Abstract
Measles and rubella are major vaccine-preventable causes of child mortality and disability. They have been eliminated from the Americas and some other regions have also come close to elimination. In this paper, we review regional progress toward measles and rubella control/elimination goals, describe the recent epidemiology of these infections and discuss challenges to achieving the goals. Globally, measles vaccination is estimated to prevent nearly 2 million deaths each year. Despite this remarkable progress, large measles outbreaks have occurred in recent years, often involving older persons who were not vaccinated in earlier years. Such an occurrence would be particularly damaging for rubella control programmes as it could lead to peaks in congenital rubella syndrome. Challenges to achieving and sustaining high vaccination coverage include civil conflict, weak health systems, geographic, cultural and economic barriers to reaching certain population groups and inadequate monitoring and use of data for action. Countries and regions aiming to eliminate measles and control rubella urgently need to improve the implementation and monitoring of both routine and mass vaccination campaign strategies.
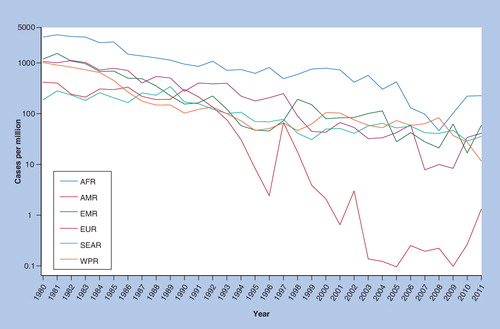
AFR: African; AMR: Americas; EMR: Eastern Mediterranean; EUR: European; SEAR: South east Asia region; WPR: Western Pacific.
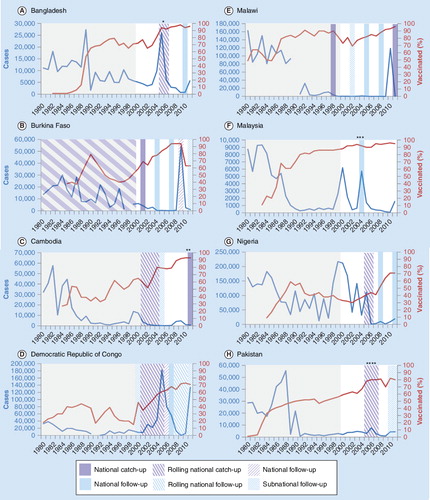
Percentage vaccinated = WUENIC MCV1. Grey shaded areas indicate period before campaigns included in WHO database. Catch-up campaigns covered 9 months–14 years and follow-up campaigns covered 9–59 months except:
*catch-up covered 9 months–10 years; **follow-up covered 7–14 years; ***catch-up covered 7–15 years; ****catch-up covered 9 months–13 years.
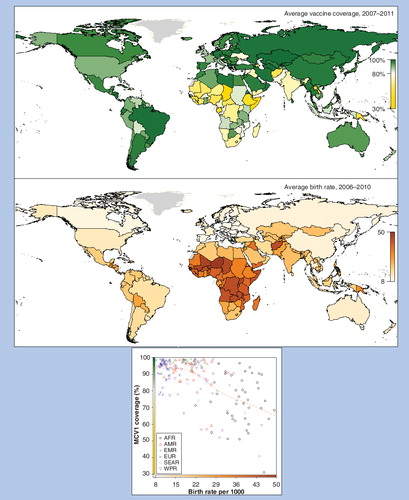
AFR: African; AMR: Americas; EMR: Eastern Mediterranean; EUR: European; SEAR: South east Asia region; WPR: Western Pacific.
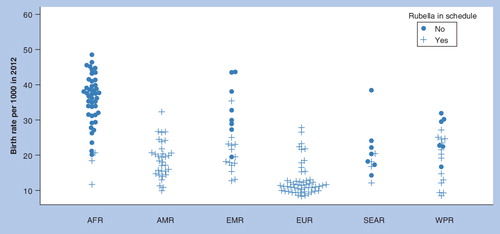
AFR: African; AMR: Americas; EMR: Eastern Mediterranean; EUR: European; SEAR: South east Asia region; WPR: Western Pacific.
