Abstract
The possibility to buy standardized external services or even new and innovative methods within drug discovery has increased dramatically during the last decades. Service providers are able to provide timely and efficient solutions to any given problem within preclinical research. The outsourcing behavior depends on the specific company type. Generally, the outsourcing level of emerging pharmaceutical and biotechnology companies is much higher than established companies due to low or missing internal resources. Whereas the “make-or-buy” decisions of large and fully integrated pharmaceutical companies are mainly competency driven, those of mid-size and small pharmaceutical, as well as biotech companies show a specific combination of cost/capacity and competency. The three different cooperation models “price competition”, “project selection,” and “strategic partnership” were identified. For all types of companies, the cooperation model of “strategic partnership” offers access to high-level expertise while reducing fixed costs and complexity. This was shown using chemical synthesis as an example but is also true for other areas of preclinical research.
1. Introduction
In the pharmaceutical industry, innovation is recognized as the cornerstone for competitive advantage and is fostered by strong investments in new technologies Citation[1]. But nowadays, costs and competitiveness issues are becoming increasingly important. Pharmaceutical companies are recognizing this imperative more and more and are exploring options to enhance the efficiency of the resources they are using and this at all stages of the value chain from discovery research to dosage form assembly or even sales and marketing. Especially the increasing costs of pharmaceutical research and development (R&D) lead to increasing pressure on output of the innovation pipeline coupled with increasing pressure of stakeholders demanding a continual earning momentum.
Out of every 10,000 substances synthesized in laboratories, on average only one or two successfully pass all the stages to become a marketable product Citation[2]. The costs for bringing new drugs to the market are still increasing and are up to 1.5 billion dollars for innovative drugs, that is, drugs with a new chemical entity, depending on the therapy area and other factors Citation[3-5]. The resulting pressure to increase the output of pharmaceutical R&D as well as the fast technological progress, for example, in genomics and proteomics, has created new needs for specialized technologies with the potential to reduce lead times and streamline the drug discovery and development process. Companies are forced to think and act much more target and result-oriented instead of reacting within existing organizational boundaries, as productivity is dependent on the internal organization of R&D Citation[6]. Pharmaceutical companies are being forced to reassess their mode of research operation including outsourcing activities Citation[7,8]. Management is increasingly recognizing that some operations can be performed more effectively by a third party by utilizing, for example, a superior scale, lower cost of capital, or access to proprietary know-how and technologies Citation[9].
This article will discuss how the challenges facing the pharmaceutical industry are shaping the outsourcing or “make-or-buy” strategies pursued by pharmaceutical companies in terms of access to know-how and technologies from specialized service providers to accelerate the drug-discovery process. After showing the role and importance of outsourcing, the requirements of the different pharmaceutical company types, and cooperation models for outsourced services, chemical synthesis is discussed as an example within preclinical research. Besides established companies, this article will also take a look at emerging companies Citation[10].
2. Role and importance of outsourcing
Outsourcing, traditionally thought of as a short-term strategy for demand realization, is now being considered to lever the core competencies to increase performance in pharmaceutical research Citation[9,10]. Cost, time, and innovation are the levers to improve research performance, and outsourcing gives the mentioned levers a positive impact. This drive toward research outsourcing has led to the increase in the number of external contract research service providers.
The decision whether or not to outsource is driven by a number of factors like technical issues (e.g., technologies involved, in-house capacity availability), product-specific considerations (e.g., volumes involved, position in the life-cycle, impact on overall product portfolio), financial considerations (e.g., investment required, respective economics of “make-versus-buy”), and type of the pharmaceutical company. The level of outsourcing can range widely between two extreme approaches: full vertical integration, where most, if not all, operations are performed in-house, and virtual operations, corresponding to full outsourcing, where all operations are systematically outsourced to third parties.
An in-between situation is most often observed where outsourcing is combined with in-house resources. An important aspect is the complementarity between in-house research and external know-how Citation[11,12], which was, for example, shown for companies in the biotechnology industry Citation[13,14]. The access to external know-how may leverage the productivity of internal research activities, at least when the organization exhibits a willingness to absorb external ideas Citation[15]. An important task in innovation management, therefore, is to integrate internal and external knowledge within the company's innovation process, in order to benefit from positive effects each activity has on the other.
