Abstract
Background: Ferric citrate (FC) is a new phosphorus binder shown to increase serum iron stores while reducing intravenous iron and erythropoiesis-stimulating agent usage. Such reductions could lower hospitalization rates and associated costs. Methods: Hospitalizations during a Phase III trial were compared between FC and active control (AC). Hospitalization costs were estimated using the 2013 US Renal Data System Annual Data Report. Results: 34.6% of FC patients were hospitalized at least once versus 45.6% of the AC group (risk reduction 24.2%; p = 0.02). There were 181 unique hospitalizations in the FC group versus 239 in the AC group, for a difference of 58 hospitalizations. Total potential savings was US$ 867,622 in hospitalization costs in the FC group. If the hospitalization reduction in our study was applied to the general end-stage renal disease population, this could translate into a savings of US$ 3002/patient/year. Conclusions: Patients receiving FC experienced fewer hospitalizations with the potential for significant savings.
In a Phase III randomized trial designed to explore the efficacy and safety of oral ferric citrate (FC) as both a phosphorus binder and a source of iron, 441 dialysis subjects with end-stage renal disease (ESRD) were randomized to receive FC or active control (AC) with calcium acetate and/or sevelamer carbonate for 52 weeks Citation[1,2]. In this trial, FC provided similar phosphorus control compared with AC Citation[2]. In addition, over 52 weeks, subjects receiving FC achieved higher mean iron parameters (ferritin = 899 ± 488 ng/ml; transferrin saturation [TSAT] = 39 ± 17%) versus subjects on AC (ferritin = 628 ± 367 ng/ml; TSAT = 30 ± 12%: p < 0.0001 for both). A significant reduction in the usage of intravenous (iv.) iron (FC median 12.95 mg/week vs 26.88 mg/week for AC; p < 0.001) and erythropoiesis-stimulating agents (ESA; FC median epoetin equivalent units/week of 5306 vs 6951 for AC; p = 0.04) while maintaining hemoglobin levels was also seen. As both iv. iron usage and ESA dose have been linked to morbidity Citation[3,4], a reduction in the use of either of these two agents could indicate a safety and economic advantage of FC. One way to evaluate this would be to compare hospitalizations and subsequent hospitalization costs between the FC and AC study populations over the course of the trial. Because all hospitalizations in the trial were captured and recorded as serious adverse events, we had the opportunity to compare this outcome between the use of FC or AC and project any cost savings associated with these findings.
The purpose of this analysis was to compare hospitalization events and their attendant treatment costs in subjects randomized to FC compared with AC in the 52-week AC period of this Phase III trial.
Methods
Setting & participants
The study design and principal results have been described in detail elsewhere Citation[1,2]. In brief, 60 sites across the USA and Israel randomized 441 adult ESRD subjects undergoing thrice-weekly hemodialysis or peritoneal dialysis for at least 3 months in a 2:1 fashion to FC or AC. AC consisted of study-supplied calcium acetate and/or sevelamer carbonate. Major inclusion criteria relevant to these analyses included known tolerability to AC drugs, a serum ferritin level <1000 ng/ml, serum TSAT <50% and a life expectancy of at least 1 year. Major exclusion criteria included parathyroidectomy in the last 6 months, malignancy in the past 5 years and actively symptomatic gastrointestinal (GI) bleeding or inflammatory bowel disease. Intravenous iron was forbidden for ferritin >1000 ng/ml or TSAT >30%. The use of ESA was at the discretion of the site. Adverse events, including hospitalizations, were routinely collected at each study visit. A hospitalization >24-h duration was considered a serious adverse event.
Outcomes & measurements
All serious adverse events were evaluated for hospitalizations. Hospitalization events were compared between subjects randomized to FC or AC. The proportion of subjects hospitalized in each study group was compared using a chi-squared test statistic. The actual trial randomization ratio was computed using the subject numbers randomized (FC 289: AC 149, 1.94:1). This achieved randomization ratio was applied to the number of hospitalizations in the AC group to estimate hospitalizations expected in the FC group. The difference was then determined between actual hospitalizations and expected hospitalizations in the FC group. An analysis of variance model was then used to calculate average hospitalizations per subject in each study group. Hospitalization costs were estimated using data from 2013 USA Renal Data System Annual Data Report (USRDS). The average inpatient cost reported was US$ 26,642 per year, or US$ 14,959 per hospitalization because of an average of 1.781 hospitalizations per patient per year Citation[5].
