Abstract
Genethon is a non-profit pharmaceutical R&D organization with the mission of developing innovative gene therapy treatments for patients affected with rare diseases. It was created by the patient organization AFM-Telethon in 1990, which has since been the source of most of its funding, thanks to the donations received during the yearly French Telethon. Genethon was a pioneer in the field of modern genetics after establishing the first maps of the human genome in the 1990s; it is now a pharmaceutical organization whose activity spans from upstream research to clinical development, encompassing innovative bioprocess development and large-scale GMP-manufacturing. It leads a diversified pipeline of products both at clinical and preclinical stages in the field of neuromuscular diseases, primary immunodeficiencies, retinal and liver diseases. The development of Genethon illustrates a unique example of patient-empowered research where a private non-profit structure, funded thanks to public generosity, favors highly innovative developments centered on the needs of patients.
1. The founding patient organization, AFM-Telethon
In order to understand the specific positioning of Genethon in the field of biomedical innovation for rare diseases, one must first look at the history of AFM-Telethon, which it was founded by. This patient organization was born in 1958 on the initiative of a group of nine families of children affected by Duchenne muscular dystrophy. This disease is characterized by a generalized muscle degeneration, leading to loss of the ability to walk between the ages of 6 and 13 years and the decline of all muscle functions, almost systematically resulting in death during adolescence or early adulthood. The scientific community showed little interest in this disease since its detailed description by the French physician Guillaume Duchenne during the second half of the nineteenth century.
The objective of AFM since its very inception has been to create a momentum for mobilization in order to improve the lives of patients and to encourage the search for therapies through an active collaboration between the families and the medical research community Citation[1].
After nearly 30 years of existence, two landmark events occurred in the history of the association. First, in 1986, the identification of the gene mutated in patients with Duchenne muscular dystrophy Citation[2] enhanced AFM’s belief that genetic research could provide a basis for therapeutic innovation. Second, the success of the collection of funds after the first French Telethon organized by the Association in late 1987 provided the means to finance an ambitious research effort. Well beyond the wildest dreams of the organizers, the first Telethon raised more than 29 million euros. In the following years, the Telethon became more and more successful with yearly donations increasing, up to a maximum of 106 million euros in 2006.
With these funds, AFM-Telethon had the means to define and lead its own research and development policy, with the conviction that the treatments could only result from a long series of significant scientific advances, requiring a long-term commitment. AFM also became convinced that beyond the financial support for existing academic laboratories, it would be necessary to establish a laboratory at the forefront of technology dedicated to the discovery of genes for rare diseases. Thus was created the Genethon laboratory, following the first discussions in 1988 between the President of the AFM at the time, Bernard Barataud, and geneticist Daniel Cohen. This laboratory was conceived as a ‘gene factory’. Also, and importantly, the therapeutic scope of the AFM from this moment would not be restricted to Duchenne muscular dystrophy, but would encompass rare genetic diseases at large.
Genethon was created in December 1990 in Evry (southern suburbs of Paris), as a non-profit association with the statutory missions to participate in localizing and identifying the genes responsible for diseases, including neuromuscular diseases, and to carry out scientific research programs applied to the development of new therapies for rare genetic diseases.
Under the scientific direction of Daniel Cohen and Jean Weissenbach, 6 months after opening Genethon had about 100 employees and featured industrial capacity of massive robotic technologies. Funding was about 85% from AFM, with the rest from government subsidies. The strategy chosen led Genethon to establish the first physical map of a human chromosome Citation[3], a first physical map of the human genome with coverage of 40% Citation[4], followed by a map covering 90% of the genome Citation[5]. In parallel, the first genetic map based on micro-satellites with 813 markers was published in 1992 Citation[6], followed by two others comprising respectively 2066 and 5264 markers Citation[7,8]. These maps became fundamental tools for the entire scientific community, and some of the data was deposited at UNESCO during an official ceremony in 1992.
2. From maps of the genome to clinical trials
Since the creation of Genethon, genetics was envisioned as a tool for the development of therapeutic strategies and not as a goal in itself. Because of this, starting in 1997, activities in genetics gradually ceased at Genethon or were transferred to the public sector and Genethon started moving towards the use of data derived from the genome to develop gene therapy approaches. Under the scientific direction of Olivier Danos, Scientific Director from 1997 to 2005, the laboratory has been involved in the field of vectorology, acquiring the tools and know-how necessary for the production of recombinant virus technology platforms, and evaluation in in vitro and in vivo disease models. Genethon made these platforms available to the scientific community through a program of large-scale distribution of gene therapy vectors through its Gene Vector Production Network, which has provided academic laboratories with >3000 recombinant vector batches for >80 disorders.
