Abstract
Introduction: The pharmaceutical industry entered the 21st century buoyed by a series of mega hits that carried it through the nineties on a wave of promise. More recently, the perceived wisdom came that the age of the mega-selling, mass-marketed drug was over; no more Prozacs, Lipitors or Viagras. With the ‘blockbuster bubble’ seemingly burst and patent protection giving way to huge rises in generic competition, the pharmaceutical industry would now seek to sustain itself by focusing on niche markets; high-value drugs for rare diseases of low prevalence, but for which a rich return on investment could be reaped. Is this picture correct?
Areas covered: This analysis looks across the rare diseases to determine the top disease targets, the most active drug developers and density by drug development phase, using data from a proprietary pipeline drug database. Examples of different development strategies (orphan expansion to other orphan designations; mainstream disease to orphan; orphan expansion to mainstream) are also highlighted.
Expert opinion: The growing trend in orphan drug designations and approvals of drugs to treat the rare diseases is likely unsustainable, largely due to pressure on health care bodies and governments to reduce costs at a time of widespread austerity. As more targeted therapies are developed along with companion diagnostics, diseases will become more segmented – to the point where a once prevalent disease meets the rare disease designation. Cancer drug development is already demonstrating this trend.
1. Introduction
In the EU, a rare disease is defined as one with a prevalence of 1 in 2000 people, and in the US, as affecting fewer than 200,000 people (equivalent to 1 in 1600 people as of 2013 population figures) Citation[1]. This report will focus on rare diseases defined as per above. By these definitions, there are between 6000 and 8000 recognized rare diseases, most of them arising from genetic origin and being chronic or life-threatening. Despite the term, rare diseases still cumulatively affect vast numbers of people, with current data indicating 30 million sufferers in the EU alone, with a further 30 million affected in the US.
2. The landscape for rare diseases
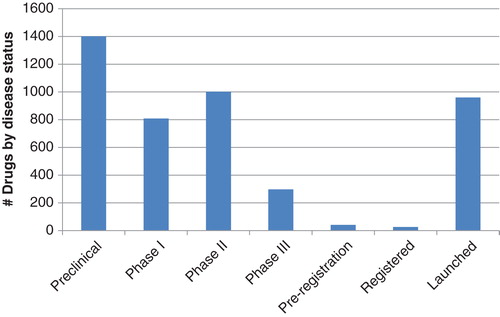
As of November 2013, Pharmaprojects data indicate there were 2907 drugs in active development for at least one rare disease (as defined by being present in either of the accepted rare diseases lists for the EU or US). In terms of disease status (the highest status reached for a particular disease), the landscape shows relatively high activity at preclinical and early-to-mid clinical trials, with less active development taking place at Phase III (). This very much mimics the pattern seen across all diseases. The data source for this analysis covers over 30 years of drug development Citation[2], and to date there have been just 657 launches for drugs used to treat rare diseases, representing 23% of the total active drugs in the development for rare disorders.
The pharmaceutical industry does appear to be taking up the mantle and that a drive toward drug development for rare diseases is certainly well underway, with active drug development in 364 rare diseases. Among these diseases, a total of 223 had nine or fewer drugs in development. Fifty-five diseases, or 15% of the rare diseases listed in Pharmaprojects, with 20 or more drugs in active development – drew the most research and development focus.
3. Therapeutic focus for rare disease development
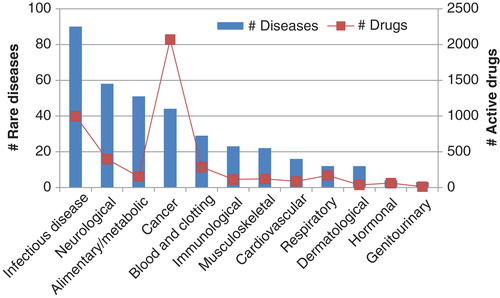
When rare disease drug development was analyzed as a function of therapeutic areas () the top five areas were determined as infectious diseases, neurological disorders, alimentary/metabolic diseases, cancers, and blood and clotting disorders.
