Abstract
Introduction: Sarcoidosis is a multi-system inflammatory disorder that may affect the peripheral nerves causing neuropathic pain. Chronic neuropathic pain is a debilitating disease and current treatment options are either of limited efficacy or are hampered by the development of serious side effects. A novel pharmacological treatment option is the non-hematopoietic erythropoietin analog ARA 290, a small peptide acting at the innate repair receptor, which is a heteromer of the erythropoietin receptor and β-common receptor. ARA 290 was recently granted designation as Orphan Drug Product by the FDA, for treatment of neuropathic pain in sarcoidosis patients.
Areas covered: This report reviews the pathophysiology of sarcoidosis and, to understand the mechanistic pathway of ARA 290, the role of the innate repair receptor in inflammation and its natural and synthetic ligands, erythropoietin and ARA 290. The results of two proof-of-concept studies are presented that show the ability of ARA 290 to induce analgesia greater than placebo in patients with sarcoidosis-induced chronic moderate to severe neuropathic pain, without safety issues.
Expert opinion: ARA 290 is a promising novel therapeutic option in the treatment of neuropathic pain in sarcoidosis. Further studies are designed to obtain additional proof of ARA 290's analgesic efficacy and ability to increase the quality of life in afflicted patients.
1. Introduction
Neuropathic pain is pain caused by a lesion or disease of the peripheral or central somatosensory nervous system (www.iasp-pain.org). It is a severe and debilitating complication associated with a variety of multi-system disorders, including diabetes mellitus (DM) and sarcoidosis, and several infectious diseases (e.g., HIV, leprosy) Citation[1]. While neuropathic pain is well recognized in DM, its common occurrence in sarcoidosis is just recently appreciated and due to awareness provoked by recent publications the number of sarcoidosis patients diagnosed with neuropathic pain is increasing Citation[2,3]. Neuropathic pain treatment is difficult and currently pharmacological interventions are based on a trial-and-error approach. Drugs being used in the management of neuropathic pain consist of topical applications (e.g., capsaicine, lidocaine), prolonged-release opioids, anticonvulsants and antidepressants Citation[1]. Irrespective of the drugs utilized, efficacy is limited both with respect to the number of patients obtaining adequate pain relief (30 – 40%) and the magnitude of effect (maximum pain relief 30 – 50%) Citation[4,5]. In the current report, we discuss a new treatment option for chronic neuropathic pain in patients with sarcoidosis: ARA 290 (). ARA 290 is an erythropoietin analog without the hematopoietic properties of EPO Citation[6-8], that is now being tested in Phase II a and b studies for treatment of sarcoidosis-related neuropathic pain. The primary action of the drug is to provide pain relief, while secondary benefits include mood enhancement and improvement of the quality of life. The choice of sarcoidosis-induced neuropathic pain is due to the fact that the initial clinical proof-of-concept trials yielded positive results in a majority of sarcoidosis patients being treated with ARA 290 for neuropathic pain relief.
Box 1. Drug summary.
2. Sarcoidosis
Sarcoidosis is a multi-system, chronic, inflammatory, orphan disease first described more than 100 years ago by dermatologists Hutchinson, Besnier and Boeck Citation[2]. Sarcoidosis affects multiple organ systems causing localized non-caseating granulomas Citation[9,10]. The lungs (> 90%) and lymphatic system are most frequently afflicted followed in frequency by involvement of cardiac, neuronal, ocular, renal, and skin tissues. The incidence of sarcoidosis varies by geographic region and ranges from 1 in 5,300 women to 1 in 6,300 men Citation[9,10]. Sarcoidosis' orphanumber is ORPHA797 (Orphanet, available at www.orpha.net) and synonyms include Besnier-Boeck-Schaumann disease and Boeck's sarcoid. The clinical course of the disease differs between patients and depends on a variety of factors such as ethnicity, genetics, site and extension of organ involvement and the underlying often-episodic inflammatory process Citation[9,10]. Symptoms may precede the diagnosis by many months due to the presentation of a variety of non-specific symptoms. In some patients the disease develops asymptomatically; in others the disease has an insidious course, which often results in a chronic relapsing form with pain and fatigue as important symptoms. In patients with an acute onset of the disease symptoms include fatigue, fever, weight loss, night sweats, erythema nodosum and a dry cough Citation[9,10]. The acute form often resolves within 24 – 36 months. In a minority of patients, the disease becomes progressive with lung fibrosis and cardiac and/or neurosarcoidosis. The progressive form of the disease has a poor prognosis with a mortality rate of up to 5%. The etiology of sarcoidosis remains unknown. Possibly exogenous toxins or antigens activate inflammatory pathways (including the release of specific cytokines from macrophages such as tumor necrosis factor (TNF)-α) that in genetically susceptible patients cause the formation of non-caseating granulomas Citation[9,10]. Specific occupations prone to being exposed to toxic materials have a higher incidence of sarcoidosis compared to the general population. For example, fire fighters involved in rescue activities during and following the 2001 World Trade Center terrorist attacks have an increased incidence of sarcoidosis Citation[9,11].