The potential value of research outsourcing arises as a key question for research managers, especially as outsourcing does not improve competitiveness automatically and is discussed as a controversial topic. Outsourcing may lead to negative effects when used only as a cost-reducing strategy to improve short-term performance. The consequence may be the loss of internal know-how and expertise as well as higher total costs in the long term. Outsourcing research also bears potential risks due to project complexity and loss of flexibility.
All these effects are becoming even more important in offshore outsourcing, that is, going to low-cost countries. Offshore outsourcing to India, China, and Eastern Europe has been popular because of relatively low costs Citation[9]. A corporation's offshore outsourcing may be seen as the result of a discrete, strategic decision, taken in response to an increasing pressure from worldwide competition. However, these countries present some challenges, particularly in terms of patent laws, cultural differences, and business complexity. Empirical evidence indicates that offshore sourcing in low-cost countries is best described as a learning-by-doing process in which the offshore outsourcing of a corporation goes through a sequence of learning steps Citation[16]. Another strategy to reduce costs, especially those of “big pharma,” was to set up own research activities in these low-cost countries.
3. Requirements regarding outsourcing
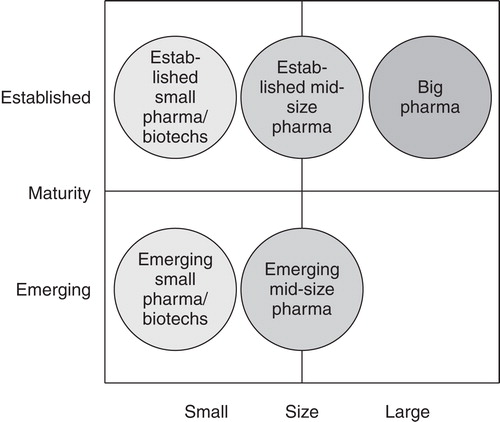
To understand outsourcing behavior the differentiation between different company types is crucial. The company types can be defined using a two-dimensional matrix with the dimensions size and maturity (). Besides large and fully integrated pharmaceutical companies (“big pharma”) there are both established and emerging mid-size and small pharmaceutical as well as biotechnology companies. “Emerging” means that the companies were founded within the last two decades with innovative approaches regarding business model, product portfolio, and operational aspects.
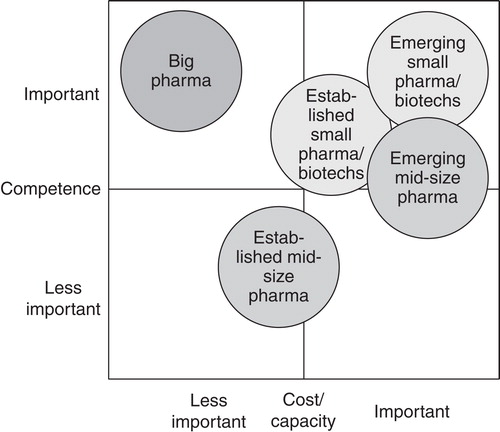
shows the importance of the two most important reasons for outsourcing for the different company types. One reason is cost/capacity which means that the pharmaceutical or biotechnology company buys low-cost and flexible additional capacities from the service provider, if internal resources are not present or temporarily too small. Important are transparent and flexible cost structures, similar or equivalent to its own in-house structures. Highly standardized cooperation models covered by general agreements with precise definition of the ownership of intellectual property (IP) should avoid additional administrative resource burdens. The other reason is competence which means that the pharmaceutical or biotechnology company buys additional expertise and know-how. The service provider must have a clear competence profile while being unique and innovative using leading-edge equipment and adhering to the highest possible technical standards. Crucial are international presence and availability of experts to support the customer worldwide as well as familiarity with the requirements of major pharmaceutical markets.