Results
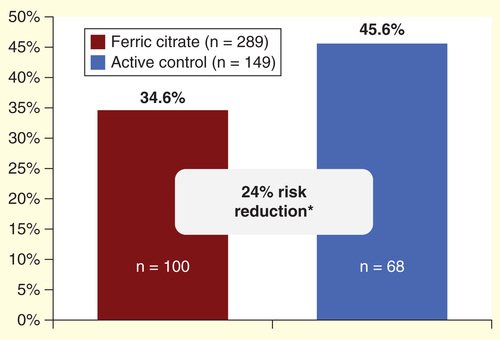
Of the 441 subjects enrolled in the trial, 438 took at least one dose of FC or AC. Approximately, 35% (100 of 289) of study subjects who received FC and 46% (68 of 149) of subjects who received AC were hospitalized at least once during the 52-week AC period of the trial, a relative risk reduction of 24.2% (; p = 0.02).
Figure 1. Proportion of subjects hospitalized at least once during the 52-week study period.
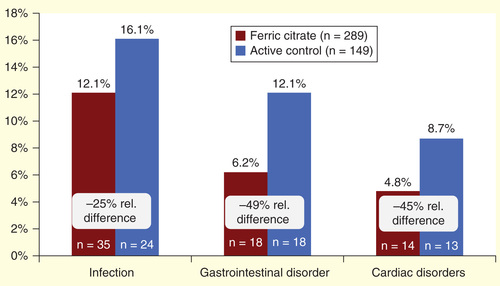
Two-thirds of the total study population was hospitalized for one of the three specific organ classifications represented in . Infections were the most common cause of hospitalization, leading to 12% of hospitalizations in the FC group and 16.1% in the AC group (a 25% relative decrease). Although not the most common cause, the largest relative difference in hospitalizations between the FC and AC groups was because of GI disorders (49% relative difference, AC: 12.1%, FC: 6.2%).
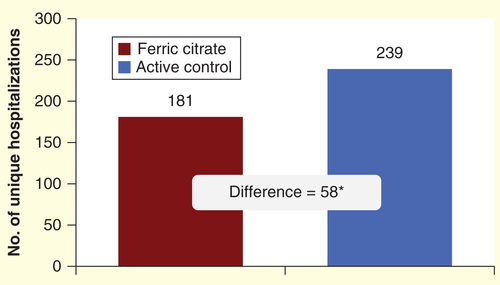
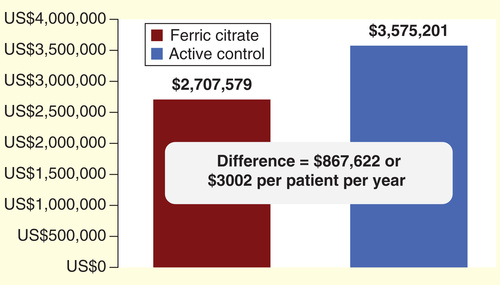
There were a total of 181 unique hospitalizations in the FC group of 289 subjects (0.63 hospitalizations per subject) and 123 hospitalizations in the AC group of 149 study subjects (0.83 hospitalizations per subject), p = 0.08. To further compare hospitalizations between subjects receiving FC and AC, we estimated that 239 hospitalizations were expected in the FC group (1.94 × 123 AC hospitalizations). The difference between expected FC hospitalizations (239) and actual FC hospitalizations (181) gives a difference of 58 fewer hospitalizations in subjects receiving FC (, a decrease of 24.1%, p = 0.08). At a USRDS average admission cost per hospitalization of US$ 14,959, this lower number of hospitalization events translates into a total savings of US$ 867,622 (58 × US$ 14,959) in hospitalization costs in the FC group. If this relative hospitalization sparing effect from FC was applied to the general ESRD population, the potential cost savings would be an estimated US$ 3002 per patient (US$ 867,622/289) over the 52-week study period .