The pain in patients with chronic sarcoidosis is often related to neuropathy of the small fibers of the peripheral nervous system Citation[2,3,12,13]. Small fiber neuropathy (SFN) is due to damage or loss of small myelinated (Aδ-) and unmyelinated (C-) fibers of the sensory and autonomic nervous system Citation[14,15]. SFN is a common manifestation in a variety of systemic disorders including diabetes mellitus, alcoholism, systemic amyloidosis, various autoimmune disorders (e.g., Sjögren's disease, systemic lupus erythematosus), genetic disorders (e.g., Farbry's disease), infectious diseases (e.g., HIV infection) and may occur as part of a paraneoplastic syndrome Citation[15]. Most studies show that in just a minority of sarcoidosis cases a generalized large fiber neuropathy is observed Citation[3,16]. Burns et al. Citation[16] showed that sural nerve biopsies in some patients with a definite diagnosis of sarcoidosis neuropathy showed varying degrees of epineural, perineural and endoneural granolomatosis causing multifocal involvement of nerve fascicles and axon loss, with occasionally concurrent vasculitis. In case of a mixed small and large fiber neuropathy also muscle weakness may become an important symptom. While until recently an incidence of 30% was documented Citation[2,3], neuropathy in sarcoidosis is increasingly diagnosed due to an increased awareness of the disease as well as improved diagnostic tests.
The most prominent clinical features of SFN include pain (often described as burning or shooting), numbness, paresthesias, allodynia (causing intolerance to touch), and hyperalgesia Citation[15]. In case of autonomic nerve involvement there may be hypo/hyperhydrosis, diarrhea/constipation, sexual dysfunction, blurry vision, and orthostatic hypotension Citation[14,15]. There is currently no specific treatment for sarcoidosis-related SFN. Sarcoidosis treatment is initiated when organ function is threatened Citation[9], with a prominent role for corticosteroids, followed by other immunosuppressive and cytotoxic agents (thalidomide, methotrexate) and antimalarial drugs (hydroxychloroquine). Also beneficial effects of intravenous immunoglobulin and TNF-α inhibition have been reported Citation[17-19]. In case of neuropathic pain, sarcoidosis patients do not seem to benefit from corticosteroid therapy Citation[3]. Chronic pain treatment in sarcoidosis patients is not different from treatment of neuropathic pain from other causes and consists of antidepressants, anticonvulsants and prolonged-release opioids. However, in common with their effects in other neuropathic pain states, these agents provide limited pain relief in just 30 – 60% of patients, at the cost of considerable side effects. These data indicate that there is an imminent need for analgesic agents with high efficacy in neuropathic pain patients without causing debilitating side effects.
Recently, we initiated a program aimed at the treatment of neuropathic pain in patients with sarcoidosis with a novel therapeutic agent, ARA 290. ARA 290 is a non-hematopoietic erythropoietin analog with potent anti-inflammatory and tissue protective properties, acting at the innate repair receptor Citation[20,21]. First studies in animals (with nerve-damage-induced neuropathic pain) and in patients with chronic neuropathic pain from sarcoidosis and diabetes mellitus indicated that ARA 290 is highly effective in causing pain relief in these neuropathic pain states. ARA 290 was recently granted designation as an Orphan Drug Product by the FDA, for the treatment of neuropathic pain in sarcoidosis patients.
3. The innate repair receptor (IRR) and ARA 290
Tissue injury initiates an innate immune response driven by pro-inflammatory cytokines, including TNF-α (see Refs. 19 and 20 and references cited therein). This pro-inflammatory process results in progressive tissue destruction but is halted in surrounding tissues by an anti-inflammatory process aimed to contain further destruction of tissue and attenuate apoptosis. Two important players in this endogenous anti-inflammatory process are the anti-inflammatory cytokine erythropoietin (EPO) and the innate repair receptor (IRR) Citation[21]. The IRR is expressed by damaged tissues and is activated by EPO produced and released into damaged tissue. The IRR is distinct from the hematopoietic EPO homodimer receptor (EPOR2) and is made up of a β-common receptor (βCR) subunit (CD131) coupled to an EPOR. The EPOR–βCR receptor complex, i.e., the IRR, is a heteromer consisting of two EPORs and two βCRs. The hematopoietic EPOR2 has a high affinity for EPO and is activated at constant low concentrations of circulating EPO (0.2 nM). Activation of the EPOR2 results in an inhibition of the apoptosis of red cell precursor cells. In contrast, the IRR has a lower affinity and requires high local concentrations of EPO (2 – 20 nM). Activation of the IRR by EPO results in activation of multiple anti-inflammatory pathways, which up-regulate survival signals and subsequently blocks inflammation-induced apoptosis, activates tissue restorative functions (e.g., stem cell differentiation) and generates nitric oxide by endothelial cells.
