Abstract
Esophageal adenocarcinoma is one of the fastest rising cancers in Western society. Incidence has increased by 600% within the last 30 years. Rates of diagnosis and death run parallel due to the poor prognosis and a lack of effective treatments. Potentially curative treatments are followed by high rates of disease recurrence. For the majority of patients, who present with advanced disease, we have no effective treatment. We discuss the key areas of progress in this demanding field and offer our views on the direction of future research and treatment.
The esophageal adenocarcinoma epidemic
Worldwide, esophageal cancer killed 395,000 people in 2010, an increase of nearly 15% from 1990 Citation[1]. Despite modern therapies, overall 5-year survival is less than 15% Citation[2]. The two dominant histological subtypes are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). The age-standardized incidence rates of EAC are rising by just under 40% every 5 years in Western countries, and EAC has become the predominant type of esophageal cancer in these areas Citation[3]. EAC arises in Barrett’s esophagus (BE), a metaplasia of the distal esophagus in response to chronic inflammation due to acid and bile exposure Citation[4,5]. The UK has the highest incidence of EAC globally, at up to 8.7 cases/100,000 of the population Citation[6], with Caucasian males being the most commonly affected ethnic group Citation[7].
Historically, ESCC of the thoracic esophagus, driven by smoking and alcohol, was more common than EAC, and this remains the case outside Western populations Citation[8]. However, countries such as Japan are beginning to observe rising rates of EAC, likely due to the ‘westernization’ of lifestyle risk factors.
Outcomes for EAC are poor because 60–70% of patients present with late-stage disease, too advanced for treatment with curative intent Citation[9]. This is partly due to the mechanically compliant esophageal anatomy, which allows symptomless tumor expansion Citation[10]. Additionally, tumor invasion and metastasis are facilitated by the absence of an outer serosal layer and the presence of a rich lymphatic plexus, and both depth of invasion and nodal metastasis are prognostic Citation[11]. Improving survival rates will require an increased understanding of the molecular mechanisms regulating these processes.
Even in those patients who are suitable for multimodal therapy (chemo/radiotherapy + surgery), 5-year survival is at best 35% Citation[9], and potentially curative treatment pathways are demanding for patients, their carers and the healthcare system.
In this review, we focus on current and future developments in the diagnosis and management of EAC. We have chosen to concentrate on the nonsurgical aspects of EAC: treatment of gastroesophageal reflux disease (GERD) and prevention of EAC, early diagnosis, endoscopic therapies for early-stage disease and advances in systemic therapies, because we believe these areas offer the greatest potential to impact on outcomes for this disease. Surgery will remain central to the treatment of esophageal cancer for the foreseeable future and the many recent advances, particularly in dramatically improved mortality rates have been reviewed in detail elsewhere Citation[12].
Heartburn cancer
EAC is predominantly a disease of middle-aged/elderly white men. EAC is caused by longstanding GERD Citation[13], and it is unsurprising that risk factors predisposing to GERD, obesity, hiatus hernia, tobacco smoking and medications compromising lower esophageal sphincter (LES) function are associated with EAC Citation[14]. Western diets high in fat, calories and cholesterol but low in antioxidants and fiber confer an increased risk of EAC Citation[15]. However, in Western countries, GERD occurs in up to 40% of the population at some point Citation[16], and it was the most common diagnosis for gastrointestinal disorders in the US outpatient clinic with almost 9 million attendances in 2009 Citation[17], but very few of these people develop EAC. We are yet to understand why only a minority of people with GERD will ever develop EAC, but evidence from next-generation sequencing studies confirms a causal link between the two. Dulak and colleagues have performed exome sequencing of 149 EAC tumor-normal pairs and whole genome sequencing of a subset (n = 15), finding a mutational spectrum unique to EAC (A > C transversions, predominantly in noncoding areas and less-expressed genes, especially at AAG trinucleotides), suggesting that it is attributable to GERD and possibly as a result as oxidative damage Citation[18]. New insights, such as these, into the genetic landscape of EAC will make it possible to determine the precise carcinogens responsible for mutagenesis. For example, is gastric acid the driver of genome-wide A > C transversions? Or, as has been suggested previously, are bile salts required for this effect Citation[19] and is mutation in esophageal epithelial cells a direct effect of the refluxate or mediated by the inflammatory response in the microenvironment? In vitro and in vivo experiments to answer these questions are now required and are important when one considers the relationship between the rise in proton-pump inhibitor (PPI) use and EAC.
Chronic GERD is thought to lead to a columnar metaplasia, commonly of intestinal phenotype, of the distal esophagus – BE, and this is the only known precursor lesion for EAC with an overall association of up to 97.4% Citation[20]. However, the majority of patients with BE will not die from EAC with the risk of progression being between 0.12–0.5% per year Citation[21]. The association between GERD, BE and EAC is well known, and therefore the development of invasive cancer is, at least in theory, preventable.
Can we prevent EAC?
Current evidence does not support prophylactic intervention at the GERD stage to prevent progression to BE and/or EAC. This is somewhat surprising, but can be explained, at least in part, by the low incidence of EAC arising in patients with GERD and the presumed length of time the distal esophagus has to be exposed to reflux for cancer to develop. Many patients with EAC have experienced GERD for several years (often decades), and trials of chemoprevention or anti-reflux surgery require large numbers and long follow-up Citation[13]. These studies have not been performed.
Will treating GERD lower the rising rates of EAC?
If EAC is caused by acid reflux, then adequate acid suppression with medical therapy or antireflux surgery should prevent it. However, current evidence does not support this, why not?
PPI therapy is good at preventing acid reflux episodes, but weakly acidic refluxes are common in patients with BE on PPI and may account for the lack of regression of BE observed in these cohorts Citation[22]. In vitro and in vivo data support the view that weakly acidic bile reflux may be at least as damaging to the esophageal mucosa as acid reflux Citation[23–25], and we believe that this leaves clinicians in a dilemma about the advice they give to GERD patients who are keen to prevent BE or EAC. Observational data are contradictory, and PPIs are not without risk. After the introduction of PPI, multiple studies showed that PPI therapy did not lead to reliable regression of BE Citation[26]. Recently, longitudinal cohort studies of patients with BE have suggested that the use of PPI may be associated with a decreased risk of dysplasia in BE patients Citation[27]. In practice, PPIs are used in patients for both symptom control and the potential decreased risk of dysplasia despite the mechanism being unclear. Long-term PPI use has been thought to be safe, but recent concerns have been prompted over side effects such as interstitial nephritis, small intestinal bacterial overgrowth, pneumonia, osteoporosis and effects on vitamin and mineral absorption Citation[28].