Figure 2. Rare disease drugs in development by therapeutic area. The left ordinate illustrates the numbers of diseases in a therapeutic area; the right ordinate illustrates the numbers of drugs in development.
Within infectious diseases, the top five rare diseases are tuberculosis (TB), malaria, tetanus, pertussis and hemophilus influenza. Interestingly, all five are only rare by Western definition; they are unfortunately all too common still in the developing world. The top five rare diseases in the neurological/sensory area are amyotrophic lateral sclerosis (ALS), Huntington’s disease, spinal cord, injury Duchenne muscular dystrophy and uveitis. For alimentary/metabolic, Gaucher’s disease, acromegaly, Pompe’s disease, Cushing’s disease and primary biliary cirrhosis are the conditions with the most active drug development. For the cancer area, pancreatic, ovarian, liver, myeloma and renal have the largest numbers of drugs in active development. Hemophilia A, hemophilia B, idiopathic thrombocytopenic purpura, sickle cell anemia and Waldenstrom’s hypergammaglobulinemia are the blood and clotting conditions with the largest number of active drugs.
Looking at the number of active drugs in development by therapeutic area, cancer leads the way, with nearly two times as many drugs compared to the second-place infectious diseases, and nearly seven times as many as there are for blood and clotting disorders.
3.1 Infectious diseases
By far, the greatest number of rare diseases being pursued is within the infectious diseases arena, with TB, malaria and tetanus having the most drugs in development, respectively (). TB is top of the list, with 88 drugs in active development. There are already a number of launched drugs, including carbocysteine, an adenosine antagonist that is also launched for other infectious diseases, including otitis media, bronchitis, bronchiectasis and respiratory tract infections. However, widespread drug-resistant TB means that there is still an urgent need for new antimycobacterial agents, and new TB vaccines. In the latter category, Cadila’s Immuvac is a mycobacterium vaccine that has completed several Phase III trials in TB patients as an adjunct to first-line therapy. Also of note is Sequella’s SQ-109, an orally active ethambutol analog targeting the MmpL3 membrane transporter of trehalose monomycolate. It is currently in a pivotal Phase II/III trial in Russia to assess efficacy in patients with multi-drug-resistant TB.
Figure 3. Infectious diseases rare drug development. The ordinate indicates the count of drugs in active development for each disease.
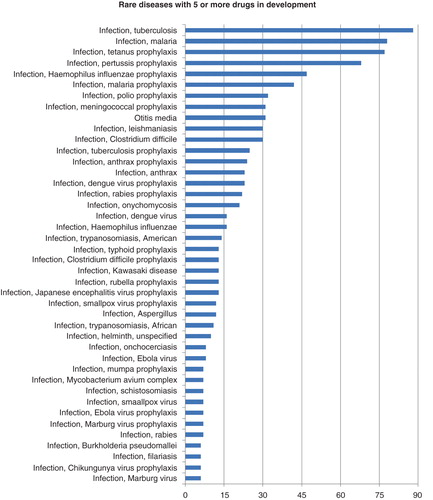
Among upcoming therapies for malaria (78 drugs in development) is artesunate, an artemisinin derivative originally developed by the Walter Reed Army Institute of Research and the US Army Medical Materiel Development Activity, and now licensed to Sigma Tau for development and manufacturing. It is awaiting approval in the US for severe, complicated Plasmodium falciparum malaria. It is available on a named-patient basis in certain EU countries Citation[3].