EPO induces tissue protection and has tissue restorative properties via an effect at the IRR Citation[22]. Recent studies in animals show that exogenous EPO improves the process of healing and effectively prevents tissue damage after injury Citation[22-27]. For example, 5000 IU/kg of recombinant human EPO (rhEPO) reduced concussive injury of the brain after a blunt trauma up to 6 h after the injury by 50 – 70% Citation[24]. However, exogenous administration of high-dose EPO will – apart from its effects at the IRR – always activate the hematopoietic receptor, activating red cell production and related side effects (e.g., thrombosis and hypertension). For example, in a clinical study infusion of EPO at 40,000 IU/week to trauma patients in the intensive care unit reduced mortality by 50% but increased the risk of thrombosis by 40% Citation[27].
Several non-hematopoietic EPO analogs have been developed that selectively activate the IRR and cause tissue protection (without any effect on the EPOR2 receptor complex and consequently without enhancement of erythropoiesis and other side effects) Citation[6,23,28,29]. These molecules that mimic the tissue protective effects of EPO have been tested in a variety of animal models including models for stoke, wound healing, cardiomyopathy and spinal cord compression, and in all of these models the non-hematopoietic EPO analogs were effective. One of these molecules is ARA 290 Citation[6-8,20,21,23]. ARA 290 is a pyroglutamate helix B surface peptide that mimics the spatial configuration of EPO that interacts with the IRR. It is as effective as exogenous EPO in its ability to reduce tissue damage from a variety of injuries and experimental animal and preclinical human data indicate that ARA 290 has no safety issues. Its mechanism of action is believed to be activation of anti-inflammatory pathways via the IRR similar to EPO Citation[6-8,20,21].
4. ARA 290: chemistry, safety, and pharmacokinetics
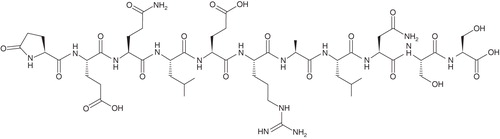
ARA 290 is an 11-amino acid, linear peptide, with a molecular weight of 1257 Daltons Citation[23]. The amino acid sequence is Pyr-Glu-Gln-Leu-Glu-Arg-Ala-Leu-Asn-Ser-Ser-OH (Pyr represents pyroglutamic acid). The chemical structure of ARA 290 is given in . Standard toxicity studies in animals revealed no adverse reactions in doses up to 1000 times the initial human dose of 1 μg/kg (Araim Pharmaceuticals, Inc., data on file). Similarly, no safety issues were identified in healthy volunteers in supra-clinical dosages, and in patients with end-stage renal disease, diabetes or sarcoidosis at therapeutic dosages (Araim Pharmaceuticals, Inc., data on file).
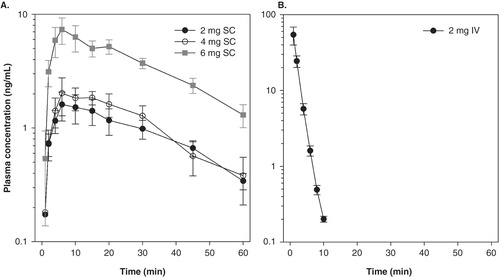
We recently performed a pharmacokinetic (PK) study on intravenous (IV) and subcutaneous (SC) administration of ARA 290 in healthy volunteers (PKARA study). Using a crossover design, healthy male volunteers received 2 mg (equivalent to ∼30 μg/kg, n = 10), 4 (∼60 μg/kg, n = 5) and 6 mg (∼90 μg/kg, n = 5) ARA 290 subcutaneously, and 2 mg (∼30 μg/kg, n = 10) intravenously. The results of the study are shown in . The 2, 4 and 6 mg SC formulation caused a dose-dependent increase in plasma CMAX (CP MAX) from 1.6 ± 0.5 ng/mL (2 mg) to 2.0 ± 0.7 ng/mL (4 mg) and 7.4 ± 1.9 ng/mL (6 mg) occurring at t = 6 min following injection () with corresponding areas-under-the-curve (AUC) of 84, 148, and 310 ng.min/mL. The elimination half-life (t½ ELIM) estimation was ∼20 min. In contrast, 2 mg IV caused a rapid peak in CP MAX at min 1 of 54.2 ± 14.5 ng/mL with a t½ ELIM of just 2 min and AUC of 313 ng.min/mL. Compared to the 4 mg SC dose, the exposure AUC of the other doses was 0.6 (2 mg SC), 2.1 (6 mg SC), and 2.1 (2 mg IV). The short t½ ELIM after the IV injection is remarkable and similar observations have been made in the rabbit and rat. These data suggest rapid passage of the drug into the effect compartment (in case of neuropathic pain probably the spinal and supra spinal CNS) and rapid activation of the receptor followed by the initiation of a sustained cascade of events involving a series of transduction factors, eventually causing the silencing or reduction of the inflammatory response (see of Citation[23]). The suggestion of a rapid passage of ARA 290 across the blood–brain barrier is in agreement with ample animal data showing passage of ARA 290 across the blood–brain barrier and eliciting a central effect despite the short plasma half-life Citation[6].