Does antireflux surgery carry an advantage over medical therapy?
Antireflux surgery offers the theoretical advantage of not only preventing acid reflux, but also establishing a physical barrier to other elements of the refluxate including bile salts. A recent large meta-analysis of studies that compared the reported incidence of EAC in subjects with BE who underwent antireflux surgery with those who had medical management showed a lower cancer incidence rate in the antireflux group (3.8 cancers/1000 patient-years vs 5.3 cancers/1000 patient-years), but this was not significant (p = 0.29) Citation[29]. The authors concluded that antireflux surgery should not be recommended as an antineoplastic procedure. But, as the authors point out in their discussion, several potential sources of bias exist in their study, including the inclusion of cohort studies with no randomization, use of different types of antireflux surgery across studies, with some not reporting the surgery used at all, and perhaps most importantly, no reporting of the competency of the antireflux procedure to prevent reflux in the long term. This is important because observational studies suggest that the quality of the antireflux procedure is vital when considering subsequent cancer risk. In a review of 14,102 patients who received antireflux surgery in Sweden, Lofdahl and colleagues found that patients who developed EAC were three-times more likely to have recurrent reflux compared with those who did not (odds ratio: 3.1; 95% CI: 1.5–6.3) Citation[30].
In favor of antireflux surgery, there are a small number of reports that indicate that antireflux surgery may have a protective effect on the esophageal mucosa at the cellular level compared with PPI Citation[31,32]. A recent study by Martinez de Haro and colleagues found that at 3, 5 and 10 years after treatment, proliferation (as measured by Ki-67) and p53 expression remained stable in patients treated with antireflux surgery, whereas a significant increase was observed in patients taking PPI Citation[32].
In general, we believe that there is a lack of good data from appropriately designed trials comparing PPI and antireflux surgery for prevention of BE or EAC. When one considers that the average follow-up from all 34 studies considered in the meta-analysis from Corey and colleagues described above is 5–6 years and that EAC occurs predominantly in the seventh and eighth decade of life, probably after many years of reflux, it is too simplistic to say that there is no place for antireflux surgery in EAC prevention.
In our opinion, the patient numbers are too small and follow-up too short in all reported studies to reliably conclude that no difference between antireflux surgery and medical therapy exists. The theoretical advantage of antireflux surgery is strong, but it is not a procedure without risk and consequence. Current evidence does not support its widespread use as an anticancer procedure. But if less invasive procedures that prevent reflux with fewer side effects were to exist, then could these be effective at preventing EAC?
A number of new interventions to treat GERD are in varying stages of development, but none has been assessed for cancer prevention. EsophyX Transoral Incisionless Fundoplication uses T tags to create an endoscopic fundoplication. Cadiere and colleagues reported 1-year results from an EsophyX Transoral Incisionless Fundoplication trial in 86 patients. GERD health-related quality of life scores improved by 50% or more in 73% of patients. Complete cessation of PPI use was reported in 81%, but importantly only 37% of patients had normalization of esophageal acid exposure Citation[33]. This would be inadequate for the prevention or treatment of BE/EAC. The LINX® reflux management system has received widespread attention. It uses a bracelet of magnets encased in titanium, which is laparoscopically placed around the LES to create a ‘magnetic sphincter’. In a study of 100 patients, normalization or improvement of distal esophageal pH at 1 year was observed in 64% of patients Citation[34]. However, the LINX device was associated with a large number of device-related consequences including dysphagia in 68% of patients, requiring endoscopic dilatation in 19 patients and a 6% explant rate. Patients with the LINX cannot undergo MRI scanning.
In our opinion, the most promising new technology is the EndoStim™ LES stimulation system that delivers electrical stimulation to the LES. EndoStim has been shown to increase resting LES pressure without impairing LES relaxation, prevent acid reflux and eliminate GERD symptoms. Importantly, LES stimulation is not associated with any sensation or side effects and the stimulation can be tailored to the patient’s symptoms. In an open-label study, median percentage of 24-h esophageal pH <4.0 was reduced from 10.1 (7.8–13.0) to 3.3 (1.8–6.9); p < 0.001 Citation[35,36]. Good quality randomized trials with appropriate follow-up are now required to determine the efficacy of these devices for GERD treatment, before considering neoplasia prevention.
Aspirin in chemoprevention
Extensive observational data show that aspirin/NSAIDs are associated with a decreased risk of EAC Citation[37]. The exact mechanism of any chemopreventive effect is unclear, and no randomized controlled trial has confirmed that this observed association is causative. However, given the poor prognosis associated with esophageal cancer, the potential use of chemoprevention has been suggested in patients with BE. The AspECT trial is currently assessing the benefits of esomeprazole, with or without aspirin, in preventing progression of BE to EAC.
Promoting awareness
In addition to addressing the rising obesity levels and presumed consequent GERD in Western populations that is contributing to the EAC epidemic, a widespread program of education is now required. The general public has a poor understanding of the symptoms of EAC. This has been recognized by the UK Department of Health who is shortly to launch a pilot awareness campaign that will focus on the importance of persistent heartburn and dysphagia via the ‘Be Clear on Cancer’ campaign Citation[38]. To advance understanding of BE/EAC among the medical community, a new British Medical Journal online learning module is available Citation[39].
The challenge of early diagnosis
Early diagnosis saves lives
EAC presents late as symptoms only manifest when tumors become large enough to cause dysphagia or pain. The stage at diagnosis of EAC is the key factor in determining prognosis. The depth of invasion through the esophageal wall determines the risk of lymph node (LN) metastasis, which, in keeping with other reports, in our cohort is the most prognostic pathological finding for poor outcome Citation[40,41]. Unfortunately, in EAC, the majority of tumors have invaded through the esophageal wall at the time of diagnosis. In a cohort of 93 patients, Schlansky and colleagues showed that 79% and 10% of symptomatic new diagnoses of EAC were T3 (tumor invades adventitia) or T4 (invasion of local structures) tumors, respectively Citation[42]. In this setting, cure is rare.