However, the transition from gene transfer approaches tested in animals to gene therapy drugs validated in patients in clinical trials required the establishment of a genuine translational and pharmaceutical R&D organization. Such an organization had to take into account the difficulties inherent in preclinical and clinical development of gene therapy products (for which to date only one, Glybera has received market authorization in Europe, none being yet approved in the USA); the complexities of production that today has not yet reached the stage of industrial maturity for gene therapies; the regulatory complexities that have arisen due to the specific mode of action of these products; and finally the difficulties in developing a drug for the targeted pathologies that are rare or ultra-rare and require demonstration of therapeutic effects in cohorts of patients very limited in numbers.
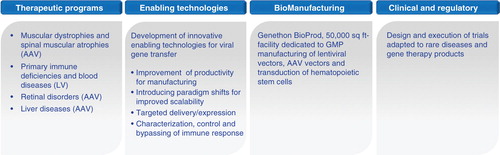
Under these conditions, the possibility of bringing such products to the clinic and ultimately ensuring their registration with regulatory agencies depended on the ability of Genethon to integrate multiple levels of innovation (): i) therapeutic innovation, that allows to design products adapted to specific diseases; ii) technological innovation, particularly in the field of bioprocess that allows development of methodologies for production, purification and characterization at scales consistent with pharmaceutical requirements; iii) bioproduction itself according to pharmaceutical standards, as the available capacity to date at the global level in this area is limiting; and iv) specific and intimate understanding of the issues and regulatory constraints for gene therapy products and the ability to participate in the creation of standards together with regulatory agencies.
Taking into account these necessary evolutions, Genethon, whose scientific director is today Fulvio Mavilio, now gathers in its laboratories nearly 200 scientists and experts, regrouped in the departments of therapeutic innovation, technological innovation, bioproduction and medical and regulatory affairs. Its operating budget in 2013 was €31.4 million, coming mainly from AFM- Telethon. In the field of bioproduction, Genethon has opened in 2013 a new fully GMP-compliant production facility, Genethon BioProd, which, with its 5000 m2 (∼ 50, 000 sq ft) of production suites and control laboratories, is one of the largest platforms in the world dedicated specifically to the production of gene therapy vectors under GMP standards.
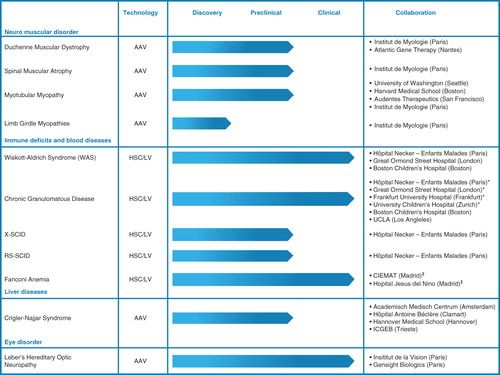
Genethon’s pipeline of therapeutic projects () includes muscle diseases (Duchenne muscular dystrophy, spinal muscular atrophy, myotubular myopathy, limb-girdle muscular dystrophy), genetic immunodeficiencies (Wiskott–Aldrich, chronic granulomatous disease, Fanconi’s anemia, X-linked severe combined immunodeficiency), pathologies of the blood, liver and retina. These projects stem from either internal research themes such as those involving the development of exon skipping therapy for Duchenne muscular dystrophy Citation[9], treatment of myotubular myopathy Citation[10] (and references therein), limb-girdle muscular dystrophy Citation[11-14] (and references therein), immunodeficiencies (Citation[15] and references therein Citation[16]) or from collaborations with academic or industrial partners in Europe, the USA or Japan.
As already mentioned, bioprocess development and bioproduction are also important components of the R&D activities at Genethon, with the aim of developing methods for production adapted to industrialization of gene therapy vector manufacturing for each project.
3. Today and tomorrow: a non-profit patient-centered pharmaceutical R&D organization
Through its successive transformations, Genethon has evolved from a pioneer in the field of genetics to a major player in the field of gene therapy for rare diseases. These developments and results were made possible by the specific governance of Genethon, a private non-profit structure with a board of director controlled by an association of patients.
The strategy of Genethon has always been measured by reference to roadmap provided by the AFM-Telethon based on the needs of patients for the emergence of treatments, without the constraints attached to expectation for financial return. The ‘return on investment’ expected by AFM-Telethon is rather measured in terms of scientific, technological and concrete clinical results and is regularly evaluated by an independent panel of experts. Scientifically, Genethon has to its credit > 1000 publications, and its current pipeline as of today consists of 14 projects, four at clinical stage as of 2014 (Genethon is sponsor of two of these trials, for Wiskott–Aldrich syndrome and chronic granulomatous disease that are being performed in European and US clinical centers). Based on preclinical studies in progress, Genethon could be involved either as a sponsor or as a partner in eight clinical trials in 2016.