5. Animal data
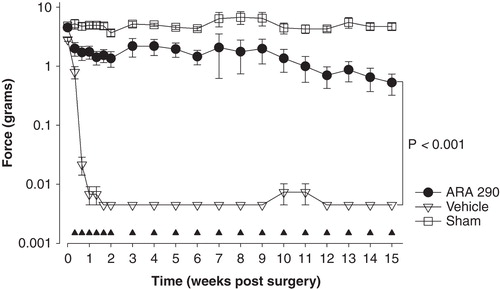
The first indication that ARA 290 causes relief of neuropathic pain comes from data obtained in rats following sciatic crush injury Citation[6]. In this model, the sciatic nerve was reversibly crushed using a ligature for the duration of 1 min. ARA 290 0.2 nmol/kg given intravenously immediately following the injury reduced damage compared to vehicle as measured by the static sciatic index (SSI). Although the SSI is a crude test of motor function, the response is certainly influenced by the pain from the nerve injury. We next performed a second series of experiments in rats using the spared nerve injury (SNI) model of neuropathic pain Citation[30]. This SNI model generates more robust and prolonged allodynia of the affected paw and involves cutting two of the branches of the sciatic nerve. ARA 290 treatment initiated within 24 h following surgery (0.030 mg/kg, five intraperitoneal injections at 2 days interval for 2 weeks, followed by one treatment/week) produced effective and long-term (> 15 weeks) relief of tactile and cold allodynia. Restricting the treatment to the first 2 weeks following surgery yielded similar results although there was a slow return of allodynia toward values observed in vehicle-treated animals. illustrates part of the results of this animal study. Subsequent studies showed a clear ARA 290 dose–response relationship and even the ability of the peptide to cause the relief of allodynia when treatment was initiated days to weeks following surgery (unpublished observations). The involvement of the βCR receptor was tested in wild type and βCR receptor knockout mice using the SNI model. A two-week treatment paradigm was applied followed by one treatment/week showing relief of allodynia in wild type animals only. In βCR receptor knockout mice, the response to ARA 290 was similar to the vehicle response (i.e., maximal allodynic paw withdrawal responses, thus no relief of allodynia).
Figure 3. Effect of treatment with ARA 290 treatment for 15 weeks (weeks 1 and 2: 0.03 mg/kg at a 2-day interval, weeks 3–15: 0.03 mg/kg once weekly) on allodynia in a spared nerve injury rat model. Allodynia was measured using von Frey filaments; the force (in grams) causing a withdrawal response of the affected paw is given on the y-axis. Open squares are sham-operated animals, open triangles vehicle-treated animals, closed circles ARA 290-treated animals (n = 8/group). The closed triangles denote the ARA 290 treatment days. Values are mean ± SEM.
The neuroanatomical site of action of ARA 290 in these experiments remains unknown. A peripheral effect is not excluded. For example, a peripheral effect from rhEPO has been observed in rats using a chronic nerve constriction injury model, where rhEPO facilitated the recovery from neuropathic pain and reduced Schwann cell TNF-α expression at the nerve injury site Citation[31,32]. However, a peripheral nerve block with a local anesthetic is unable to prevent the development of peripheral neuropathy following SNI Citation[33]. This suggests that central effects are predominant in the development of allodynia following peripheral nerve injury. Indeed, following peripheral nerve injury, an innate immune response is triggered in the spinal cord dorsal horn with the release of pro-inflammatory cytokines (including TNF-α) Citation[32,34-38]. This neuroinflammatory response is self-amplifying with collateral damage to surrounding neurons causing central sensitization of primary and secondary neurons (causing allodynia and hyperalgesia). While currently no data are available on the effects of ARA 290 on the inflamed spinal cord, there are data showing that rhEPO reduces allodynia following L5 spinal nerve transaction concomitant with a reduced activation of glia cells and reduced expression of pro-inflammatory cytokines and NF-κB activation at central sites Citation[39,40]. These data confirm a central site of action of rhEPO. Taken the fact that ARA 290 is an EPO analog acting at the EPO–βCR receptor complex, and that it rapidly passes the blood–brain barrier, we argue that ARA 290 has a predominant anti-inflammatory and neuroprotective mode of action at spinal and supraspinal sites. Studies are ongoing to confirm our hypothesis of a central site of action of ARA 290.