Discussion
We conclude that the use of FC as a phosphorus binder was associated with reduced hospitalization events among patients with ESRD compared with the use of calcium acetate and/or sevelamer carbonate. The FC-treated group had fewer subjects with one or more hospitalizations (a 24.2% improvement) and less hospitalizations per subject (a 24.1% improvement). The hospitalization advantage for FC is particularly noteworthy, given that a requirement for being enrolled into the trial was having already demonstrated the ability to tolerate the binders that were used in the AC subjects. Thus, although the potential corresponding cost savings of the reduced hospitalization events seem to be substantial, they may represent conservative estimates.
Given the presence of phosphorus in food and its dependent excretion by the kidney in addition to relatively poor removal with dialysis treatments, well-nourished patients with ESRD typically require oral phosphorus binders to achieve desired serum phosphorus levels. Iron deficiency is likewise a well-recognized clinical problem in the dialysis population, interfering with the response to ESAs. In light of dose-limiting GI tolerance to ferrous salts given in the fasting state Citation[6–8], the majority of dialysis patients receive iron supplementation via the iv. route Citation[9].
FC is effective as both a phosphate binder and a source of iron, and its use is associated with significant reductions in the usage of both iv. iron and ESAs Citation[2]. This is consistent with the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) study, which found an association between higher iron storage levels and reduced ESA use Citation[10]. Such effects alone could be expected to confer cost benefits, not only related to the direct cost of these drugs Citation[11,12] but also potentially through a reduction in the ancillary services (nursing and pharmacy) associated with the preparation and administration of these medications.
Adding to these direct drug-related cost savings, this analysis predicts a reduction in hospitalization costs. When hospitalizations were categorized by key organ system classes, approximately two-thirds of hospitalizations were attributable to infections, GI disorders and cardiac disorders. Infections were the most common cause of hospitalization, accounting for 12% of hospitalizations in the FC group compared with 16.1% in the AC group – corresponding to a 25% reduction. The ability to prevent infection-related hospitalizations is of particular importance, given that there has been a 30% increase in this specific outcome among patients with ESRD (45% increase in those undergoing hemodialysis) since 1993 as well as an increase in the infection-related hospital days per patient per year, which has seen a 19.2% increase for hemodialysis patients Citation[5]. We found that corresponding reductions in hospitalization rates for GI disorders and cardiac disorders approached 50%.
The reasons behind the reduction in hospitalization rates in subjects receiving FC are not known. However, there are data to suggest that this is not because of random chance as both iv. iron and ESA dose have been linked to morbidity and mortality Citation[3,4]. Although iron is critical for the ability to carry oxygen in the blood, the iv. route for administration of iron leads to elevated levels of free non-protein-bound (catalytic) iron that induces oxidative stress. For example, 100 mg of iron sucrose causes a fourfold increase of non-transferrin-bound iron and increases superoxide generation by up to 70%. This oxidative stress has been linked to cardiovascular disease in multiple animal models. In addition, iron is a key cofactor for many microbes, and the human body normally sequesters iron in the setting of infection Citation[4]. The iv. injection of iron, as opposed to the oral route of delivery, introduces the risk of transient bacteremia, as well. Also, the direct injection of iv. iron bypasses the normal GI regulatory control of iron absorption. In addition, catalytic iron in the blood has been shown to be toxic to lymphocytes. When iron is delivered through the GI tract, iron absorption is highly regulated and nonbound iron in the blood is prevented Citation[13]. Because subjects receiving FC received less iv. iron compared with AC subjects and higher percentages of FC subjects received no iv. iron after 3 months in the study, this may have accounted for some of the reduction in hospitalizations.
ESA dose has also been independently linked to morbidity and mortality Citation[3]. Although it is unclear whether it is the higher hemoglobin levels that accompany higher ESA dosing or the ESA itself that is potentially harmful, a recent meta-regression analysis suggested the latter Citation[14]. ESAs are known to induce vasoconstriction and elevate blood pressure. ESA use and dose has been linked to stroke and other cardiovascular events in addition to its use being questioned in other non-ESRD populations of patients Citation[15–17].