There are 77 drugs in development for tetanus prophylaxis, including Shantha Biotechnics’ Shan5, a pentavalent vaccine containing both tetanus and diphtheria toxoids, and under development for several other infectious diseases, including diphtheria, hemophilus influenza, hepatitis B and pertussis. It is in a Phase III non-inferiority trial in India in 1100 children and infants. Also showing promise for tetanus prophylaxis is GC-1107, a triple toxoid vaccine under development by Green Cross. Having completed Phase II trials for prophylaxis of tetanus and diphtheria, the vaccine is currently in a randomized, double-blind Phase II/III trial in 171 healthy children, to assess immunogenicity and safety.
3.2 The oncology landscape for rare diseases
Among the drugs in development for rare cancers, the most activity is for pancreatic and ovarian cancer, with 290 and 279 drugs in active development, respectively (). Roche’s bevacizumab, a blockbuster anti-VEGF mAb, has been on the market for non-rare cancers such as colorectal and non-small-cell lung cancer. It is also marketed in the EU countries as Avastin since 2011 for ovarian cancer, with a US filing expected to follow in 2013. Bevacizumab is in a European Phase III trial (AURELIA) in 361 patients with ovarian, fallopian tube or peritoneal cancer to assess its efficacy and safety in combination with chemotherapy Citation[3]. A further Phase III trial in patients with epithelial ovarian cancer is also ongoing Citation[4]. Avastin was also in development for pancreatic cancer; however, a Phase III trial investigating its efficacy in combination with standard chemotherapy agents did not meet the primary end points in terms of overall- and progression-free survival Citation[5].
Figure 4. Drugs in active development for rare cancer indications. The abscissa indicates the count of drugs by disease.
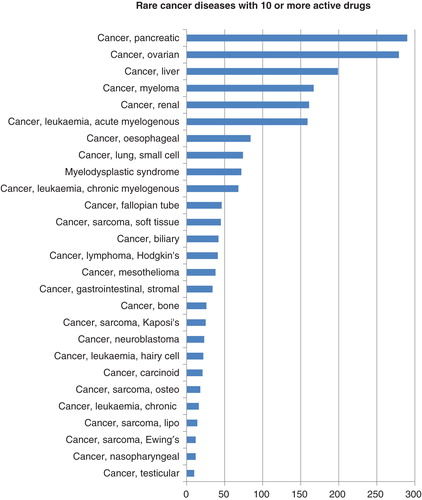
Amgen’s trebananib targets TIE-2 tyrosine kinase, and is being developed in partnership with Takeda and Dyax. Trebananib is currently in a Phase III trial (TRINOVA-2) in 380 women with ovarian, peritoneal or fallopian tube cancer to assess efficacy in combination with liposomal doxorubicin. Completion is expected in 2014 Citation[6]. The drug is also in Phase I trials for pancreatic cancer, as well as for gastrointestinal stromal tumors (GIST). Trebananib represents a good example of a targeted therapy that has shown potential across several rare diseases.
Another kinase inhibitor in the pipeline for rare cancers is Bayer’s regorafenib. Acting on a broad spectrum of kinase targets, the drug is already launched for GIST in Canada, Japan and the US, and is one of only three drugs to gain approval thus far for this rare cancer. It is currently in a Phase III trial (RESORCE) in patients with hepatocellular carcinoma, with completion expected in 2015 Citation[7]. Regorafenib is also in a Phase II trial for advanced renal cell cancer, where preliminary results have demonstrated a disease control rate of 81% Citation[8]. With activity against several rare cancers as well as already having achieved approval, regorafenib is another good prospect in the landscape for rare cancer indications.
Among drugs for some of the lesser pursued rare cancers, Takeda is developing alisertib, an orally available aurora kinase inhibitor. The drug is being investigated in a Phase II trial in neuroblastoma, a rare childhood cancer with an incidence of just 1 in 200,000 in the US. To date, there are only two drugs available for the treatment of neuroblastoma, these being Novartis’ teniposide and Baxter’s trofosfamide.