6. Clinical trials
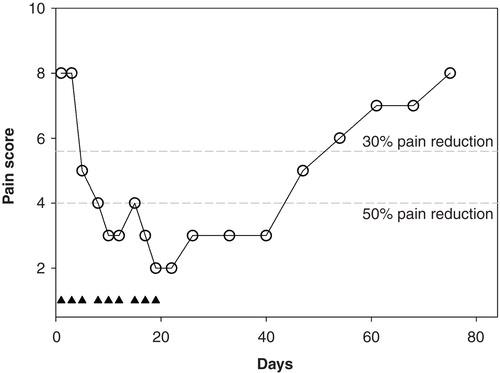
In light of the success of the animal studies, we performed two proof-of-concept clinical trials in patients with chronic neuropathic pain at the Leiden University Medical Center. Here, we will present the unpublished data of the first trial and give a summary of the results of the second trial. The first trial was an open-label study aimed to obtain an indication of efficacy and safety of ARA 290 in patients with sarcoidosis (n = 10) and DM (n = 10). The trial is registered under NTR3081 at trialregister.nl. Patients were enrolled in the trial when they were diagnosed with sarcoidosis or DM type 1 or 2 with a pain score of at least 5/10 (). Patients were treated in house with intravenous ARA 290 injections (2 mg in 9 mL saline, given over 2 min). A total of three injections were administered at 2-day intervals (i.e., on Monday, Wednesday, and Friday). Pain scores before, during and for one month following treatment were collected. All patients concluded the study without side effects and no safety issues were apparent. Patient characteristics were similar () and pretreatment pain scores were 7.0 ± 2.0 and 7.1 ± 2.7 in sarcoidosis and DM patients, respectively. The results of the study on the total population (n = 10 + 10) are summarized in (data presented as percentage of pre-treatment pain scores [worst pain in last 24-h], closed circles; see for patient characteristics). The one-week ARA 290 treatment in sarcoidosis patients caused a reduction in pain scores of 40%; the effect lasted for the 3 days following the end of treatment (i.e., until day 8). Similarly, in DM patients the treatment resulted in a reduction in pain scores of 30% at least until day 8. In the study, responders were defined as those patients that had pain relief of 2 points or more. The number of responders was 6/10 in the sarcoidosis group and 5/10 in the DM group. Their responses are given in (open squares), showing responses of about 50% in both populations and again lasting for three days following the end of the ARA 290 injections. To better understand these data, we performed a time-series analysis using non-linear mixed effects modeling (NONMEM). This analysis described the change of pain scores in terms of an exponential function with half-life T½ (see explanation in Citation[41]). T½ averaged to 2.6 ± 0.2 days (median ± SE). This means that after the last treatment the effect returns toward baseline with a half-life of almost 3 days (i.e., after 3 days the response has returned by 50%, after 6 days by 75%, etc.). No difference in T½ was observed in sarcoidosis and DM patients. The conclusion of this first trial was that ARA 290 is safe and produces effective pain relief but that the duration of effect following a 1-week treatment is rather limited. To test the effect of a longer treatment duration, one of the sarcoidosis patients was retreated, but now for 3 weeks instead of just one week. The results of ARA 290 treatment (2 mg IV for three weeks with 3 injections/week: on Monday, Wednesday and Friday) are given in , showing a large effect on pain scores (a decrease from 8 to 2 points at the end of the 3-week treatment period). Also the duration of effect was extended with an estimated value of T½ of 24 days. Since this is an n = 1 trial, some caution is warranted in the interpretation of the data.
Table 1. Study design of trial #1, An open-label study on the effect of a 1-week treatment with intravenous ARA 290 on pain scores in chronic neuropathic pain patients.
Table 2. Patient characteristics of trial #1 (see ).
Figure 4. Effect of ARA 290 treatment on neuropathic pain in 10 sarcoidosis patients (A) and 10 patients with diabetes mellitus (B). Responders are defined as patients with a reduction in pain score ≥ 2 points within the initial 10 days following the start of treatment. Star: p < 0.05 vs. baseline pain score (analysis on all patients). The closed triangles denote the ARA 290 treatment days. Values are mean ± SEM. The broken grey lines are the 30% and 50% response lines. For study design see and for patient characteristics see .
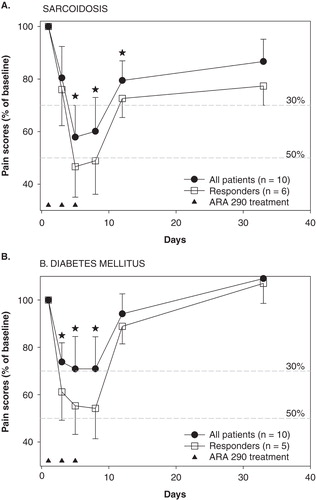
Figure 5. Effect of a 3-week ARA 290 treatment in one sarcoidosis patient with severe neuropathic pain. The closed triangles denote the ARA 290 treatment days. The broken grey lines are the 30% and 50% response lines.