Early EAC, defined as that limited to the muscularis mucosa (T1), does not normally metastasize to LNs Citation[43] and consequently, early-stage cancers, ≤T1, carry an excellent prognosis with a greater than 90% 5-year survival Citation[44]. Unfortunately, the number of tumors detected at this stage is low (<1%) Citation[42]. The challenge we face is how to detect EAC at an earlier stage, which would allow a better chance of survival and potentially more localized and less invasive endoscopic treatment. Intuitively, large-scale population-based screening might seem attractive.
Screening for EAC?
The diagnosis of EAC is currently made with white light endoscopy combined with biopsy for histological confirmation, and no alternative method has yet been validated. Despite high sensitivity and specificity, this is an invasive and expensive test with small but significant risk. Current endoscopy frameworks could not support national screening programs, which would be prohibitively expensive. These factors combined with the relatively low incidence of EAC do not currently justify population-based mass-screening programs. Is there justification for screening and surveying high-risk groups?
Identification & surveillance of BE
If BE is the precursor for EAC, then should we be screening for and monitoring those with BE? This may be particularly important given that the majority of patients with EAC present de novo with no previous history of BE.
Symptomatic GERD is common, but only 8–20% of patients with GERD have BE, and the incidence is similar in asymptomatic patients undergoing endoscopy Citation[45]. The diagnosis of BE is made by endoscopy and subjective histological assessment. It is subject to both sampling and reporter error. Endoscopic screening of patients with GERD for BE is not recommended by worldwide gastroenterology associations.
The American Society of Gastroenterology and the British Society of Gastroenterology guidelines support the surveillance of those with histologically confirmed BE. However, there is a lack of robust evidence for this practice, and the cost-effectiveness of routine surveillance in Barrett’s patients has been questioned Citation[46]. The benefits of surveillance in BE are currently being assessed in the BE Surveillance Study, a randomized trial comparing 2 yearly endoscopy and biopsy versus endoscopy at the time of need, as determined by the patient. The not insignificant issue of interval cancers (those occurring between 2 yearly routine endoscopies) will also be captured by this study and it may shed light on the optimum time interval for surveillance.
The Benign Barrett’s and Cancer Taskforce consensus group is seeking to formulate clear evidence-based factors to risk stratify BE. It is likely that a combination of patient-related factors (genetic predisposition, body mass, etc.), environmental factors and disease-specific factors (mutational burden in BE, dysplasia, etc.) will be combined to deliver more accurate risk prediction and targeting of surveillance and interventions for BE. Currently, the low risk of Barrett’s progression to EAC means that Barrett’s surveillance submits many patients to the psychological distress and physical discomfort of endoscopy with benefit for only a few. Is there an alternative to endoscopic surveillance and can we utilize biomarkers to predict those at risk of progression to EAC?
Cytosponge™: a viable alternative to endoscopy?
Cytosponge™ is an ingestible capsule that has shown early promise in the primary care setting. The capsule is swallowed with water, dissolving in the stomach to expand to a sponge like mesh 3 cm in diameter. This is withdrawn, harvesting cells for analysis by immunofluorescence for trefoil factor 3. Trefoil factor 3 is a marker highly specific for cells with an intestinal phenotype. It is surprisingly well tolerated by patients and compared with gastroscopy; the sensitivity and specificity of the test were 73.3% (95% CI: 44.9–92.2%) and 93.8% (91.3–95.8%), respectively, for 1 cm or more circumferential BE, increasing to 90.0% (55.5–99.7%) and 93.5% (90.9–95.5%), respectively for clinically relevant segments of BE 2 cm or more in length Citation[47]. It is currently undergoing further assessment in the BE Screening Trial 2. BE Screening Trial 2 is designed to compare Cytosponge™ and molecular analysis with endoscopy to determine the specificity and sensitivity of Cytosponge™, its differential sensitivity in dysplastic and nondysplastic BE and its ability to risk stratify patients with BE. It also aims to see if high throughput and automated analysis of Cytosponge™ specimens are possible.
Cytosponge™ is an evolving technology, but one we believe has the potential to change the way we diagnose and survey those with BE and EAC .
How do we identify the patients with BE who will progress to EAC?
The ability of biomarkers to select out those at greatest risk of progression and/or early cancers is an attractive proposition. In current clinical practice, dysplasia is the biomarker for risk of EAC progression, but this is subject to sampling error and interpretation bias. It does not help in the risk stratification of the majority with nondysplastic BE. Loss of heterozygosity, DNA content abnormalities, TP53 and Cyclin-A have been used to predict the risk of EAC progression in BE Citation[48,49]. All biomarkers that have progressed beyond Phase II studies can be linked to cellular proliferation, but none has entered clinical practice.
A possible explanation for this has recently become apparent. The traditional model of EAC development is via increasing cellular dysplasia after metaplasia in the distal esophagus (BE) in response to persistent acid reflux and inflammation. A step-wise accumulation of DNA mutations during these processes has been suggested to be required for the development of invasive cancer, akin to the adenoma–carcinoma sequence in colorectal cancer Citation[2]. In a recent exome-sequencing study by Agrawal and colleagues, of the 11 EAC samples and 2 adjacent areas of BE, the majority of somatic mutations observed in EAC were present in histologically normal BE Citation[50]. This discovery suggests that the mutational burden of EAC may already be established in nondysplastic BE Citation[18,50], meaning that the drivers for disease progression in BE–EAC lie beyond gene mutations in the epithelial cells. Suspicion must now fall on the esophageal microenvironment with the possibility that nonmutated stromal cells play a critical role in EAC development and progression, a theory that would be consistent with long-term acid and bile salt exposure leading to a proinflammatory milieu. This possibility is supported by the finding of a prognostic stromal signature identified by Saadi and colleagues in esophageal cancer patients that contained a predominance of inflammation- and TGF-b-related genes Citation[51].