Also being investigated for neuroblastoma is erismodegib, under development by Novartis. The drug targets the hedgehog pathway. It is currently in a Phase II trial for several cancer indications, including neuroblastoma and other rare childhood cancers, including rhabdomyosarcoma and osteosarcoma. It was also in development for Gorlin syndrome, but development was terminated during Phase III trials. If development continues as planned, erismodegib is shaping up to be a major player in the treatment of rare cancers, particularly those in children.
3.3 Major players in rare cancers
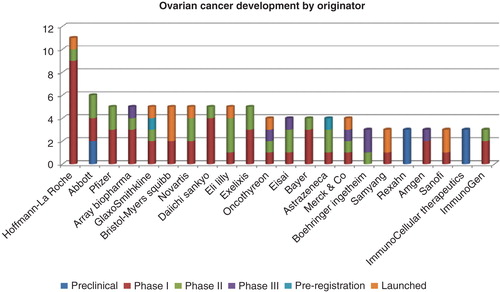
In ovarian cancer, Roche leads in terms of numbers of drugs, with 11 drugs in active development. This includes one launched drug, bevacizumab (see above), and pertuzumab, which is currently in Phase II for this disease (). A large proportion of Roche’s rare disease pipeline for ovarian cancer is currently in Phase I trials, including the phosphoinositide-3 kinase/mammalian target of rapomycin kinase inhibitor apitolisib, anti-IG1 antibody vesencumab, and the immunotoxin DMUC-5754A. Abbott has six drugs in active development; of these, volociximab and veliparib are the most advanced candidates. Volociximab, a chimeric monoclonal antibody targeting α5–β1 integrin has completed a Phase II trial in combination with liposomal doxorubicin, in patients with advanced epithelial ovarian or peritoneal cancer. Results showed partial responses and a median progression-free survival time of 193 – 221 days Citation[9,10]. Veliparib is an orally available PARP inhibitor under development by AbbVie, following its separation from Abbott. It has completed a Phase II trial in patients with ovarian, fallopian tube and peritoneal cancers.
Figure 5. Top 15 originator companies developing drugs for ovarian cancer. For each company, a count of drugs by development phase is indicated on the ordinate.
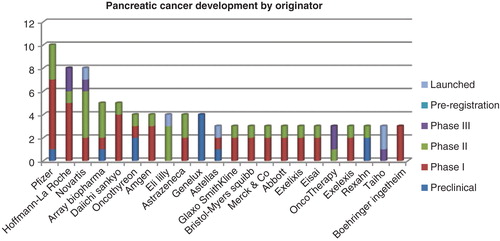
In the landscape for pancreatic cancer, Pfizer leads with a total of 10 active drugs, 6 of which are in Phase I and 3 in Phase II. Roche is in second-place, with eight drugs currently in active development (). Its most advanced candidate is the oral hedgehog pathway inhibitor, vismodegib. The drug is in a Phase II trial in patients with metastatic pancreatic cancer in combination with gemcitabine Citation[11]. Roche currently has five candidates in Phase I trials for pancreatic cancer. These include vesencumab, an anti-IG1 antibody that has completed a Phase I in advanced or metastatic solid tumors, including ovarian cancer. Also in the Phase I pipeline is DMOT-4039A, for which the mechanism of action is as yet undisclosed. The drug is in a Phase I trial in both pancreatic and ovarian cancer, with completion expected in 2015 Citation[12].
4. Recurring candidates
Among the drug landscape for rare diseases, there are some drugs that stand out in that they are under investigation for multiple rare diseases (). Not surprisingly, the greatest activity involves oncology drugs, with many companies focusing simultaneously on a number of rare cancer indications. The top drugs being tested for efficacy in rare diseases include: tivantinib, rigosertib, cabozantinib, everolimus and bevacizumab. ArQule’s MET tyrosine kinase inhibitor tivantinib is in development for 13 rare cancers; biliary, esophageal, non-small-cell lung cancer, Merkel cell carcinoma, mesothelioma, ovarian, liposarcoma, pancreatic, renal, soft tissue sarcoma, testicular, liver and oral cancer. The drug is currently in a pivotal Phase III trial in patients with inoperable hepatocellular carcinoma. Filings in several territories are expected in 2015 Citation[13]. The drug also has EU orphan drug status for soft tissue sarcoma.
