to changes made in response to the first validation study. This open-label study was performed in four cities and in a similar environment to the first validation study. The participants were different to those included in earlier studies. A subset of the simulation injection scenarios was selected for testing. Participants were trained and assessed using the modified pen and revised IFU, but without the 7 – 10-day delay before testing. After completing the simulated injection scenarios in both validation studies, participants were asked for a subjective assessment of the IFU’s directions on the 10 critical dosing steps.
3. Results
3.1 Validation study
The first validation study recruited 18 infertility patients, 15 patients’ significant others, and 16 FNs (). During testing, two critical use errors occurred, both of which were in the FN group (): one related to setting the dose (37.5 IU instead of 75 IU) and the other to completing an incomplete dose (completed dose used was original dose instead of remaining dose). No use errors were observed in either the infertility patient or significant other groups. Two close calls (near errors) occurred: a wrong dial up of remaining dose, and a mathematical error when dividing a dose between two pens. Both were subsequently corrected by following the IFU when instructed to confirm the dose before administering the medication.
Table 1. Demographic characteristics and prior injection experience of participants in the validation studies.
Table 2. Critical use errors in A) validation study and B) mitigation validation study.
To address the two critical use errors observed, modifications to the device design and IFU were implemented. To mitigate the use errors related to setting the dose, design improvement included centering and double etching of the dose numbers; the latter was to increase the contrast between the dose numbers and display background. To address the use error related to completing the dose, a revision to the IFU included instruction to confirm the dose with that recorded in the treatment diary: “Check that the dose you have set in the dose display matches what you wrote in your treatment diary”. Additional minor revisions to the IFU included modification to the ‘Welcome’ text with additional information on the new attributes of the redesigned pen; instruction to wait at least 5 s before needle removal to ensure delivery of full injection; removal of text instructing to hold down the dose knob when removing needle; addition of text stating not to re-cap the pen before needle removal; and re-ordering sentences on steps after injection to improve clarity of wording.
3.2 Mitigation effectiveness validation study
A mitigation effectiveness validation study was conducted to confirm that the risk mitigations implemented after the first validation study were successful. For this testing, 16 infertility patients, 15 significant others, and 16 FNs were recruited. During this validation test, two additional critical use errors occurred (): inaccurate dosing (underdosing: 75 IU instead of 187.5 IU) and a needle stick injury following needle re-capping, during which the needle pierced the cap. The incorrect dosing error was not due to an error in the user–pen interface but instead was caused by the participant (FN) not recalling a verbally given dose and instead using a previous dose from her diary. The cause of the needle stick injury (patient) was considered to be related to the lack of a solid backstop to use for re-capping the needle. A significant other also experienced a needle piercing the outer needle cap due to the lack of a solid backstop but did not receive a needle stick injury (close call; ). Wording was added to the IFU to “use a hard flat surface, such as a wall, when re-capping the used needle”.
No use difficulties observed in the initial validation study were repeated in the mitigation validation study and no use errors required further mitigation.
3.3 Post-testing assessment of IFU steps
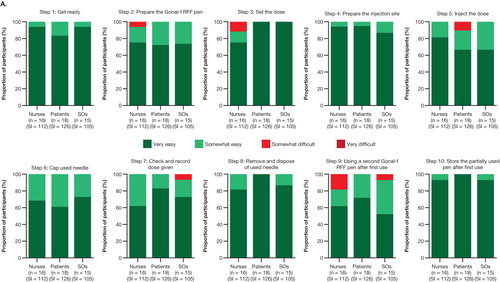
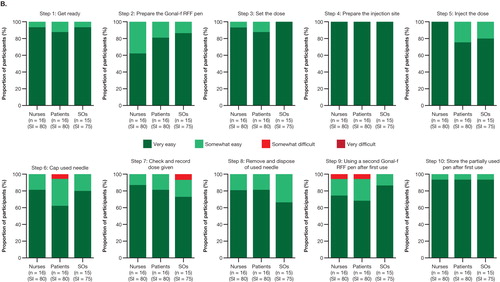
At the end of the initial validation study involving 18 patients, 15 significant others, and 16 FNs, with 343 combined simulated injections, the participants mostly rated the 10 criticial steps of the IFU as ‘very easy’ or ‘somewhat easy’ (, indicated by dark green and light green); no participants rated any steps as ‘very difficult.’ Two nurses did not agree that the IFU provided all the information needed to use the pen safely and effectively. Following the mitigation validation study involving 16 patients, 15 significant others, and 16 FNs (235 combined simulated injections), the total number of ‘somewhat difficult’ ratings across the 10 IFU steps were reduced from 10 in five steps to 4 in three steps. All users agreed that the IFU provided all the information needed to use the pen safely and effectively.