The results of the first proof-of-concept study were encouraging and in agreement with the animal data. The next trial was aimed to assess whether ARA 290 treatment produces analgesia greater than placebo in sarcoidosis-related neuropathic pain trial register # (NTR3081) Citation[42]. This double-blind, randomized, placebo-controlled trial was performed with 22 sarcoidosis patients, of which 12 received a 4-week ARA 290 treatment (2 mg IV with 3 injections/week: on Monday, Wednesday, and Friday); the others received normal saline instead of ARA 290. All patients had a diagnosis of SFN and pain scores ≥ 5. The preliminary data analysis revealed that ARA 290-treated patients had a significant greater decrease in neuropathy symptoms as determined by the small fiber neuropathy screening list score and a significant improvement in pain score as determined from the short form of the RAND36 (a quality of life questionnaire) during the 4-week treatment period. No safety issues or side effects were noted by clinical or laboratory assessments (e.g., as expected no changes in hemoglobin concentration occurred). The study was successful and indicates that ARA 290 indeed produces analgesia greater than placebo in the sarcoidosis patient population with moderate to severe neuropathic pain. No data on the offset of effect were available from this study.
A next Phase 2b study is designed to assess the efficacy of a more frequent treatment paradigm (one injection/day). In contrast to the two previous trials, a subcutaneous formulation of ARA 290 will be used allowing the patients to inject themselves daily (comparable to daily insulin injections). This double-blind, randomized, placebo-controlled trial (the NERVARA study) will be performed in Q3 and 4 of 2012 and will monitor the effect of ARA 290 on pain scores as well as on the neurohistology of small fiber neuropathy. In 2013/2014 multicenter trials in the US are being planned.
In conclusion, despite the relatively small size of the proof-of-concept studies, the picture that emerges from the data is that ARA 290 produces the relief of neuropathy-related symptoms in sarcoidosis patients with chronic moderate to severe neuropathic pain greater than placebo with a response rate of about 50%. No safety issues have emerged in patients or volunteers treated with the ARA 290 peptide. The duration of effect seems to depend on the extent of treatment and possibly also on the frequency of exposures during treatment. These items will be examined further in upcoming trials.
7. Conclusions
ARA 290 is a non-hematopoietic EPO analog acting at the innate repair receptor (an erythropoietin – β-common receptor heteromer) that was recently designated as an Orphan Drug Product by the FDA, for the treatment of neuropathic pain in sarcoidosis patients. Animal data indicate that this 11-amino acid peptide effectively counteracts tissue damage under conditions of inflammation and/or injury through its actions at the IRR, mimicking the effect of locally released erythropoietin. Recently, a program was initiated aimed at using ARA 290 for treatment of neuropathic pain due to small fiber neuropathy in patients with sarcoidosis. Initial open-label and double-blind, placebo-controlled randomized proof-of-concept trials indicate the ability of ARA 290 to produce analgesia, significantly greater than placebo, in patients with sarcoidosis-induced chronic moderate to severe neuropathic pain. No side effects or toxicity issues were noted. Additional studies are planned to further substantiate the efficacy of ARA 290 to produce long-term pain relief and improvement of the quality of life in patients with neuropathic pain from sarcoidosis.
8. Expert opinion
Despite the fact that the number of chronic pain patients has increased over the last decades and most probably will continue to increase in an aging population, the success of current treatment is disappointing. Treatment of chronic pain with a neuropathic component using current treatment options (i.e., antidepressants, anticonvulsants, prolonged release opioids, and local applications) is not truly efficacious Citation[4,5]. Small fiber neuropathy is a neuropathic pain disorder that is associated with a variety of diseases with worldwide a high frequency of occurrence (e.g., DM, leprosy, and HIV-infection) but also in other less common diseases (such as sarcoidosis) causing debilitating chronic pain and a significant reduction of the quality of life Citation[15]. It is therefore not surprising that physicians and industry alike are actively searching for innovative treatment strategies that are distinct from the classical therapies (i.e., aimed at different pathways) Citation[43].
SFN is due to damage of the peripheral nerves. However, a large portion of the chronification of neuropathic pain is related to central (i.e., at spinal and supraspinal sites) processes with most importantly central sensitization causing reduced pain thresholds in the areas of the affected nerves and secondary spreading of this process across the spinal cord dorsal horn Citation[1,44]. This latter process causes an extended and often spreading area of allodynia and hyperalgesia. Various mechanisms are involved in central sensitization, for example spinal up-regulation of excitatory receptors, inability to engage central inhibition and inflammation in the spinal cord with activation of glia cells releasing pro-inflammatory cytokines which maintain and reinforce the process of central sensitization Citation[45,46].