We used the USRDS figure of US$ 14,959/ESRD hospitalization for our calculations. We did not collect the true costs for the hospitalizations in this trial. This number, however, does not seem unreasonable as this is an average of costs associated with prolonged complicated hospitalizations (not unusual for a patient with ESRD), in addition to the costs associated with less complicated admissions for dialysis access-related issues or for patients needing emergent dialysis for fluid overload; hospitalizations that may last only a day or two.
Although bearing many similarities to the US general ESRD population, the Phase III trial population was younger (44% of the subjects were <65 years of age) than the general USRDS population (21% <65 years of age) to which we applied the cost-saving data Citation[2]. We cannot be sure that the hospitalization sparing effect we found in subjects taking FC would apply to the general dialysis population. However, there is no reason to think that any of these binders would be tolerated any differently in the moderately older general ESRD patient population. In addition, our hospitalization data are derived from the 52-week AC period of the trial, thus we do not have data to allow us to predict whether this hospitalization sparing effect would persist in subsequent years.
In 2011, ESRD cost Medicare US$ 34.3 billion and accounted for 6.3% of the Medicare budget Citation[5]. The potential total economic effect of using FC would require a formal pharmacoeconomic evaluation that included the costs of ESAs and iv. iron between the treatment groups, the costs of the study drugs, and any survival data between the groups. Modeling data from the Phase III trial demonstrated that after year one of therapy, the FC study subjects would require 129,000 fewer units of ESA/year and reduction in iv. iron/year dosage of 1960 mg while maintaining the same hemoglobin levels. Applying 2013 Medicare pricing, this would save approximately US$ 2000/patient/year: US$ 1585 in ESAs and US$ 516 in iv. iron. For managed care plans, these savings could be doubled. Citation[11,12]. The phosphorus levels were similar in both groups, and the pill number necessary to achieve these goals was essentially the same (7.7 for calcium acetate, 8.0 for FC and 9.0 for sevelamer) Citation[2]. This study does not have the long-term survival data to determine whether life expectancy is changed and thus determine the quality-adjusted life-years gained or cost/life year gained, as is often the case when determining the true cost–effectiveness of a new drug. These data would require a much larger study cohort and a longer follow-up to determine these factors. The sole purpose of this study was to focus on any potential savings associated with hospitalizations. The results of this study predict a savings of approximately US$ 3000/patient in hospitalizations (for at least 1 year), that are in addition to the predicted approximately US$ 2000/year savings that results from reductions in the use of ESAs and iv. iron Citation[11].
The findings of this study must be interpreted in the context of its design-related limitations. This study was a post hoc analysis of a Phase III randomized trial that was not designed or powered to assess hospitalization events between cohorts. In addition, costs were estimated using USRDS summary hospitalization cost information. These costs were not specific to the study population and may be under- or overestimated. Extrapolation of the results may be limited to patients with similar demographic and treatment characteristics. For instance, the study population is about 10 years younger than the average USRDS population, and hospitalizations in older patients may be longer. However, this is not a cost to hospital evaluation, but a cost to Medicare evaluation and because Medicare’s hospitalization payments are diagnosis-related group based, length of stay will not affect Medicare’s costs. On the contrary, the cost/hospitalization that we use is USRDS derived and reflects an older population with an average age of 65 years. Perhaps if we had the average hospitalization cost/year for our 55-year-old average age study subject, this number might be less and thus we could be overestimating the savings. Regardless, even with these limitations, any dollar savings even close to these estimations are significant.
In conclusion, this study demonstrates that the use of FC as a phosphorus binder is associated with fewer hospitalizations that should result in significant savings. Results from this analysis are important, given the high cost of treating patients with ESRD.
Key issues
Ferric citrate (FC) is a new and unique phosphorus binder, which in a Phase III clinical trial of 441 dialysis subjects over a 52-week active control (AC) period provided similar phosphorus control while increasing serum iron stores when compared with an AC group using sevelamer carbonate and/or calcium acetate.