Figure 4. Assessment of IFU steps in the (A) validation study and (B) mitigation validation study. No responses of ‘very difficult’ were reported.
4. Discussion
An iterative approach was taken to evaluate the safety and accuracy of the redesigned follitropin alfa pen during simulated normal use in HFE studies. A risk assessment was performed, a control strategy was put in place, and testing was conducted to confirm that the control strategies, proposed in the risk management plan to minimize risk, were effective. When a control strategy failed, it was re-examined and revised further to control risks, and re-testing was conducted to demonstrate that the implemented control strategies were effective in reducing hazards to as low as reasonably possible. In this way, a series of human factors evaluations of the redesigned follitropin alfa pen were performed through a number of studies, which culminated with the simulated-use validation study and the mitigation validation study. This iterative process performed over a number of studies is a key strength of the evaluations.
It is important that HFE studies are adequately representative of the real-world setting. This was achieved by simulation of the anticipated use environment, and testing the performance of users to administer follitropin alfa doses with the device. Originally, two user groups, infertility patients and FNs, were planned. However, based upon the input from a group of fertility specialists (reproductive endocrinologists and FNs), significant others were added. Feedback from the fertility specialists suggested the spouse/significant other be encouraged to participate in the injection process, as an additional way to be involved in the treatment process. The infertility patient and significant other user groups included participants who were naive to device use, as well as those with experience of using devices; the latter group was included in the assessment to observe whether there were any influences of use of other devices that could interfere with correct and safe use of the redesigned pen. The user groups included participants with a range of educational levels and ages. FN participants had a wide range of years of experience, had been active instructors of gonadotropin medication administration, and may, or may not, have administered medication. To ensure alignment of HFE device use testing with real-world experience, advice was obtained from currently practicing FNs both on pen training and on the scenarios of pen use.
The HFE studies of simulated-use testing of the redesigned follitropin alfa pen reported here evaluated the safety, efficiency, and robustness of the redesigned follitropin alfa pen. In the course of the studies, modifications were made to the pen design, outer needle cap, and the IFU. Our studies have shown that intended users of the redesigned follitropin alfa pen can safely and effectively perform essential and critical tasks with the pen. In line with recommendations, the validation studies were performed in user groups of about 15 intended users Citation[12]. The use of smaller groups may not give accurate information; for example, in a study published in 2003, some randomly selected sets of five participants found only 55% of the problems Citation[13]. However, with 10 users, the lowest percentage of problems revealed by any one set was 82% (mean 94.7%); with 15 users, this variable was 90% (mean 97%), and increasing to 20 users resulted in a small further increase to 95% (mean 98.4%) Citation[13].
Although results were generally positive in the first validation study, observation of even one use error resulted in consideration that the proposed risk management plan did not sufficiently mitigate risks/hazards and that additional control measures should be considered. To that end, device design and IFU modifications were implemented and then re-assessed during the second validation study.
The second validation (mitigation effectiveness) study performed with these modifications to the pen and IFU demonstrated that improvements to the dose display had mitigated the previously identified risk related to the display legibility, and that the clarification of the IFU had mitigated the potential for use errors when completing dosing with a second pen. Thus, the issues and the residual risk associated with each use error identified during the validation study were addressed and the redesigned pen was deemed to be acceptable. The iterative process used worked well to optimize simulated use of the redesigned pen. Most users found that the steps in the administration process were ‘very easy’ or ‘somewhat easy’ to use; few had any difficulty with the steps in the IFU. For any future design modifications of the follitropin alfa pen, human factors testing will play an important role early in the design process so as to optimize the device design earlier in device development.
HFE validation studies, such as those performed here, have some limitations: the tests were simulation tests rather than real clinical practice use, and the study design of the validation studies was observational and not interventional – observers questioned subjects regarding certain operational steps in the injection process once all injection simulations were completed. In addition, safety data other than accidental needle sticks and dosing errors were not captured. However, the HFE validation studies were designed to demonstrate optimal user–device interactions, and the simulation testing conducted here is defined as an acceptable method for assessing safe and effective use of a pen device according to FDA guidance.
5. Conclusion
Through an iterative process involving a number of different simulated-use human factors studies, design modifications were made to the redesigned follitropin alfa pen, including changes to the outer needle cap to reduce the risk of needle stick injuries and improvements to the clarity of the dose display to avoid dosing errors. Validation studies showed that the changes made were effective in reducing needle exposure and dosing errors. In usability studies, most users found the steps involved in using the redesigned pen ‘very easy’ or ‘somewhat easy’ to perform.