One of the first attempts to employ a new treatment strategy in neuropathic pain is the use of the anesthetic ketamine Citation[46]. This treatment is aimed at antagonism of one of the important players in the process of central sensitization, the excitatory glutamatergic N-methyl-D-aspartate receptor (NMDAR). Ketamine is an antagonist of the NMDAR and produces prolonged pain relief (up to months following treatment), probably by desensitizing the NMDAR Citation[47], although an anti-inflammatory effect cannot be excluded. Despite its efficacy, the use of ketamine is currently restricted for various reasons. Treatment is only successful when the drug is infused for days rather than hours and thus cumbersome and expensive Citation[46,47]. Treatment is only possible in an in-house setting as the drugs need to be administered via the intravenous route and continuous monitoring of side effects and toxicity is required. Side effects include psychosis-like behavior, nausea/vomiting, tachycardia/hypertension, and after repetitive treatments liver enzyme elevation. All of these items reduce compliance of physicians to administer the treatment.
In the current report, a novel therapy aimed at the inhibition of the pro-inflammatory response associated with neuropathic pain is addressed. ARA 290, derived from the EPO molecule, and acting solely at the innate repair receptor has been tested positively in various animal models of inflammation and tissue destruction. The possible ability of ARA 290 to reduce chronic pain from peripheral nerve damage was deduced from earlier studies showing an effect of (high dose) EPO in animal models of neuropathic pain. The animal data and the two proof-of-concept studies in sarcoidosis patients with neuropathic pain that we present here indicate that: i) ARA 290 produces effective relief of pain from peripheral neuropathy in sarcoidosis patients greater than placebo, and ii) ARA 290 treatment up to 4 weeks is safe and no side effects have been reported. Overall, these data are promising but, since sample sizes were small further trials are required. One objection of the initial trails was the need for the IV injection of the drug. To overcome this issue, a subcutaneous formulation has been developed that will make at-home treatment possible, very similar to the use of insulin. This formulation will be applied in future trials. These trials will not only have to address the efficacy of ARA 290 on pain symptoms, but evenly important need to assess its ability to improve other sarcoidosis-related symptoms such as fatigue, malaise and depression. Finally, it may well be that long-term ARA 290 treatment may have a beneficial effect on the underlying inflammatory process of the disease, similar to anti-TNF treatment. This awaits further study.
An important property of ARA 290 is that that despite its short half-life (t½ ELIM ∼2 min), ARA 290 produces long-term analgesic effects, indicating that its efficacy is not driven by PK. Most likely its effect is related to a sustained cascade of events of which the first step is activation of the IRR receptor. This receptor seems to work as an on/off switch. ARA 290 puts the IRR in the on mode after which transduction factors are released that will eventually silence or reduce the inflammatory response and cause prolonged analgesia. In this respect, ARA 290 is similar to ketamine. Ketamine produces long-term analgesia not driven by PK (analgesia persists despite a rapid elimination of the drug from the system). It is believed that ketamine initiates a cascade of events ultimately causing a desensitized NMDAR and possibly a reduced inflammatory response in the spinal cord.
ARA 290 is currently being developed specifically for treatment of neuropathic pain in sarcoidosis. The reason for this is the success of ARA 290 treatment in the initial trials in a sarcoidosis patient population and the lack of effective pain treatment registered for this affliction. Still, it may well be that ARA 290 is effective in other neuropathic pain disorders as well. Indeed in the first proof-of-concept study, patients with SFN from DM responded well to ARA 290 treatment. Further planned studies will address the ability of ARA 290 to reduce pain in DM neuropathy and Complex Regional Pain Syndrome type 1.
Declaration of interest
A Cerami, A Dunne and M Brines are all employees of Araim Pharmaceuticals (the manufacturer of ARA 290) and they hold stock in the company. The other authors declare no conflicts of interest.