“Big pharma” companies normally have their own in-house capacities for preclinical research. They are less interested in buying services for cost and capacity reasons due to sufficient in-house capacities. Also for established mid-size companies cost and additional capacity is not a reason for outsourcing as almost all companies have sufficient in-house resources in drug discovery. Competence is also not a main reason for outsourcing, as these companies are very much focused on special fields with huge in-house competence. In contrast, “big pharma” companies with diverse product lines have a high interest in additional, external know-how which is not available in-house or too expensive, if it were to be built up internally Citation[17]. Expanding in-house capabilities by external expertise is seen as the most important advantage of using external services. Higher attention is given to the quality of the services, which should be world class. Cost reduction (reducing fixed costs or reducing people on the payroll) has minor relevance for the outsourcing of services compared to smaller companies.
Small pharma and biotech as well as emerging mid-size pharma companies have limited internal resources and competencies. In contrast to “big pharma” and established mid-sized companies they depend on external services within preclinical research and see outsourcing as an effective method to capture capacity and expertise without investing much money in in-house resources Citation[10]. In particular, emerging biotechnology companies lack experience and expertise in specific fields, which consequently forces them to relay on external service providers. They have some clear preferences for providers: lean and flexible development capacities, easily adaptable to smaller demands, full service range and know-how with capabilities for the support of project management.
4. Cooperation models for outsourced services
In the area of drug discovery, three different cooperation models have been identified. “Price competition” means that service providers are systematically put into competition in order to secure lowest purchasing prices. This model is less strategically oriented, but rather serves to achieve the demand for the most cost-efficient fulfillment. It can be applied successfully only if the outcome can be measured easily. The service providers within the cooperation model “project selection” are selected based on a project-by-project basis from a core list of preselected service providers. The service providers are engaged according to the fit of their core competence to the specific project requirements. The cooperation model “strategic partnership” is realized with a handful of preferred service providers, who are given preferential “right of first refusal.” A framework contract covers all the relevant aspects of the cooperation. Analyzing the relevance of these cooperation models for the different outsourcing areas within preclinical research shows that the “strategic partnership” model is used mainly in the areas of discovery research and chemical synthesis. Due to the economic pressure there has been an increase in creative outsourcing solutions to reduce costs for pharmaceutical companies during the last years, such as the use of dedicated facilities and employees of service providers within strategic partnerships. “Price competition” is mainly used for services in the area, for example, of analytics. The cooperation model “project selection” has limited relevance for all areas and is more relevant for drug development activities (clinical trials, process, and formulation development) Citation[18] as well as manufacturing and distribution activities (drug substance production, packaging, and logistics Citation[19].
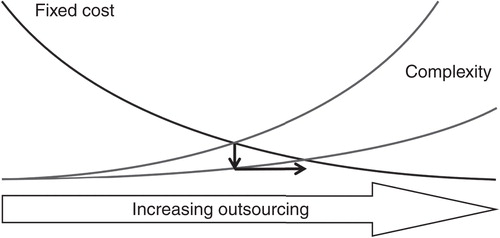
Outsourcing decisions are often a balance between fixed costs and the complexity of processes and organizational structures (). Creating and running internal R&D capacities demand a higher level of in-house competencies while causing increasing fixed costs. Alternatively, outsourcing of activities has the potential to reduce fixed costs, but increases complexity due to more and new interfaces as well as intermediaries with different interests and culture. Very simply put, the optimum level of “make versus buy” is defined by the minimum of fixed costs and complexity. Following the schematic mechanism for the understanding of the outsourcing level in , service providers must reduce the level of complexity to increase share of outsourcing.
Pharmaceutical companies think intensively about the optimal supplier structure, if they decide to outsource services. Outsourcing in pharmaceutical research requires specialized partners that understand the strict regulatory barriers and high risk associated with pharmaceutical R&D. Pharmaceutical companies invest a lot of management capacity choosing appropriate service providers and committing them to the company to achieve goal congruence. Stringent inspection of the supplier's facility, quality, best practices, trained staff, and certified processes is crucial in the selection process Citation[9]. Assessment of the service provider's financial stability is imperative during the selection process. Financial stability is required for the smooth turnaround of the projects within the proposed timeline. As these suppliers work with various projects from pharmaceutical companies, it becomes crucial to ensure that there is no backlog of projects due to financial constraints.