Figure 7. The most novel drugs that are in development for the most rare diseases. For each drug candidate, a count of rare diseases in active development is found on the ordinate.
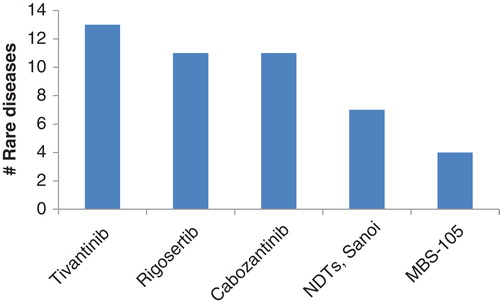
Another drug showing promise in rare diseases is Oncanova’s rigosertib. This small-molecule polo-like kinase inhibitor is in development for 11 forms of rare cancer, the most advanced indications being chronic myelomonocytic leukemia, pancreatic cancer and myelodysplastic syndrome, all of which are in Phase III trials. The drug also boasts Phase I development for leiomyosarcoma.
Another kinase inhibitors, Exelixis’ cabozantinib is also in development for a number of rare cancers. Having recently been launched in the US for thyroid cancer, this spectrum-specific kinase inhibitor is in active development for 10 rare cancers. The most advanced of these are liver, bone and renal cancers, for which Phase III trials are ongoing. Earlier stage trials for other rare cancers, including GIST and leiomyosarcoma, are also underway, giving the drug a potentially diverse use across a wide range of rare cancers.
With infectious disease being a far second to oncology for development of drugs for rare diseases, several drugs again stand out as being major players. Sanofi, in collaboration with the Drugs for Neglected Diseases initiative, has a project to develop therapies for several rare infectious diseases, including both American and African trypanosomiasis, filariasis and onchocerciasis Citation[14]. In total, seven rare infectious diseases are under investigation, with the project currently in early preclinical development.
Microbion’s MBS-105 also features here, focused on the prophylaxis and treatment of TB and anthrax. The broad spectrum antimicrobial is intended for use against Gram-negative pathogens in the bio-defense space, and is also under investigation for Brucella and Yersinia pestis infections. Development is currently at the lead series stage, and Microbion is seeking partners for commercialization Citation[15].
This ‘promiscuity’ of certain drugs is particularly apparent in oncology, where multiple drugs are in development across a range of rare cancer indications. This can be explained if we consider mechanisms of the drugs in question. It stands to reason that drugs developed to attack a particular disease pathway will later be found to be active against other diseases with similar etiology. Accordingly, the most promiscuous drugs for rare cancer indications include specific disease pathway-targeting drugs such as mitogen activated kinase and polo-like kinase inhibitors, as well as specifically targeted monoclonals. Conversely, more traditional cancer drugs such as classical cytotoxics do not feature heavily. Therefore, it would seem that the drive toward developing drugs for rare diseases could be happening in tandem with a move within the pharma industry toward developing mechanistically targeted drugs with activity in multiple diseases within a particular therapeutic space.
5. What’s happening elsewhere?
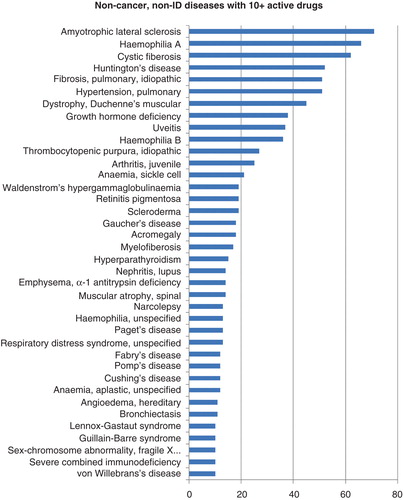
Beyond infectious diseases and oncology, 38 rare diseases can count 10 or more drugs in active development ().