The overall risk management plan was effective, as residual risk associated with each potential use error identified during the human factors validation studies was deemed to be acceptable. Thus, the human factors simulated-use studies showed that the redesigned follitropin alfa pen can be used to administer treatment safely and accurately by intended users.
Acknowledgments
The authors thank Jane Davies of Caudex Medical, Oxford, UK, and Michele Springer of Caudex Medical, New York, NY, USA (supported by EMD Serono, Inc., Rockland, MA, USA, a subsidiary of Merck KGaA, Darmstadt, Germany) for their assistance in the preparation of this manuscript, and Deborah Rice, RN and Gladys Lopez, RN for consultant advice on medical training practices and review of the Instructions for Use. The authors would also like to thank the following centers for recruiting participants to the study: Boston IVF, Waltham, MA, USA; Fertility Centers of Illinois, Chicago, IL, USA; San Diego Fertility Center, San Diego, CA, USA; and Texas Fertility Center, Austin, TX, USA.
Declaration of interest
MC Mahony, B Hayward, and D Green are employees of EMD Serono, Inc., Rockland, MA, USA (a subsidiary of Merck KGaA, Darmstadt, Germany). P Patterson is an employee of Agilis Consulting Group, LLC, Cave Creek, AZ, USA. R North is an employee of Human Centered Strategies, LLC, Colorado Springs, CO, USA. This study was sponsored by EMD Serono, Inc., Rockland, MA, USA, a subsidiary of Merck KGaA, Darmstadt, Germany. EMD Serono contracted with Agilis Consulting Group, LLC, who designed and performed the study, monitored and analyzed the data, and wrote the study report.
Notes
Bibliography
- Perfetti R. Reusable and disposable insulin pens for the treatment of diabetes: understanding the global differences in user preference and an evaluation of inpatient insulin pen use. Diabetes Technol Ther 2010;12:S79-85
- Kadiri A, Chraibi A, Marouan F, et al. Comparison of NovoPen 3 and syringes/vials in the acceptance of insulin therapy in NIDDM patients with secondary failure to oral hypoglycaemic agents. Diabetes Res Clin Pract 1998;41:15-23
- Kang HJ, Kim CH, Ahn JW, et al. Comparison of follitropin beta administered by a pen device with follitropin beta administered by a conventional syringe in patients undergoing IVF-ET. Clin Exp Reprod Med 2011;38:37-41
- Merck Serono. News release: Merck Serono Received European Approval for Three Pre-filled, Ready-To-Use Pen Injectors for Fertility Treatment. 2011. Available from: http://www.prnewswire.co.uk/news-releases/merck-serono-received-european-approval-for-three-pre-filled-ready-to-use-pen-injectors-for-fertility-treatment-145115315.html [Last accessed 4 February 2014]
- PR Newswire. Merck Serono Announces US FDA Approval for Fertility Pen. 2013. Available from: http://www.prnewswire.co.uk/news-releases/merck-serono-announces-us-fda-approval-for-fertility-pen-228355641.html [Last accessed 4 February 2014]
- Christen M, Schertz JC, Arriagada P, et al. The redesigned follitropin alpha pen injector for infertility treatment. Expert Opin Drug Deliv 2011;8:833-9
- US Food and Drug Administration. Draft Guidance for Industry and Food and Drug Administration Staff - Applying Human Factors and Usability Engineering to Optimize Medical Device Design. 2011. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm259748.htm [Last accessed 4 February 2014]
- National Center for Human Factors in Healthcare. Education: what is Human Factors in Healthcare? 2014. Available from: http://medicalhumanfactors.net/education.html [Last accessed 4 February 2014]
- Association for the Advancement of Medical Instrumentation. AAMI TIR49:2013. Design of training and instructional materials for medical devices used in non-clinical environments. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2013
- Institute for Healthcare Improvement. Failure Modes and Effects Analysis (FMEA) tool. 2011. Available from: http://www.ihi.org/knowledge/Pages/Tools/FailureModesandEffectsAnalysisTool.aspx [Last accessed 28 November 2013]
- US Food and Drug Administration. Products and Medical Procedures: what to Do if You Can’t Find a Sharps Disposal Container. 2014. Available from: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/Sharps/ucm263259.htm [Last accessed 4 February 2014]
- Association for the Advancement of Medical Instrumentation (AAMI). Human factors engineering - Design of medical devices. 2010. Report No.: ANSI/AAMI HE75:2009
- Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput 2003;35:379-83