Before measles vaccine was introduced, measles was one of the most severe childhood illnesses leading to at least 2 million deaths per year Citation[1,2], and it was a major cause of blindness in low income countries Citation[3]. The acute illness is characterized by fever and rash with coryza, cough or conjunctivitis. Measles virus is a potent immune modulator and common complications that often require hospital care include pneumonia, diarrhea and dysentery. Clinical, virological and pathological features are reviewed elsewhere Citation[4]. Since licensure of live attenuated measles vaccine 50 years ago, estimated global measles mortality has fallen to <7% of its pre-vaccination levels Citation[5]. Elimination of measles is biologically feasible Citation[6], and programmatic feasibility has been demonstrated in the Americas, where the last indigenous case occurred in November 2002 Citation[7].
Rubella infection is another cause of fever and rash in children which is usually mild when acquired postnatally Citation[8] but may result in fetal loss or severe disability after primary infection in the first trimester of pregnancy Citation[8–10]. Estimates of the global burden of congenital rubella syndrome (CRS) derive from models of the risk of infection in pregnancy, using serological data on the age-specific prevalence of rubella antibodies. Based on literature reviewed in 1996, approximately 110,000 cases of CRS (uncertainty bounds ranging from ~14,000–308,000) were estimated to occur each year in developing countries which did not vaccinate against rubella Citation[11], with highest numbers predicted in Africa and south east Asia. A review of data up to 2010 resulted in estimates of a similar order of magnitude Citation[12].
Measles and rubella vaccines are live, attenuated viral vaccines Citation[4,13] which are most effective when administered after maternal antibody is lost (this passively acquired antibody declines exponentially from about age 2–3 months onward and is undetectable in most infants by 9–12 months of age). They are available as separate or combined vaccines and administered by subcutaneous or intramuscular injection, although alternative routes of vaccination such as aerosol or intranasal have shown promise Citation[13–18]. Sabin and colleagues pioneered the use of aerosol measles vaccines in Mexico in the 1980s Citation[19]. Subsequently, trials in South Africa and Mexico showed that sero-responses and persistence of boosted antibody levels were better following measles vaccination of school children by aerosol than subcutaneous vaccination Citation[20–22] and some studies in infants have also shown good responses Citation[14,17,18,23]. Aerosol administration of dry-powder measles vaccine is also being evaluated Citation[24]. Cost–effectiveness analyses Citation[24] and stakeholder opinion Citation[25] suggest that the aerosol route could be efficient and help programs to achieve measles control goals. A recent pivotal Phase II/III trial conducted in India under the auspices of the WHO measles aerosol project, however, showed that following vaccination at age 9 months, the per-protocol seropositivity in the aerosol arm was 85.4% (95% CI: 82.5–87.9%) as compared to 94.6% (95% CI: 92.7–96.1%) in the subcutaneous arm, with the difference in seropositivity and the upper limit of the confidence interval both being greater than the non-inferiority margin of 5% defined in the study protocol. SAGE members concluded that the tested aerosol vaccine may not be suitable for primary vaccination of infants against measles but recommended that further research on measles-rubella (MR) aerosol be conducted Citation[26].
Combination MR vaccines used in industrialized countries usually also include mumps vaccine, but the burden of mumps infection is poorly described in low income countries. Based on mortality and disease burden, WHO considers measles control and the prevention of CRS to be higher priorities than the control of mumps Citation[27]. We therefore do not discuss mumps in this article.