In terms of drugs in development, ALS boasts 71 drugs in active development. Despite this, there is currently only one drug on the market to treat ALS: Sanofi’s riluzole. This glutamate antagonist was launched for ALS in 1996, for which it gained US orphan drug status. It is now widely launched for the disease worldwide. In Phase III for ALS is free radical scavenger edaravone, under development by Mitsubishi Tanabe Pharma. Already launched in Japan for cerebral infraction, edaravone is currently in a Japanese Phase III trial in ALS patients Citation[16].
Another non-cancer rare disease with substantial drug development activity is cystic fibrosis (CF). The multi-system genetic disorder currently has 66 drugs in active development, including 4 drugs on the market. These include Genentech’s aerosolized DNase therapy, which was first launched in 1994 and is now in widespread use, as well as Dompe’s Flutifort, a carbocysteine lysine salt that has been available since 1995. More recently launched drugs for CF include ivacaftor, developed by Vertex Pharmaceuticals. The drug targets the source of CF directly by potentiating the gating activity of the defective chloride channel behind the disease, cycstic fibrosis membrane conductance regulator (CFTR) Citation[17]. Currently launched in several markets, ivacaftor represents an innovative therapy for the treatment of CF. Vertex Pharmaceuticals is also developing a combination product, combining ivacaftor with another CFTR-targeting drug, lumacaftor. A Phase III program is currently underway, with several further Phase III trials in CF patients planned. The active development of ivacaftor and lumacaftor now makes Vertex Pharmaceuticals a major player in the future of CF treatment.
Drug development outside oncology and infectious diseases spans a wide range of rare diseases. Other diseases under investigation to a lesser extent include Sjögren’s syndrome. To date, just one drug is available for this autoimmune disease. Cevimeline is a muscarinic agonist and has been launched since 2000. In clinical development for the disease now is belimumab. Originally developed by Human Genome Sciences (now GlaxoSmithKline), the anti-B-lymphocyte stimulator antibody completed a Phase II proof-of-concept trial in patients with Sjögren’s syndrome.
6. Conclusion
Since the blockbuster days of the 1990s, drug development and the pharma industry have undergone a big change. As Novartis’ CEO Joseph Jimenez recently put it, ‘the definition of a blockbuster is changing’ Citation[18]. The proposed solution is to produce drugs for smaller disease segments rather than chasing big markets. Examining the market picture for rare diseases, it seems that adoption of this strategy is already well underway. Progress in genomics and biomedical science over the past 20 years now means that the molecular basis of diseases is far better known, giving companies a better ‘roadmap’ to develop molecular targeted drugs, which means rare diseases can be addressed much more effectively. Today, the genetic etiology of 4500 diseases is known, compared to 50 such diseases 20 years ago.