5. Chemical synthesis services as example
Within the traditional outsourcing model around chemical synthesis a few years ago only the product was sold exclusively or semi-exclusively to the customer. Synthesis know-how and process design was further owned by the provider. In a number of cases, royalty payments were part of the contract. Very often, customers were forced to buy further scale-up and manufacturing services from the same provider. Opposing views on IP and rigid customer provider relations provoked rather complex contracts and parallel structures on both sides.
This view has changed totally with the emergence of the “strategic partnership” cooperation model. Service contracts without paying royalties are absolutely preferred, as pharmaceutical companies wish to have a reliable cost base versus unknown revenue sharing due to royalty payments. In addition, pharmaceutical companies want to keep IP in-house to maintain flexibility and to be independent from third parties as much as possible. Exclusivity is favored from partners, as single source means the provision of trust and undivided attention Citation[20,21].
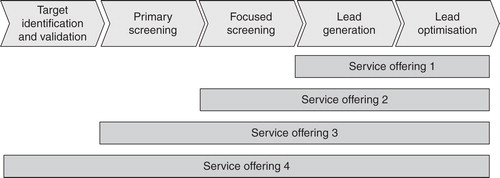
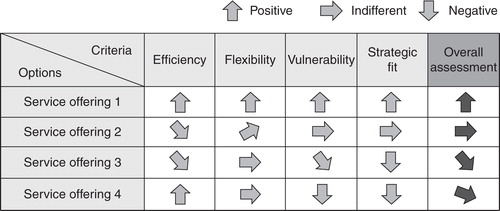
One major question for service providers as well as pharmaceutical companies is how the optimal service offering should look based on the “strategic partnership” cooperation model. Taking a closer look at chemical synthesis shows that service providers prefer different options for positioning chemical synthesis services within the drug-discovery process ().
Service offering 1: The lead generation and optimization process is located within the pharmaceutical company but regarding synthesis, the pharmaceutical company concentrates on medicinal chemistry expertise and outsources synthetic chemistry work for which in-house resources are not available. The service provider synthesizes the substance for further lead optimization or in larger quantities for preclinical testing. It requires no medicinal chemistry expertise and can concentrate on supporting the pharmaceutical company with inexpensive and reliable capacity. This service requires only a simple interface and provides high flexibility, with low risks. It is the approach recommended in stable and predictable situations.
Service offering 2: The synthesis service provider supports the pharmaceutical company to synthesize compounds for focused screening. Involving the service provider into the focused screening process requires an intensive information exchange between the pharmaceutical company and the service provider (e.g., validating results). The synthesis service provider has to have medicinal chemistry expertise and the customer has to manage a complex interface. The situation regarding IP and payment of royalties becomes a big issue. The two companies must have a common goal and precise contractual agreements.
Service offering 3: The synthesis service provider supplies compounds for libraries and high-throughput screening and is fully integrated into the drug-discovery process which maximizes the interface and the need for information exchange between pharmaceutical company and service provider. The situation regarding IP is rather complex and has to be clarified in the contracts. This service needs deep reciprocal trust at all levels of the companies.
Service offering 4: This is a radical approach including all steps of the drug-discovery process where the pharmaceutical company outsources the complete discovery process. The service provider has research teams covering biological, medicinal chemistry expertise and independently creates leads which are licensed to pharmaceutical companies. This approach can be recommended under very heavy time and capacity pressure.
The different service offerings have their specific advantages and disadvantages. Engaging a synthesis specialist with service offering 1 reduces the complexity and increases the flexibility of pharmaceutical companies, but only if there are sufficient in-house resources for the early drug-discovery process. The need for intensive information exchange between the pharmaceutical company and the service provider in service offering 2 increases complexity, but additional external know-how is made available. Involving the service provider also in the primary screening process in service offering 3 maximizes the interfaces between pharmaceutical company and service provider. With this option, the pharmaceutical company can speed up the whole drug-discovery process. Outsourcing the complete drug-discovery process in service offering 4 is suitable for emerging small pharma and biotech companies without or with very limited own resources. This could make the company vulnerable as critical know-how about targets and lead compounds is given out of the company before patent applications secure this know-how.