A single dose of measles-containing vaccine (MCV1) induces antibody responses among 85–90% of infants at age 9 months and over 95% of infants vaccinated at age 11 months or above (reviewed in Citation[28]). Field studies show median vaccine effectiveness (VE) of 84.0% (interquartile range [IQR]: 72.0–95.0%) and 92.5% (IQR: 84.8–97.0%) after vaccination at 9–11 and >12 months, respectively Citation[29]. Infants may respond to vaccine at an earlier age, once most mothers have vaccine-induced rather than natural immunity, since the former results in lower antibody levels being transferred in utero Citation[30]. In low transmission settings, it is feasible to vaccinate at the age of 15 months when effectiveness is higher Citation[31,32]. By contrast, the WHO recommends vaccination at age 9 months in countries with ongoing transmission in which the risk of measles mortality among infants remains high Citation[28,33,34]. To eliminate measles, a second dose of vaccine is recommended Citation[33]; field studies show median VE for two doses of 94.1% (IQR: 88.3–98.3%) compared with no vaccination Citation[29]. Responses to measles vaccine are lower in HIV-infected children Citation[35–37], but their high mortality has reduced any resulting effect on population immunity Citation[38,39]. The potential role of revaccination of HIV-infected children on antiretroviral therapy in low-income countries is under evaluation Citation[40]. Rubella-containing vaccines (RCV) currently distributed in most countries contain the RA 27/3 strain prepared in human diploid cell culture Citation[13]. Seroconversion rates of 94–98% have been reported after vaccination at the age of 9 months or older (reviewed in Citation[41] and Citation[42]).
Immunity is long-lasting among persons who develop a primary immune response to measles and rubella vaccines. Antibody levels wane after vaccination Citation[43], but are boosted rapidly on exposure to infection which may be subclinical or associated with mild illness. For rubella, such re-infection of pregnant women has very low risk of transmission to the fetus Citation[44,45]. Antibody levels are boosted after revaccination of persons whose antibody levels have waned but fall again quickly Citation[28].
Rubella vaccination is cost effective in industrialized and middle-income countries Citation[46]. In 2010, however, over two-thirds of the global birth cohort lived in countries that did not include rubella vaccination in their national immunization programme, due to a lack of empirical data on disease burden (only a minority of cases are seen at medical facilities having the capacity to diagnose CRS Citation[47,48]), the increased cost of combined MR vaccine compared to single-antigen measles vaccine, and concerns about potential increases in CRS if adequate coverage of rubella vaccination could not be assured and sustained Citation[49].
As interest in measles elimination increases Citation[50], there are calls to include rubella in measles control and elimination activities Citation[51–53]. The GAVI alliance, a public-private partnership of charitable organizations, national governments and international organizations such as WHO and UNICEF, has supported the introduction of new vaccines in low-income countries for more than a decade and has also contributed funding for measles campaigns. There are currently 56 GAVI-eligible countries, although not all are available for all types of support Citation[201]. Recently, GAVI has made funding available for MR catch-up campaigns in countries that can achieve 80% immunization coverage (via routine, or routine and campaigns); and can finance the introduction of rubella vaccine into their routine program immediately following the catch-up campaign Citation[54]. GAVI will also fund second dose measles vaccine (MCV2) costs in eligible countries, though not the rubella component. In 2012, WHO and partners published the first global combined strategic plan for measles and rubella control and elimination Citation[202]. In this paper, we briefly review some principles of measles and rubella vaccination and describe progress toward measles control and elimination and current challenges in different WHO regions. We then discuss the implications of the experience with measles vaccination programmes for rubella control and elimination.
Principles of measles & rubella control and elimination
The crucial factor determining the spread of infections transmitted person-to-person, such as measles and rubella, is the number of secondary cases caused by each infectious person Citation[55]. The basic reproduction number, R0, is a measure of the transmissibility of an infection within a population, defined as the average number of secondary infections produced by a typical infective person in a totally susceptible population. It depends on the characteristics of the infectious agent (e.g., infectivity and duration of infectiousness) and of the population (e.g., population density and social mixing patterns). The R0 of measles has been estimated as 14–18 in England and Wales, 12.5 in North America, and 10 in Niger Citation[56]. The R0 of rubella is generally lower (4–7) Citation[57], however in high density settings (e.g., in Addis Ababa, Ethiopia) R0 values as high as 11.8 have been estimated Citation[58].