Future directions in the early diagnosis of EAC
The holy grail of translational research in screening for EAC is to be able to see and assess the patient with GERD in the primary care setting, where an easy, cost-effective test can detect the presence of abnormalities and the risk of progression to EAC. We believe that the first step will be using technologies such as the Cytosponge™ as a screening tool to target endoscopy. This will require the chosen biomarkers to be highly sensitive (it is our opinion that trefoil factor 3 alone is not sufficient in this regard), which could be at the expense of modest specificity, as long as patients with positive results could rapidly access definitive endoscopy.
In addition, if a panel of validated biomarkers can be developed that can clearly define the stage boundaries between BE, high-grade dysplasia (HGD) and EAC, then the simplicity of Cytosponge™ can be combined with the ever increasing ‘horsepower’ of next-generation sequencing to offer near patient testing and rapid diagnosis in the BE–EAC sequence. Modern information technology opens the possibility of large-scale screening for GERD patients in the primary care setting and targeted selection of those who need further investigation with endoscopy, with the potential of cancer prevention. However, this is only to be recommended if acceptable and successful treatments for HGD and early EAC are available.
Early treatment
Early treatment aims for esophageal preservation with avoidance of esophagectomy and its associated morbidity and mortality. This can be achieved by novel endoscopic therapies that may be more acceptable to patients and more inclusive of those deemed medically unfit for esophageal resection. Some of these techniques are promising, but have, as yet, no long-term data.
Endoscopic therapies
Numerous endoscopic techniques are available for HGD and early EAC including: photodynamic therapy (PDT), laser, multipolar electrocoagulation, argon plasma coagulation, radiofrequency ablation (RFA), cryotherapy, as well as endoscopic mucosal resection (EMR). We believe that EMR in combination with RFA is the current endoscopic therapy with most promise.
All ablative techniques and EMR have the potential to miss occult EAC and leave behind cancer or intestinal metaplasia. This is reported after PDT in up to 51.5% Citation[52]. The significance is not yet determined, but EAC arising under squamous re-epithelialization has been reported in up to 4.6% of cases after PDT Citation[53]. EMR followed by RFA (EMR/RFA) has also been compared with stepwise radical endoscopic resection (SRER). They provide complete removal of intestinal metaplasia in 96% and 92% of cases respectively. In a randomized controlled trial comparing SRER and EMR/RFA, by van Vilsteren and colleagues, SRER required a median of six sessions including dilatation for strictures in 88% of cases, whereas EMR/RFA has a median of three sessions and 14% stricture rate Citation[54].
Despite convincing trial data and widespread uptake, it is not yet proven that ablative techniques can eliminate the risk of cancer, decrease the need for surveillance and be cost-effective. It would be sensible for ablative and mucosal resection techniques to be compared with modern series of esophagectomy for HGD or T1 cancer (including minimally invasive operative techniques) rather than using the morbidity and mortality of historical series covering all stages of disease.
Endoscopic therapy is attractive due to the presumed reduction in morbidity and mortality compared with surgery. The risk of under staging esophageal cancer and potential risk of occult EAC requires close assessment. Until robust trial data are available, it is important that the treatment for HGD and early neoplasia is discussed in a balanced fashion, and all data are entered into appropriate registries Citation[55].
High-grade dysplasia
Up to 59% of cases of HGD progress to EAC Citation[56]. Esophagectomy has been the treatment of choice for those fit for surgery with HGD; Williams and colleagues demonstrated a series of 38 patients, with HGD, treated with esophagectomy. There was no in-hospital or 30-day mortality, 100% disease-free survival (DFS) and 97% overall survival at 32 months Citation[57]. Occult adenocarcinoma was found in 29% of cases of HGD following resection.
Shaheen and colleagues showed complete eradication of HGD in 81% of patients at 1 year using endoscopic RFA Citation[58]. Three adverse events occurred in 284 treatments performed in 84 patients (mean 3.5 treatments per patient) and the stricture rate was 6%. Similar outcomes have been published from the UK National HALO RFA Registry with 81% of patients also clear of dysplasia at 1 year Citation[55].
The consensus of the Barrett’s Dysplasia Cancer Taskforce is that endoscopic treatment should be preferred to surgical treatment in most patients with HGD in BE Citation[59]. The high risk of EAC in HGD is an indication to stop surveillance and commence endoscopic treatment. RFA in combination with EMR, for raised or suspicious lesions, is the treatment of choice and is the current practice in our unit.
The treatment of T1 EAC
T1 tumors can be subdivided into T1a (invades lamina propria or muscularis mucosa) and T1b (invades the submucosa). Distinction is important because of the increased risk of LN involvement corresponding to the depth of tumor invasion. T1a tumors are associated with a risk of LN metastases of 5% or less; in T1b reported rates of LN metastases varies from 23 to 40% Citation[60,61]. This low risk of LN involvement, in combination with EUS and PET CT to improve accuracy of staging, means T1a EAC can be treated with endoscopic therapy. In the authors unit, it is our practice that, after counseling, patients with T1a EAC are offered EMR/RFA, while T1b EAC is treated with esophagectomy due to the risk of LN metastases.
In the future, it may be possible to offer EMR/RFA combined with sentinel LN biopsy (SLNB) to T1b patients. SLNB has become gold standard in surgery for breast cancer, and its use in other solid tumors remains debated. A recent study showed SLNB in esophageal cancer to have a sensitivity of 90% and specificity of 96% Citation[62]. Such a protocol would have the theoretical benefits of allowing us to predict which patients will benefit from esophagectomy and lymphadenectomy due to occult LN metastasis. This would spare those with localized disease a morbid operation. Advances in thoracoscopic skills will make SLNB for early-stage disease a viable option. While EMR/RFA could avoid esophageal resection and its associated risks, it must not compromise long-term survival.