Notes
Bibliography
- Baron R, Binder A, Wasner G. Neuropathic pain. Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807-19
- Hoitsma E. Small fiber neuropathy: a novel finding in sarcoidosis. PhD thesis, University of Maastricht; 2005
- Tavee J, Culver S. Sarcoidosis and small-fiber neuropathy. Curr Pain Headache Rep 2011;15:201-6
- Dworkin RH, O'Connor AB, Audette J, Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3-14
- Finnerup NB, Otto M, McQuay HJ, Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118:289-305
- Brines M, Patel NS, Villa P, Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci USA 2008;105:10925-30
- Ahmet I, Tae HJ, Juhaszova M, A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage. Mol Med 2011;17:194-200
- Polgarova K, Luthje P, Cerami A, Brauner A. The erythropoietin analogue ARA 290 modulates the innate response and reduces Escherichia coli invasion into urothelial cells. FEMS Immunol Med Microbiol 2011;62:190-6
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. New Engl J Med 2007;357:2153-65
- Costabel U. Sarcoidosis: clinical update. Eur Respir J 2001;18(Suppl 32):56s-68s
- Izbicki G, Chavko R, Banauch GI, World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest 2007;131:1414-23
- Hoitsma E, Marziniak M, Faber CG, Small fibre neuropathy in sarcoidosis. Lancet 2002;359(9323):2085-6
- Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med 2011;105:95-100
- Hoitsma E, Faber CG, van Kroonenburgh MJ, Association of small fiber neuropathy with cardiac sympathetic dysfunction in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:43-50
- Hoitsma E, Reulen JP, De BM, Small fiber neuropathy: a common and important clinical disorder. J Neurol Sci 2004;227:119-30
- Burns TM, Dyck PJB, Aksamit AJ, Dyck PJ. The natural history and long-term outcome of 57 limb sracoidosis neuropathy cases. J Neurol Sci 2006;244:77-87
- Baughmann RP, Iannuzzi M. Tumour necrosis factor in sarcoidosis ands its potential for targeted therapy. BioDrugs 2003;17:425-31
- Baughmann RP, Drent M, Kavuru M, Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795-802
- Hoitsma E, Faber CG, van Santen-Hoeufft M, Improvement of small fiber neuropathy in a sarcoidosis patient after treatment with infliximab. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:73-7
- Cerami A. TNF and EPO: major players in the innate immune response: their discovery. Ann Rheum Dis 2012;71(Supp II):i55-9
- Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med 2012;18:486-96
- Brines M, Grasso G, Fiordaliso F, Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA 2004;101:14907-12
- Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Int Med 2008;262:405-32
- Brines ML, Ghezzi P, Keenan S, Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 2000;97:10536-1
- Grasso G, Graziano F, Sfacteria A, Neuroprotective effect of erythropoietin and darbepoietin alfa after experimental intracerebral hemorrhage. Neurosurg 2009;65:763-9
- Liao ZB, Zhi XG, Shi QH, He ZH. Recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur J Neurol 2008;15:140-9
- Corwin HL, Gettinger A, Fabian TC, Efficacy and safety of epoietin alfa in critically ill patients. N Engl J Med 2007;357:965-76
- Leist M, Ghezzi P, Grasso G, Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 2004;305:239-42
- Erbayraktar Z, Erbayraktar S, Yilmaz O, Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing. Mol Med 2009;15:235-41
- Swartjes M, Morariu A, Niesters M, ARA 290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and beta-common receptor knockout mice. Anesthesiology 2011;115:1084-92
- Bianchi R, Buyukakilli B, Brines M, Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA 2004;101:823-8
- Campana WM, Li X, Shubayev VI, Erythropoietin reduces schwann cell TNF-alpha, Wallerian degeneration and pain-related behaviors after peripheral nerve injury. Eur J Neurosci 2006;23:617-26
- Suter MR, Papaloizos M, Berde CB, Development of neuropathis pain in the rat spared nerve injury model is not prevented by a peripheral nerve block. Anesthesiology 2003;99:1402-8
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 2004;45:397-407
- Empl M, Renaud S, Erne B, TNF-alpha expression in painful and nonpainful neuropathies. Neurology 2001;56:1371-7
- Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor [alpha] to the normal and mechanically compressed lumbar ganglia in the rat. Pain 2002;95:239-46
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 2004;45:89-95
- Zhang L, Berta T, Xu ZZ, TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain 2011;152:419-27
- Jia H, Feng X, Li W, Recombinant human erythropoietin attenuates spinal neuroimmune activation of neuropathic pain in rats. Ann Clin Lab Sci 2009;39:84-91
- Jia H, Jin Y, Ji Q, Effects of recombinant erythropoietin on neuropathic pain and cerebral expressions of cytokines and nuclear factor-kappa B. Can J Anesth 2009;56:597-603
- Dahan A, Olofsen E, Sigtermans M, Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur J Pain 2011;15:258-67
- Dahan A, Heij L, Niesters M, Safety and efficacy of ARA 290 in neuropathy associated with sarcoidosis: a pilot study. In preparation
- Burgess G, Williams D. The discovery and development of analgesics: new mechanisms, new modalities. J Clin Invest 2010;120:3753-9
- Kuner R. Central mechanisms of pathological pain. Nat Med 2010;16:1258-66
- Woolf CJ. What is this thing called pain? J Clin Invest 2010;120:3742-4
- Noppers I, Niesters M, Aarts L, Ketamine for treatment of chronic non-cancer pain. Expert Opin Pharmacother 2010;11:2417-29
- Sigtermans M, van Hilten JJ, Bauer MCR, Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain 2009;145:304-11