In the context of a vaccination programme, the effective reproduction number, R, is key for predicting and preventing outbreaks Citation[55]. R is the average number of infectious individuals resulting from a single infective introduced into the population, given the population mix of vaccine-acquired and naturally acquired immunity at that time Citation[59], and decreases as population immunity increases. If R is below 1, then the average case will give rise to less than 1 case and transmission will eventually cease. Consequently, elimination programmes aim to keep R below 1. Births and immigration increase population susceptibility (and hence R), while vaccination slows the rate of this increase. High vaccine coverage induces a period of low incidence termed the ‘honeymoon period’ Citation[60] during which cohorts of susceptible children who were not immunized in the early years of the programme can reach older ages before being exposed. Susceptible persons gradually accumulate until ‘post-honeymoon’ outbreaks occur Citation[61]. Although the proportion of cases in older children increases after vaccination, the absolute number of such cases may still fall because of the overall reduction in incidence. For rubella, it is crucial to achieve and sustain adequate coverage to avoid an increased incidence in adults, as discussed later in this paper.
Definitions of measles goals & targets
Measles elimination is defined as the absence of endemic measles transmission in a defined geographical area (e.g., region) for ≥12 months in the presence of a well performing surveillance system Citation[62]. As of 2011, the WHO Region of the Americas had eliminated measles, and four of the remaining five regions had adopted a measles-elimination goal; the Americas and Europe included a rubella elimination goal. Standardized definitions and indicators have been developed to monitor progress towards elimination, including clinical, laboratory and epidemiological definitions which lead to 12 possible categories for classifying measles cases Citation[62]. Similar indicators are likely to be developed and used for rubella elimination.
Progress in measles control & elimination
Progress in the Americas
The USA established its first measles elimination goal in 1966 Citation[63]. Measles elimination appeared close in 1983, but subsequently outbreaks occurred among highly vaccinated school-age populations Citation[64], leading to expensive outbreak control activities Citation[65,66]. In 1989, a two-dose measles vaccination strategy was recommended Citation[67]. From 1989–1991 there was a large measles resurgence in the USA (rubella and CRS also increased at that time) Citation[68]. Almost one half of all measles cases and 90% of deaths occurred in unvaccinated preschool children Citation[65,69,70]. Control required immense efforts to deliver the first dose on time Citation[71], demonstrating the critical importance of achieving and sustaining high and timely routine coverage.
The goal of eliminating measles from the Americas was set in 1994, the year when the region was declared polio-free Citation[7]. In Latin America and the Caribbean, routine services aimed to ‘keep-up’ high population immunity by vaccinating over 90% of each birth cohort and increasing the age for first dose to 12 months Citation[53]. In the early 1990’s ‘catch-up’ campaigns, usually targeting children aged 9 months to 15 years were conducted, aiming to immunize all susceptible children who had accumulated over the previous years of routine vaccination. After approximately 4 years, ‘follow-up’ campaigns were done among children aged 1–4 years, to sustain high population immunity. Both types of campaigns included community-based ‘mop-up’ activities in areas where monitoring showed that campaign coverage was below 95% Citation[7]. Finally, once the goal of rubella elimination was added in 2003 Citation[53], ‘speed up’ campaigns were done among adults (of both sexes in all but three countries) up to age 40 years, to quickly reduce transmission. Although rubella was the primary motivator for the speed-up campaigns, all countries used combined MR or MMR vaccines Citation[53]. The age range for rubella vaccination campaigns was wider than that for measles as the lower R0 of rubella meant that a larger number of adults had been susceptible to rubella than for measles. Programmes were guided by close monitoring of progress including routine coverage, campaign coverage, case-based disease surveillance and virus surveillance Citation[7,53]. The last endemic case of measles was documented in November 2002, and that of rubella in 2009 Citation[53].
Progress in other regions
The success of measles elimination in the Americas encouraged the adoption of measles elimination goals in the eastern Mediterranean (EMR, 1997), European (EUR, 1998), western Pacific (WPR, 2005) and African (AFR, 2011) regions, with varying target dates for elimination. The south east Asia region (SEAR) retains a measles mortality reduction goal, but elimination is under discussion.
By 2000, through global use of at least one routine dose of MCV, estimated measles mortality had fallen about 75% to 535,300 deaths (95% CI: 347,200–976,400). This fell to 139,300 (95% CI: 71,200–447,800) in 2010 through further increases in routine MCV1 coverage and campaigns Citation[5]. The Measles Initiative, launched in 2001 with a goal of reducing measles mortality, reports that its assistance has led to vaccination of over a billion children in catch-up and follow-up campaigns in 47 GAVI-eligible countries at an average cost of just under one dollar per dose administered Citation[203]. Reported measles incidence reached all-time lows in the European region in 2006, when cases were less than 1% of those reported in 1980, and in EMR in 2010, when cases were less than 3% of those reported in 1980 Citation[204]. Despite this encouraging progress, no other regions have shown the uniformly high and sustained impact of the Americas on reported measles incidence .