Advances in neoadjuvant & adjuvant therapies
Neoadjuvant therapy
Neoadjuvant therapy followed by surgery is established as the gold standard in the management of patients with locally advanced adenocarcinoma of the esophagus/EGJ. In the UK, neoadjuvant chemotherapy (NAC) in conjunction with transthoracic esophagogastrectomy is the current standard of care for these patients Citation[63]. Proposed benefits of neoadjuvant therapy include: downstaging of the primary tumor Citation[64] and LNs Citation[65], an increase in the resectability of the tumor Citation[66], elimination of micrometastases Citation[67] and improved survival Citation[68]. However, the most recent meta-analysis to compare NAC versus surgery alone in 2062 patients suggests only 5.1% survival advantage at 2 years for patients treated with NAC for adenocarcinoma Citation[68]. NAC is demanding, may render the patient physiologically compromised and delays surgery; it does not benefit many.
It has been suggested that the UK lags behind the rest of the world in that as a medical community, we are yet to widely adopt neoadjuvant chemoradiotherapy (nCRT) in the neoadjuvant setting. In the recent Dutch CROSS trial, enrolling 368 patients, convincing evidence for the role of nCRT in esophageal cancer over surgery alone was put forward. However, the major benefit was seen for ESCC and not EAC. A direct comparison of NAC versus nCRT followed by surgery is now required for EAC. The All Ireland Cooperative Research Oncology Group is currently recruiting to a randomized clinical trial of neoadjuvant and adjuvant chemotherapy (MAGIC regimen) versus neoadjuvant chemoradiation (CROSS protocol) for patients with adenocarcinoma of the esophagus and esophagogastric junction. The trial design has received criticism because of a perception of inadequate power and the likelihood that few patients will complete the MAGIC regime; despite this, a number of UK centers are involved.
No matter what type of treatment is given, patients who have a significant pathological response to neoadjuvant therapy have consistently been shown to have an improved survival compared with poor responders Citation[69]. We have recently identified a hitherto unrecognized group of patients who derive benefit from NAC by virtue of LN downstaging despite little or no pathological response in the primary tumor. In this group, disease free survival (DFS) is increased from 1.114 years (95% CI: 0.961–1.2675) to 5.533 years (95% CI: 3.558–7.531); p < 0.0001 Citation[41].
But, for patients without a significant pathological or nodal response, the delay to surgery may outweigh the benefits of neoadjuvant therapy and these patients may be needlessly exposed to toxic treatments. Both NAC and surgery are associated with considerable morbidity and mortality Citation[70]. We urgently need mechanisms to predict response to neoadjuvant therapies at the patient level to allow optimal tailored treatment.
Predicting response to neoadjuvant therapy
We have proposed predictive markers of response to chemotherapy, and like many other groups have shown potential biomarkers Citation[40]. Serum albumin was shown to be the only predictor of pathological response to chemotherapy. Neutrophil–lymphocyte ratio is predictive of overall and DFS in EAC but not response to NAC. No biomarker has been robustly validated or has reached sufficient sensitivity or specificity to be of clinical value Citation[71].
The most promising method to assess response has been PET–CT. The Metabolic response evalUatioN for Individualization of neoadjuvant Chemotherapy in Esophageal and esophagogastric adeNocarcinoma II trial looked at tumor glucose uptake with 18F-FDG PET CT before and 14 days after initiation of chemotherapy Citation[72]. A subgroup of PET nonresponders was identified whose prognosis was poor. Salvage chemoradiotherapy was used in those where PET predicted a lack of response and this yielded an increased histological response but not an increased RO resection rate which formed the primary endpoint of this trial. Metabolic response evalUatioN for Individualization of neoadjuvant Chemotherapy in Esophageal and esophagogastric adeNocarcinoma looks at PET–CT response following NAC. Worldwide nCRT reflects the standard pattern of care, and there is no evidence yet to support PET–CT in predicting response to nCRT. We await the results of the Dutch NEOadjuvant therapy monitoring with PET and CT in Esophageal Cancer trial specifically powered and designed to answer this question Citation[73].
Other solid tumors lead the way in predicting the benefit of neoadjuvant therapy, and it is likely that a panel of markers will be required to predict the benefit for EAC patients. The search remains to identify a robust predictor of response to neoadjuvant therapy as a first step to truly personalized therapy.
Adjuvant therapies
The role of adjuvant therapy in esophageal cancer is controversial. There are concerns over the additional risk, versus benefit, of postoperative treatment over neoadjuvant therapy alone Citation[70]. This has resulted in a lack of adoption in the UK Citation[63]. In addition, the reclassification of junctional tumors that invade the esophagus as esophageal tumors in the TNM 7 staging system makes decision making regarding adjuvant therapy for these patients difficult, and recent evidence suggests that the site of the tumor relative to the gastroesophageal junction is important for outcomes in current cohorts Citation[74].
The MRC ST02 (MAGIC) trial, evaluating Epirubicin, Cisplatin and 5-Fluorouracil perioperatively (pre- and post-surgery), was originally designed to study gastric cancer. However, a significant number of patients with distal esophageal and junctional cancers were recruited. When compared with surgery alone, patients who received perioperative chemotherapy had a better overall survival (5-year survival 36 vs 23%) and progression-free survival Citation[70]. The contribution of the postoperative component of chemotherapy was difficult to assess because a significant proportion of the group failed to complete the chemotherapy regimen.
It makes sense that patients who have a partial response to NAC may be the most appropriate to be considered for trials of adjuvant treatment. Data from other disease sites suggest only patients responding to neoadjuvant treatment benefit from further treatment Citation[75].
Additional adjuvant therapies in esophageal cancer are scarce, but therapies are being suggested and assessed following their routine clinical use in other solid tumors. The AddAspirin trial is looking at the benefit of aspirin to improve survival after standard primary therapy in common early stage solid tumours, including EAC. The use of agents, such as trastuzumab and lapatinib, which inhibit the growth cascade of human EGF 2 (HER2), in breast cancers that overexpress HER2 is well established. HER2 overexpression is linked to poor prognosis in breast and ovarian cancer Citation[76]. Over expression of HER2 is seen in other cancers including esophageal cancer. HER2 overexpression is reported in 17–32% of esophageal junctional adenocarcinomas, and this would suggest a potential role for HER2 inhibitors in EAC Citation[77]. Supportive evidence comes from the trastuzumab for gastric cancer study, a Phase III randomized controlled trial in patients over expressing HER2, which randomized patients to receive capecitabine and cisplatin or fluorouracil (5-FU) with cisplatin (given every 3 weeks for six cycles) or chemotherapy in combination with intravenous trastuzumab. Intention-to-treat analysis showed improved median survival of 2.7 months with trastuzumab. Pathological response rate, time to tumor progression and duration of response were significantly higher in the trastuzumab-plus-chemotherapy group Citation[78]. Lapatinib, a small molecule dual tyrosine kinase inhibitor of EGF receptor and HER2, is used in breast cancer resistant to trastuzumab. A Phase II study with lapatinib monotherapy in EGF receptor ± HER2 positive EAC on patients with disease progressing on previous therapies showed no objective response Citation[79].