Companies have to choose between these alternatives and the decision, whether to outsource and which activities to outsource, depends on the individual situation of the pharmaceutical company. An example to identify the best service offering is given in for a large fully integrated pharmaceutical company. After a systematic evaluation of all options using the criteria efficiency of the discovery process, flexibility and vulnerability of the pharmaceutical company, and strategic fit to the current drug-discovery philosophy, service offering 1 increases flexibility with moderate risk and also enables access to innovative synthetic methods Citation[22]. In this case, a common interface between a pharmaceutical company and a synthesis service provider is the “chemical structure written on paper.”
6. Conclusion
Outsourcing has traditionally been thought of as a short-term strategy in order to cut back expenses or to provide a company with additional capacities. This understanding has changed throughout the last few years. If a corporation's outsourcing is driven by a desire for cost minimization, after a time the outsourcing experience lessens the advantages that can be achieved through outsourcing: a strategic partner could not only offer cost advantages, but also quality improvement and innovation. Today, outsourcing is seen as a method to increase performance in pharmaceutical research via leveraging of core competencies. This is not only true for the area of chemical synthesis but also for other areas of preclinical research.
The conclusion is that within pharmaceutical research the tendency to outsource services is high, if competencies, know-how, or technologies are not available in-house or where in-house buildup would be too expensive or time consuming. The positive effects of outsourcing are enhanced, if the supplier is actively used to supplement existing core competencies. The “strategic partnership” model guarantees a high internal competence level in the long term. Thus, there is a far greater performance improvement potential in investing in, rather than divesting, research. If, however, there is high complexity in the processes between service provider and customer, or interfaces, which are difficult to define, the benefits of outsourcing decline.
7. Expert opinion
If outsourcing is solely used as a strategy in order to improve short-term performance through a reduction of expenses, it will lead to negative effects. The analysis of pharmaceutical companies' outsourcing in chemical synthesis proves that loss of internal know-how and expertise and higher total expenses in the long term can be crucial consequences. Cost, time, and innovation are the levers to improve R&D performance and R&D outsourcing could give the mentioned levers a positive impact. Besides limiting fixed costs, service providers can often provide the expertise and know-how in a more flexible and cost-effective way than internal resources. Positive effects of outsourcing can be enhanced, if service providers, as strategic partners, offer unique know-how. In that case, their services can be used to supplement existing core competencies (i.e., to free resources in order to invest in higher internal capability). The complementarity between in-house R&D and external know-how creates additional benefits regarding the quality of research and services. Therefore, the right strategic partner could not only offer cost advantages, but also quality improvement and innovation, and the strategic partnership model guarantees a high internal competence level in the long term.
Thus, pharmaceutical companies have to choose outsourcing strategies dependent on their specific situation regarding the long-term R&D strategy (“How does the outsourcing strategy fit to the R&D strategy?,” “Which areas of preclinical research are core and which are non- core activities?”) and correlating R&D capabilities and resources (“Is primarily additional competence/know-how or capacity needed?,” “Which costs are accepted to build up and maintain internal capabilities/resources?”). After systematically evaluating all outsourcing options based on company-specific criteria, the outsourcing strategy should be defined and executed based on a longer time horizon. Short-term adjustments are necessary, but the well-known changes in the outsourcing “philosophy” from one extreme to the other every few years are counterproductive.
Higher complexity combined with reduced efficiency and flexibility, difficulties to maintain and transfer know-how and issues with the IP situation are seen as major obstacles to outsourcing chemical synthesis. Service providers, if not already done, should react to these concerns of pharmaceutical customers and take attention to some important aspects. The complexity of the cooperation can be reduced through highly standardized and transparent processes and contracts, leaving all critical IPs at the pharmaceutical company, and including clear and transparent rules regarding the engagement in projects of direct competitors. In the pharmaceutical industry the willingness to pay royalties is low. The companies prefer contracts based on a fee-for-service model with fixed prices (e.g., attached to milestones), which allow a better cost calculation of the project. The communication between pharmaceutical company and service provider should be very intensive with the employees responsible for the project management, including stringent quality control, in close vicinity to the pharmaceutical company. This is especially important, if the service provider uses offshore lab resources (e.g., in China or India).