For the giants of Big Pharma, the area of rare diseases represents a less-tapped resource and opportunities to turn their established drugs against diseases for which there is an unmet need, drawing on previously proven safety and efficacy data. As previously mentioned, rare diseases are of low in prevalence but not impact on those affected. Targeting rare diseases, often with existing drugs, allows pharma companies to further increase market penetration. There are other reasons behind companies’ wishes to develop drugs for diseases of low prevalence. In the US, the Orphan Drug Act of 1983 sought to bolster development for orphan drugs by offering incentives, including a 7-year period of market exclusivity following launch (regardless of patent life), a waiver on FDA fees and a 50% tax credit on clinical studies. Similarly in the EU, Regulation 141/2000 offers 10-year market exclusivity, with tax credits offered by individual EU markets. These incentives have had the desired effect, with the number of treatments for rare diseases rising from 10 to over 400 in the period since the legislation was passed. The Prescription Drug User Fee Act also leverages development of drugs for rare diseases, making it possible for drugs to gain priority review status. Priority review status reduces FDA review time from 10 to 6 months, potentially decreasing the time to approval. Faster approvals for such drugs may reduce time-to-market, allowing a drug targeting a rare disease to gain a market foothold Citation[19,20]. Add to this, the 2012 FDA Safety and Innovation Act that has made it easier for drugs for rare diseases to progress through clinical trials, and the legislative framework with its ‘developmental drivers’ is now much more conducive toward development of drugs for rare diseases than in decades past. This has translated into the market, with global sales of orphan drugs increasing nearly 10% a year between 2005 and 2011, resulting in over 400 approvals for orphan drugs in the US and the EU. The outlook for drug development in rare diseases is inherently linked to drug development as a whole. Pharmaprojects data show the highest activity in terms of drugs for rare diseases to be within oncology. This is perhaps testament to the changing strategy within the pharma industry. Cancer by definition represents a huge market with high commercial gain, meaning that drug development activity in general is high. This provides a high starting ‘pool’ of targeted anticancer drugs, which once proved safe and efficacious, can then be directed against rare forms of cancer. However, among the 100 cancer diseases included in Pharmaprojects, a significant number, 42, fall within the definition of rare diseases, and able to benefit from regulatory bodies’ orphan drug acts. For other less prevalent disease areas where the commercial incentives for drug development may be lower, there may be fewer drugs in development that can be targeted against the rare diseases in these therapeutic spaces, and development for rare diseases may depend on standalone targeting of a rare disease rather than repurposing of existing drugs. The knock-on effect of this may be that development for rare diseases may be less.
7. Expert opinion
In recent times, there has undoubtedly been an increase in drug development for rare diseases. With the seeming demise of the traditional ‘market conquering’ blockbuster drug and advances in biomedical science and genomics, the concept of significant return on investment in the rare diseases market is conceivable. The regulatory concessions incumbent with development for a rare disease would mean that subsequent development for other diseases, whether rare or non-rare, would not incur the further costs of additional safety data. However, the relatively low prevalence and high costs associated with clinical trials and approvals in multiple countries make the prospect of a true blockbuster drug centered purely on rare diseases a debatable one. While increased development is promising, the flip side of this increased development drive and the raised profile of rare diseases is the continuing trend of pharma companies seeking to command very high prices for such drugs, propagated by the often life-threatening nature of these diseases and the few alternatives available in terms of drug therapy. Development of orphan drugs may be on the rise, but the final decision on whether these new drugs are funded lies with individual health care systems and providers, and government programs. While the benefit of such orphan drugs in terms of quality of life and in many instances, life-saving potential cannot be denied, several new orphan drugs approved in 2013 were priced at least $150,000 per patient per year, with three of these coming in at an annual cost of $300,000. While this is often comparable to the high cost of other blockbuster drugs (anticancer biologics, for example), the cost of these orphan drugs may be considered too high to sustain due to the lower prevalence of the diseases being treated, even though in some cases the benefits to patients may be comparable or even greater than that seen for drugs treating non-rare diseases. At a time when there is global pressure to reduce the economic cost of health care, governments and health care bodies are now starting to question reimbursement of expensive orphan drugs, the overall cost of which must be spread over a relatively small patient population. This reluctance to reimburse these drugs may in the end slow the continued advance of the ‘bull’ rare diseases market.
Progress in systems biology has shown that ‘pathways’ are not siloed, but rather their component enzymes, metabolites or signaling messengers participate/converge in multiple functionalities. As genomics, systems biology and translational medicine continue apace, repurposing of rare disease therapies may be possible, and perhaps help drug developers recover their research costs across wider patient populations. But until this happens, the economics of pricing for rare diseases is at risk.