Further clinical trials assessing adjuvant treatments will continue to report, and the hope is that they will show some marginal benefits to prolong survival. New agents are urgently needed that improve the outlook for patients with unresectable EAC.
The tumor microenvironment in EAC
The major focus of cancer research over the past three decades has been the cancer cells themselves. However, tumor cells do not exist in isolation, and attention is increasingly being paid to the tumor microenvironment (stroma). Early data from whole-genome sequencing studies suggest that the mutational burden of EAC may already exist at the time of metaplastic transformation, and the relationship with the stroma may provide the impetus for malignant transformation Citation[18,50]. Convincing evidence exists that supports the role of the stroma in cancer progression.
Cancer-associated fibroblasts (CAF) form the major cellular component of the tumor stroma. CAF are a complex heterogeneous group of cells, and they are shown to derive from resident fibroblasts as well as other mesenchymal cell types (endothelial, pericytes, stellate cells, preadipocytes) and bone marrow-derived cells (fibrocytes, mesenchymal stem cells). Activated CAF have a contractile, myofibroblastic phenotype and express alpha-smooth muscle actin (α-SMA), they promote tumor cell growth, invasion and metastasis Citation[80].
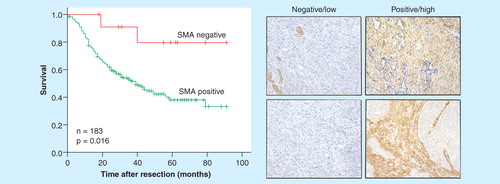
High levels of α-SMA expression predict poor outcome in several cancer types Citation[81]. We have validated this in our own EAC cohort showing α-SMA expression to be more accurate than T, N, M or R status in predicting survival [Underwood et al., Unpublished Data].
Figure 2. CAF in the tumor microenvironment predict survival in EAC. Left panel – Kaplan-Meier survival curve following esophagectomy in α-SMA positive and negative tumors. Median survival α-SMA positive tumors = 39 months. Hazard ratio: 7.1 (1.7–29.4; p = 0.007). More predictive than T, N, M or R status. Right panel – representative immunohistochemistry.
TGF-β is a key, and well documented Citation[82], player in CAF recruitment and function. It represents an appealing target and several TGF-β inhibitors, such as Lerdelimumab and Metelimumab, have been developed and are in early phase trials Citation[83]. However, the role of TGF-β is complex, and it is clearly tumor suppressive at defined points in EAC development. Therefore, strategic targeting of TGF-β inhibitors is required if we are to maximize it as a therapy.
CAF have also been shown to influence the local immune system in the microenvironment through their secreted cytokines, chemokines and extracellular matrix proteins. These influence the recruitment, functional status and retention of myeloid and lymphoid cells. CAF have been immunotherapeutic targets through the use of fibroblast-activating protein vaccinations, prolonging survival when used in combination with chemotherapy in murine models of breast and colorectal cancer Citation[84]. Novel targeting of CAF is likely to be either via decreasing the CAF population through targeting of expansion, recruitment or transdifferentiation or alternatively blockade of tumor promoting secretions from CAF.
In addition to the potential to develop treatments, it would also seem logical to include biomarkers from the tumor microenvironment in the staging of cancer to more accurately reflect the aggressiveness of tumors. The classification of cancer may therefore be better served by not only information of the tumor and its cells but also utilizing the tumor microenvironment and the host. There are now increasing calls to adopt this strategy with particular focus on the local immune infiltrate and the host inflammatory response Citation[85].
Expert commentary & five-year view
Esophageal cancer is a significant global health burden. It is diagnosed late, and overall survival remains stubbornly poor. We are witnessing a silent epidemic of adenocarcinoma of the distal esophagus and gastroesophageal junction that is only now being given the attention that it deserves. It is clear that western lifestyles are driving the rapid increase in EAC, with obesity and GERD the most convincing causative factors. We are likely to see a continued increase in the incidence of EAC as Western populations continue to become more obese.
So if we are unable to easily turn back the tide of obesity what can be done to improve outcomes for EAC patients? We and others believe that early diagnosis is vital for the future. Mass screening of symptomatic GERD patients with endoscopy is not feasible, and novel techniques that are inexpensive, minimally invasive and applicable to primary care will be required. The Cytosponge™ shows promise in this arena. Public awareness of the symptoms of EAC is equally important.
There is developing evidence that medical therapies for the treatment of GERD, PPI in particular, are not as safe in the long term as first thought, and it is likely that a growing number of GERD patients will demand alternative therapies. Antireflux surgery is effective and theoretically offers the advantage of preventing bile reflux. If we are to prove that antireflux surgery prevents BE/EAC, then we will need well-constructed studies that incorporate assessment of the efficacy of the antireflux procedure in the long term. Such studies are likely to be expensive and difficult to manage. They will require national/international recruitment and standardization of surgery, but this has been proven possible by the LOTUS trial collaborators Citation[86].
Endoscopic therapies already exist for premalignant and early-stage EAC, and we are likely to see a substantial increase in their application as and when diagnosis moves away from patients with dysphagia and advanced incurable disease. It is important to remember that some patients will still be better served (and may desire) definitive surgery and our efforts to make esophagectomy safe and efficient must continue.