This effect of FC on iron stores led to a significant reduction in intravenous iron (median 1.85 mg/day FC, 3.84 mg/day AC, p < 0.001) and erythropoiesis-stimulating agent (median epoetin equivalent units/day: FC, 758 vs AC, 993, p = 0.043) usage in the FC group while maintaining hemoglobin levels.
Because both intravenous iron usage and erythropoiesis-stimulating agent dose have been linked to morbidity, a reduction in either agent could result in decreased hospitalization rates that would translate into subsequent cost savings.
On the basis of our analysis, based on the aforementioned Phase III trial, we found that 34.6% of the FC patients were hospitalized at least once during the trial compared with 45.6% of the AC group (risk reduction 24.1%, p = 0.02).
Adjusting for the 2:1 FC to AC study subject randomization, there were 181 unique hospitalizations in the FC group compared with 239 in the AC group for a difference of 58 hospitalizations. At US$ 14,959/admission, this translates into a total potential savings of US$ 867,622 in hospitalization costs in the FC group.
If the reduction in hospitalization events seen in our study subjects taking FC was to be applied to the general ESRD population, and if it was to persist beyond the 52-week study period, this could translate into a savings of US$ 3002/patient per year.
Overall, patients receiving FC experienced fewer hospitalizations with the potential for significant savings. Results from this analysis are important, given the high cost of treating patients with ESRD.
Financial & competing interests disclosure
The study was funded by Keryx Biopharmaceuticals, Inc. All the authors declare that they have received research grants from or acted as a consultant to Keryx, and received travel support from Keryx. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Umanath K , Sika M , Niecestro R , et al. Collaborative Study Group . Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int 2013;17:67-74
- Lewis JB , Sika M , Koury M J, et al, for the Collaborative Study Group. Ferric citrate binds phosphorus and delivers iron to end-stage renal disease patients. J Am Soc Nephrol 2014. [Epub ahead of print]
- Chaknos CM , Berns JS . Erythropoiesis-stimulating agents on trial: are higher dosages causing harm? Am J Kidney Dis 2013;61:6-8
- Fishbane S , Mathew A , Vaziri ND . Iron toxicity: relevance for dialysis patients. Nephrol Dial Transplant 2014;29:255-9
- U.S. Renal Data System , USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013
- Macdougall IC , Tucker B , Thompson J , et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 1996;50:1694-9
- Fudin R , Jaichenko J , Shostak A , et al. Correction of uremic iron deficiency anemia in hemodialyzed patients: a prospective study. Nephron 1998;79:299-305
- Besarab A , Coyne DW . Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol 2010;6:699-710
- Fuller DS , Pisoni RL , Bieber BA , et al. The DOPPS Practice Monitor for US dialysis care: trends through December 2011. Am J Kidney Dis 2013;61:342-6
- Kapoian T , O’Mara NB , Singh AK , et al. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol 2008;19:372-9
- Rodby R , Umanath K . Hsieh A, et al, for the Collaborative Study Group: phosphorus binding with ferric citrate reduces erythropoiesis-stimulating agent (ESA) and IV iron usage and cost in patients with ESRD. Am J Kid Dis 2014;63:A95
- Rodby R , Hsieh A . Niecestro R, et al, for the Collaborative Study Group: phosphorus binding with ferric citrate reduces managed care costs through reduced use of iron and erythropoiesis-stimulating agents (ESAs). J Manage Care Pharm 2014;20:S53
- Rooyakers T , Stroes E , Kooistra M , et al. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Invest 2002;32(Suppl 1):9-16
- Koulouridis I , Alfayez M , Trikalinos TA , et al. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am J Kidney Dis 2013;61:44-56
- Bohlius J , Schmidlin K , Brillant C , et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009;373:1532-42
- Corwin HL , Gettinger A , Fabian TC , et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007;357:965-76
- Stowell CP , Jones SC , Enny C , et al. An open-label, randomized, parallel-group study of perioperative epoetin alfa versus standard of care for blood conservation in major elective spinal surgery: safety analysis. Spine (Phila Pa 1976 2009;34:2479-85