If these aspects are handled properly, the professional market for highly specialized services and the flexible structures within the services networks make pharmaceutical research more efficient. Consequently, in the future, highly specialized research service providers will play an even more important role as integrative part of the processes in the whole pharmaceutical industry.
Article highlights.
Outsourcing is seen as a method to increase performance in pharmaceutical research via leveraging of core competencies.
Service providers are able to provide timely and efficient solutions to any given problem along the whole value chain of pharmaceutical R&D.
The possibility to buy standardized external services or even new and innovative methods within drug discovery has increased dramatically during the last decades.
The outsourcing behavior of established large and mid-size pharma companies is different to that of small pharma and biotech as well as emerging mid-size pharma companies.
The cooperation model of “strategic partnership” offers access to high-level expertise while reducing fixed costs and complexity.
Declaration of interest
The author is the CEO of Festel Capital.
Notes
This box summarizes key points contained in the article.
Bibliography
- Achilladelis B, Antonakis N. The dynamics of technological innovation: the case of the pharmaceutical industry. Res Policy 2001;30(4):535-88
- European Federation of Pharmaceutical Industries and Associations (EFPIA). The pharmaceutical industry in figures. EFPIA, Brussels: 2008
- Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006;25(2):420-8
- DiMasi J, Hansen R, Grabowski H. The price of innovation: new estimates of drug development costs. J Health Econ 2003;22(2):151-85
- Levine DS. New Estimate of Drug Development Costs Pegs Total at $1.5 Billion. Available from: http://www.burrillreport.com/article-new_estimate_of_drug_development_ costs_pegs_ total_at_1_5_billion.html [Last accessed 14 May 2013]
- Cockburn I, Henderson R. Absorptive capacity, coauthoring behavior and the organisation of research in drug discovery. J Ind Econ 1998;46:157-82
- Quinn JB. Strategic outsourcing: leveraging knowledge capabilities. Sloan Manage Rev 1999;40(4):9-21
- Quinn JB. Outsourcing innovation: the new engine of growth. Sloan Manage Rev 2000;41(4):13-28
- Findlay SM. Outsourcing in pharma. Pharm Technol Eur 2007;19(5):13-14
- Van Arnum P. Outsourcing strategies of emerging pharma. Pharm Technol 2008;32(10):48-53
- Cassiman B, Veugelers R. Complementarity in the innovation strategy: internal research, external technology acquisition, and cooperation in research. IESE Business School Working Paper. PJB Publications; 2002. p. 457
- Veugelers R, Cassiman B. Make and buy in innovation strategies: evidence from belgian manufacturing firms. Res Policy 1999;28(1):63-80
- Arora A, Gambardella A. Complementarity and external linkages: the strategies of the large companies in biotechnology. J Ind Econ 1990;38:361-79
- Arora A, Gambardella A. Evaluating technological information and utilizing it: scientific knowledge, technological capability and external linkages in biotechnology. J Econ Behav Organisation 1994;24(1):91-114
- Veugelers R. Internal research expenditures and external technology sourcing. Res Policy 1997;26(3):303-16
- Maskell P, Pedersen T, Petersen B, Dick-Nielsen J. Learning paths to offshore outsourcing: from cost reduction to knowledge seeking. Industry Innov 2007;14(3):239-57
- Phlippen S, Vermeersch A. Complementarities and research boundaries of the firm: a project level study on pharmaceutical research. Tinbergen Institute Discussion Paper TI 2008-022/3 Pharmaceutical Strategies
- Festel G. Outsourcing chemical synthesis in the drug discovery process. Drug Discov Today 2011;16(5-6):237-43
- Festel G, Schicker A, Boutellier R. Performance improvement in pharmaceutical R&D through new outsourcing models. J Bus Chem 2010;7(2):89-96
- Falk M, Festel G. New ways to buy in chemistry. Scrip Mag 2002;58-9
- Festel G, Falk M, Hofmeier U. Leased competence: a new R&D model in outsourcing chemical synthesis. Chim Oggi Chem Today Outsourcing Compend 2003;21:1
- Festel G, Maas R, Bellof M. Getting access to new technologies – how chemical synthesis outsourcing can tweak the process of drug discovery. Chim Oggi Chem Today 2013;31(1):16-18