Although the operational structure of Genethon resembles that of a biotech or of an R&D unit of pharma, its approach to innovation is quite different. To meet the needs of patients, Genethon’s mission is by necessity to explore highly innovative therapeutic approaches, to overcome significant scientific and technological obstacles, and to undertake projects for which the result is inherently uncertain. This willfully risk-taking posture is for Genethon and its parent patient organization a necessary prerequisite for the delivery of high-value therapies to patients. It differs from conventional strategies of pharmaceutical corporations that, focused on short-term revenues, are more generally risk-averse Citation[17].
Through its pipeline of projects, the goal of Genethon now is to provide patients with drugs that will have received marketing authorization. Some of these drugs will undergo registration based on Genethon’s own resources; in other cases, partnerships are built with private entities under the condition that through their own finances and their skills, they can then accelerate product development for the benefit of patients (as in the case of the ongoing developments of therapies for Leber’s hereditary optic neuropathy and myotubular myopathy).
Beyond the involvement of Genethon in the field of rare diseases, it is also important to realize that research led by Genethon contributes in developing tools and methodologies that will undoubtedly prove useful for the treatment of frequent disorders. Gene therapy does indeed represent a promising therapeutic option for several frequent disorders such as cancer, heart failure, neurodegenerative and metabolic diseases Citation[18].
In conclusion, Genethon’s path illustrates a striking example where the combat of a patient organization has turned into innovative science and therapeutic innovation, in the general interest.
Declaration of interest
The author is CEO of Genethon who funded this paper. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgements
The author wishes to acknowledge the support of Susan Cure in preparing this manuscript.
Notes
Bibliography
- Guthleben D, Le Faou O. Une course pour la vie, L’AFM et la recherche biologique et médicale. Armand Colin; Paris: 2011
- Monaco AP, Neve RL, Colletti-Feener C, et al. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 1986;323(6089):646-50
- Chumakov I, Rigault P, Guillou S, et al. Continuum of overlapping clones spanning the entire human chromosome 21q. Nature 1992;359(6394):380-7
- Bellanné-Chantelot C, Lacroix B, Ougen P, et al. Mapping the whole human genome by fingerprinting yeast artificial chromosomes. Cell 1992;70(6):1059-68
- Cohen D, Chumakov I, Weissenbach J. A first-generation physical map of the human genome. Nature 1993;366(6456):698-701
- Weissenbach J, Gyapay G, Dib C, et al. A second-generation linkage map of the human genome. Nature 1992;359(6398):794-801
- Gyapay G, Morissette J, Vignal A, et al. The 1993-94 Généthon human genetic linkage map. Nat Genet 1994;7(2 Spec No):246-339
- Dib C, Fauré S, Fizames C, et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 1996;380(6570):152-4
- Goyenvalle A, Vulin A, Fougerousse F, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 2004;306(5702):1796-9
- Childers MK, Joubert R, Poulard K, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med 2014;6(220):220ra10
- Richard I, Broux O, Allamand V, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995;81(1):27-40
- Fougerousse F, Bartoli M, Poupiot J, et al. Phenotypic correction of α-sarcoglycan deficiency by intra-arterial injection of a muscle specific AAV1 serotype vector. Mol Ther 2007;15(1):53-61
- Roudaut C, Le Roy F, Suel L, et al. Restriction of calpain3 expression to the skeletal muscle prevents cardiac toxicity and corrects pathology in a murine model of limb-girdle muscular dystrophy. Circulation 2013;128(10):1094-104
- Herson S, Hentati F, Rigolet A, et al. A phase I trial of adeno-associated virus serotype 1-γ-sarcoglycan gene therapy for limb girdle muscular dystrophy type 2C. Brain 2012;135(Pt 2):483-92
- Charrier S, Stockholm D, Seye K, et al. A lentiviral vector encoding the human Wiskott-Aldrich syndrome protein corrects immune and cytoskeletal defects in WASP knockout mice. Gene Ther 2005;12(7):597-606
- Galy A, Thrasher AJ. Gene therapy for the Wiskott-Aldrich syndrome. Curr Opin Allergy Clin Immunol 2011;11(6):545-50
- Moors EH, Cohen AF, Schellekens H. Towards a sustainable system of drug development. Drug Discov Today 2014. [Epub ahead of print]
- Kaufmann KB, Büning H, Galy A, et al. Gene therapy on the move. EMBO Mol Med 2013;5(11):1642-61