In summary, the previously neglected rare disease market has seen somewhat of a revival in terms of drug development, owing to advances in biomedical science and genomics, and also in part to the financial and regulatory incentives available to pharma companies who set out to develop drugs for these conditions. This is good news for the thousands of patients worldwide who suffer from these often life-threatening or life-limiting diseases. But there is a threat to the longevity of this trend. At a time when confidence in the financial markets is still recovering from the worldwide economic downturn, government health care bodies and regulatory agencies are placing increasing constraints on pricing of these drugs, that may prove to be a limiting factor in the continued growth in drug development for rare diseases.
Article highlights.
The drug development landscape for drugs to treat rare diseases is robust, with the highest activity focusing on rare cancer disorders. The most frequently targeted diseases include pancreatic cancer, ovarian cancer, liver cancer, myeloma and renal cancer.
Fifteen percent of all rare diseases (55) have 20 or more drugs in active development.
Companies developing these therapies very often target multiple rare cancer disorders simultaneously.
For the infectious disease therapy area, significant development for rare diseases targets several of the more common non-developed world conditions like tuberculosis and malaria.
The need for new therapies for rare diseases is driving high prices, but this may not be sustainable long-term, given the measures increasingly put in place by health care bodies and government departments.
Declaration of interest
Both authors are employees of Citeline (an informa business). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
This box summarizes key points contained in the article.
Bibliography
- Rare Disease Day. Available from: www.rarediseaseday.org/article/what-is-a-rare-disease
- Citeline®. Pharmaprojects. 2013. Available from: www.citeline.com/products/pharmaprojects/[Last accessed 4 October 2013]
- ClinicalTrials.gov. Available from: http://clinicaltrials.gov/ct2/show/NCT00976911
- Roche investor. 2010. Available from: www.roche.com/investors/ir_update/inv-update-2010-10-11b.htm [Last accessed 8 May 2014]
- Roche press release. 2010. Available from: www.roche.com/media/media_releases/med-cor-2010-02-23.htm [Last accessed 8 May 2014]
- ClinicalTrials.gov. Available from: http://clinicaltrials.gov/show/NCT01281254
- ClinicalTrials.gov. Available from: http://clinicaltrials.gov/ct2/show/NCT01774344
- Press releases, Bayer, 30 May & 22 Sep 2009
- 20th EORTC-NCI-AACR Symp Molec Targ Cancer Ther; Geneva; 2008; Abs 512
- 45th ASCO; Orlando; 2009: Abs 5560
- ClinicalTrials.gov. Available from: http://clinicaltrials.gov/show/NCT01064622
- ClinicalTrials.gov. Available from: http://clinicaltrials.gov/ct2/show/NCT01469793
- Kyowa Hakko Kirin interim results. Available from: http://v4.eir-parts.net/v4Contents/View.aspx?template=ir_material_for_fiscal_ym&sid=6441&code=4151 [Last accessed 13 May 2014]
- DNDi. 2014. Available from: http://dndi.org/diseases-projects/diseases.html
- Microbion pipeline. 2011. Available from: www.microbioncorp.com/health/pipeline/
- ClinicalTrials.gov. Available from: http://clinicaltrials.gov/ct2/show/NCT00424463
- Press release, Vertex. 2012. Available from: http://files.shareholder.com/downloads/VRTX/3028312001x0x538345/514a2201-5b2f-4c48-ba96-944d7ae49ace/VRTX_News_2012_1_31_Webcasts.pdf [Last accessed 13 May 2014]
- CNN Money News story ‘Novartis CEO: we need to re-think the blockbuster. Available from: http://management.fortune.cnn.com/2013/03/04/novartis-ceo-joseph-jimenez/ [Last accessed 8 May 2014]
- FDA. Fast track, breakthrough therapy, accelerated approval and priority review. Available from: www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/SpeedingAccesstoImportantNewTherapies/ucm128291.htm#priorityreview [Last accessed 8 May 2014]
- FDA. Prescription Drug User Fee Act (PDUFA). Available from: www.fda.gov/forindustry/userfees/prescriptiondruguserfee/default.htm [Last accessed 8 May 2014]