But what of new treatments for established disease? We are currently limited to the blunt tools of conventional chemotherapy, radiotherapy and surgery. Understanding the fundamental biology of EAC is critical to the development of new therapies. Current efforts to define the genetic landscape of EAC and understand what determines germ-line susceptibility to BE and EAC are beginning this process. The clinical utility of whole-genome sequencing projects will be the development of molecular–phenotype therapeutics. To achieve this, model systems that faithfully represent the tumor of origin are required. Our group has developed the first such system to come from the Esophageal International Cancer Genome Consortium project, a novel EAC tumor cell line derived from 55-year-old male (pT4N3M0) who died from disease recurrence 7 months after NAC and esophagectomy [Underwood et al., Unpublished Data]. Authentication of this cell line using whole-genome sequencing, genome-wide microarrays and in vitro and in vivo functional studies confirms that it is a representative invasive tumor model and contains somatic deletions/mutations in the most commonly mutated genes in EAC (p16, SMAD4 & TP53). Models such as this will become a test bed for new targeted therapies that will be included into adaptive trials of combinations of existing therapies determined by the patient’s own DNA and their tumors’ molecular phenotype. This genetic information will be used to prevent patients receiving toxic treatments that will have no benefit; for instance, if we can find reliable markers of tumor resistance to platinum compounds from pretreatment biopsies in real time, then we will be able to reasonably withhold this treatment. It is also vital that we deepen our understanding of the relationship between EAC and the host environment; our group and others are leading efforts in this regard.
Once a bastion of nihilism, we believe the future to be much more optimistic for patients diagnosed with EAC. It is not unreasonable to think that in addition to the aggregation of marginal gains observed in the recent past, a ‘game-changing’ development in the management of EAC might be around the corner.
Key issues
The incidence of esophageal adenocarcinoma is rising worldwide but most rapidly in the west.
Trials assessing screening, surveillance and chemoprevention near their conclusion.
New endoscopic therapies seem promising, but lack long-term efficacy, cost-effectiveness and comparison with established treatment modalities.
Neoadjuvant therapy is now established in treating patients with locoregional esophageal cancer, but as yet the optimum treatment algorithm is undefined.
There are no clinically useful predictors of response to neoadjuvant therapy.
Morbidity and mortality continue to fall, following surgery with continued innovation in the perioperative treatment pathway.
The International Cancer Genome Consortium and similar consortia are defining the genetic landscape of esophageal adenocarcinoma providing the potential to develop personalized medicine as well as novel therapies.
Financial & competing interests disclosure
A Cowie is funded by a grant from the Royal College of Surgeons of England; F Noble is funded by a grant from Cancer Research UK; T Underwood is funded by a grant from the Medical Research Council. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2095-128
- Koppert LB, Wijnhoven BP, van Dekken H, et al. The molecular biology of esophageal adenocarcinoma. J Surg Oncol 2005;92(3):169-90
- Lepage C, Rachet B, Jooste V, et al. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol 2008;103(11):2694-9
- Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 2012;21(1):36-51
- Saadi A, Shannon NB, Lao-Sirieix P, et al. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci USA 2010;107(5):2177-82
- Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001;92(3):549-55
- Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol 2007;21(6):921-45
- Law S, Wong J. Changing disease burden and management issues for esophageal cancer in the Asia-Pacific region. J Gastroenterol Hepatol 2002;17(4):374-81
- Clinical Effectiveness Unit R, AUGIS, BSG, NCASP. National Oesophago-Gastric Cancer Audit. National Report. The NHS Information Centre, Leeds, UK; 2010 IC15100510
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381(9864):400-12
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002;95(7):1434-43
- D’Amico TA. Outcomes after surgery for esophageal cancer. Gastrointest Cancer Res 2007;1(5):188-96
- Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340(11):825-31
- Wild CP, Hardie LJ. Reflux, Barrett’s oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer 2003;3(9):676-84
- Mayne ST, Risch HA, Dubrow R, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 2001;10(10):1055-62
- Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 1976;21(11):953-6
- Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179-87. e1-3
- Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45(5):478-86
- Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol 2008;295(2):G211-18
- Theisen J, Stein HJ, Dittler HJ, et al. Preoperative chemotherapy unmasks underlying Barrett’s mucosa in patients with adenocarcinoma of the distal esophagus. Surg Endosc 2002;16(4):671-3
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365(15):1375-83
- Frazzoni M, Savarino E, Manno M, et al. Reflux patterns in patients with short-segment Barrett’s oesophagus: a study using impedance-pH monitoring off and on proton pump inhibitor therapy. Aliment Pharmacol Ther 2009;30(5):508-15
- Farre R, van Malenstein H, De Vos R, et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 2008;57(10):1366-74
- Farre R, Fornari F, Blondeau K, et al. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut 2010;59(2):164-9
- Cronin J, Alhamdani A, Griffiths AP, et al. In vitro and ex vivo models of extended reflux exposure demonstrate that weakly acidic mixed reflux heightens NF-kB-mediated gene expression. Dis Esophagus 2010. [Epub ahead of print]
- Cooper BT, Neumann CS, Cox MA, Iqbal TH. Continuous treatment with omeprazole 20 mg daily for up to 6 years in Barrett’s oesophagus. Aliment Pharmacol Ther 1998;12(9):893-7
- El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99(10):1877-83
- Johnson DA, Oldfield EC 4th. Reported side effects and complications of long-term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol 2013;11(5):458-64. quiz e37-8
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003;98(11):2390-4
- Lofdahl HE, Lu Y, Lagergren P, Lagergren J. Risk factors for esophageal adenocarcinoma after antireflux surgery. Ann Surg 2013;257(4):579-82
- Babar M, Ennis D, Abdel-Latif M, et al. Differential molecular changes in patients with asymptomatic long-segment Barrett’s esophagus treated by antireflux surgery or medical therapy. Am J Surg 2010;199(2):137-43
- Martinez de Haro LF, Ortiz A, Parrilla P, et al. Long-term follow-up of malignancy biomarkers in patients with Barrett’s esophagus undergoing medical or surgical treatment. Ann Surg 2012;255(5):916-21
- Cadiere GB, Van Sante N, Graves JE, et al. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication using EsophyX. Surg Endosc 2009;23(5):957-64
- Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013;368(8):719-27
- Rodriguez L, Rodriguez P, Gomez B, et al. Long-term results of electrical stimulation of the lower esophageal sphincter for the treatment of gastroesophageal reflux disease. Endoscopy 2013;45(8):595-604
- Rodriguez L, Rodriguez P, Gomez B, et al. Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: final results of open-label prospective trial. Surg Endosc 2013;27(4):1083-92
- Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124(1):47-56
- England PH. Be clear on cancer: Public Health England. Available from: http://campaigns.dh.gov.uk/category/beclearoncancer/ [Last accessed 20 January 2014]
- Varghese S, Cowie A, Underwood TJ, Fitzgerald RC. Barrett’s oesophagus - diagnosis and management. 2013. Available from: http://learning.bmj.com/learning/module-intro/barrett’s-oesophagus – diagnosis-and-management-.html?moduleId=10045036 [Last accessed 20 January 2014]
- Noble F, Hopkins J, Curtis N, et al. The role of systemic inflammatory and nutritional blood-borne markers in predicting response to neoadjuvant chemotherapy and survival in oesophagogastric cancer. Med Oncol 2013;30(3):596
- Noble F, Nolan L, Bateman AC, et al. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J Gastroenterol 2013;19(48):9282-93
- Schlansky B, Dimarino AJ Jr, Loren D, et al. A survey of oesophageal cancer: pathology, stage and clinical presentation. Aliment Pharmacol Ther 2006;23(5):587-93
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15(11):3278-88
- Wang VS, Hornick JL, Sepulveda JA, et al. Low prevalence of submucosal invasive carcinoma at esophagectomy for high-grade dysplasia or intramucosal adenocarcinoma in Barrett’s esophagus: a 20-year experience. Gastrointest Endosc 2009;69(4):777-83
- Modiano N, Gerson LB. Barrett’s esophagus: incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag 2007;3(6):1035-145
- Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol 2008;168(3):237-49
- Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372
- Varghese S, Lao-Sirieix P, Fitzgerald RC. Identification and clinical implementation of biomarkers for Barrett’s esophagus. Gastroenterology 2012;142(3):435-41. e2
- Lao-Sirieix P, Lovat L, Fitzgerald RC. Cyclin A immunocytology as a risk stratification tool for Barrett’s esophagus surveillance. Clin Cancer Res 2007;13(2 Pt 1):659-65
- Agrawal N, Jiao Y, Bettegowda C, et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov 2012;2(10):899-905
- Saadi A, Shannon NB, Lao-Sirieix P, et al. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci USA 2010;107(5):2177-82
- Ban S, Mino M, Nishioka NS, et al. Histopathologic aspects of photodynamic therapy for dysplasia and early adenocarcinoma arising in Barrett’s esophagus. Am J Surg Pathol 2004;28(11):1466-73
- Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett’s esophagus: follow-up in 100 patients. Gastrointest Endosc 1999;49(1):1-7
- van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60(6):765-73
- Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology 2013;145(1):87-95
- Al-Kasspooles MF, Hill HC, Nava HR, et al. High-grade dysplasia within Barrett’s esophagus: controversies regarding clinical opinions and approaches. Ann Surg Oncol 2002;9(3):222-7
- Williams VA, Watson TJ, Herbella FA, et al. Esophagectomy for high grade dysplasia is safe, curative, and results in good alimentary outcome. J Gastrointest Surg 2007;11(12):1589-97
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360(22):2277-88
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143(2):336-46
- Bollschweiler E, Baldus SE, Schroder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006;38(2):149-56
- Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 2008;112(5):1020-7
- Thompson SK, Bartholomeusz D, Jamieson GG. Sentinel lymph node biopsy in esophageal cancer: should it be standard of care? J Gastrointest Surg 2011;15(10):1762-8
- Allum WH, Blazeby JM, Griffin SM, et al. Guidelines for the management of oesophageal and gastric cancer. Gut 2011;60(11):1449-72
- Langer R, Ott K, Feith M, et al. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol 2009;22(12):1555-63
- Bollschweiler E, Holscher AH, Metzger R, et al. Prognostic significance of a new grading system of lymph node morphology after neoadjuvant radiochemotherapy for esophageal cancer. Ann Thorac Surg 2011;92(6):2020-7
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339(27):1979-84
- Matsuyama J, Doki Y, Yasuda T, et al. The effect of neoadjuvant chemotherapy on lymph node micrometastases in squamous cell carcinomas of the thoracic esophagus. Surgery 2007;141(5):570-80
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12(7):681-92
- Korst RJ, Kansler AL, Port JL, et al. Downstaging of T or N predicts long-term survival after preoperative chemotherapy and radical resection for esophageal carcinoma. Ann Thorac Surg 2006;82(2):480-4. discussion 4-5
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355(1):11-20
- Fareed KR, Kaye P, Soomro IN, et al. Biomarkers of response to therapy in oesophago-gastric cancer. Gut 2009;58(1):127-43
- zum Buschenfelde CM, Herrmann K, Schuster T, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 2011;52(8):1189-96
- van Heijl M, Omloo JM, van Berge Henegouwen MI, et al. NEOadjuvant therapy monitoring with PET and CT in Esophageal Cancer (NEOPEC-trial). BMC Med Phys 2008;8:3
- Curtis NJ, Noble F, Bailey IS, et al. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J Surg Oncol 2014;109(3):202-7
- Collette L, Bosset J-F, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? a trial of the european organisation for research and treatment of cancer radiation oncology group. J Clin Oncol 2007;25(28):4379-86
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244(4905):707-12
- Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res 2012;18(2):546-54
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742):687-97
- Hecht JR, Urba SG, Koehler M, et al. Lapatinib monotherapy in recurrent gastrointestinal malignancy: phase II efficacy and biomarker analyses. Proceedings of the American Society of Clinical Oncology, Gastrointestinal Cancers Symposium; 2008
- Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Canc Res 2011;1(4):482-97
- Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol 2011;223(4):470-81
- Naber HP, ten Dijke P, Pardali E. Role of TGF-beta in the tumor stroma. Curr Cancer Drug Targets 2008;8(6):466-72
- Bonafoux D, Lee WC. Strategies for TGF-beta modulation: a review of recent patents. Expert Opin Ther Pat 2009;19(12):1759-69
- Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 2006;116(7):1955-62
- Galon J, Pages F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Translat Med 2012;10:1
- Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011;305(19):1969-77