In AFR, measles transmission may have been interrupted briefly in southern Africa Citation[72] and Uganda Citation[73], but since 2009 outbreaks have occurred across the region Citation[74]. Large outbreaks were reported in the Democratic Republic of Congo (DRC: 133,802 cases, 2011), Malawi (118,712 cases, 2010), Burkina Faso (57,489 cases, 2009), Zambia (28,989 cases, 2010–2011) and Nigeria (18,843 cases, 2011). Of these, Malawi, Burkina Faso and Zambia reported both high routine coverage and regular measles vaccination campaigns. Vaccination and long lulls between outbreaks increase the average age of infections Citation[75]. Between 2002 and 2009, the median age for a reported measles case was 36 months in countries with MCV1 coverage of less than 50%, vs 49 months in those with MCV1 coverage of 75% or greater Citation[76]. In Burkina Faso, Malawi and Zambia there were large numbers of cases among teenagers and adults, and the majority of cases in Malawi’s 2010 outbreak were over 5 years of age Citation[77].
Nine countries in EMR reported measles incidence of <one case per million persons in the presence of a sensitive and well-functioning nationwide surveillance system in 2011 Citation[78]. In 2011, however, a major resurgence of measles occurred in conflict-affected and poorer countries with 35,923 cases reported to WHO-more than in 1997. Of these, almost half were from Somalia, and between 2600–5600 from each of Afghanistan, Pakistan, the Sudan and YemenCitation[204]. In Somalia, low MCV coverage in areas where immunization services could not be provided for nearly 2 years led to a massive measles outbreak, primarily among children aged <5 years. Population movements led to measles virus transmission among refugees, and outbreaks in Ethiopia and Kenya Citation[79].
In Europe, Finland implemented a two dose schedule early in the programme and is the only country to have sustained measles elimination Citation[80]. Catch-up campaigns, some going up to age 40 years, were implemented mainly in central and eastern Europe (CEE) and the newly independent states (NIS). From 2004–2006 outbreaks in CEE and NIS involved a high proportion of older and previously vaccinated persons Citation[81] whereas most transmission since then has been in unvaccinated persons. From 2009–2011, large outbreaks occurred in western Europe, as well as Bulgaria and Ukraine, and outbreaks continue to occur in several countries at the time of writing. These often began among groups having low coverage such as Roma and Sinti communities, Irish Traveller communities, anthroposophic groups and ultra-orthodox Jewish communities Citation[82–86] and spread to the wider population and to other countries Citation[86–89]. The majority of cases were among the unimmunized population, in infants younger than one year, adolescents and young adults. Low vaccine effectiveness, possibly related to cold chain failures and/or use of a more thermo-labile vaccine, was reported in Ukraine Citation[90] and nosocomial transmission contributed in France and elsewhere Citation[86].
SEAR has implemented accelerated measles control strategies. India was the last country to offer a second opportunity for measles vaccination Citation[202]. A routine second dose has now been implemented in states with reported MCV1 coverage ≥80% Citation[91]. In states with reported MCV1 coverage <80%, catch-up campaigns reportedly reached almost 40 million children aged 9 months to 10 years (86–89% of target) in 2010–2011, with the final phase of the campaigns scheduled for completion by April 2013. The two other large countries in the region, Bangladesh and Indonesia, have much higher routine coverage but gaps remain, particularly in Indonesia.
WPR reported by late 2012 that the region was approaching interruption of endemic measles transmission Citation[91]. China has had 99% MCV1 WHO-UNICEF estimates of coverage (WUENIC) since 2010. It conducted subnational campaigns from 2003–2009 and a massive national catch-up campaign in 2010, reporting 95% coverage. China reported just under 10,000 cases to WHO in 2011 compared to 38000–131,000 cases per year in the previous decade and over a million cases in 1980 and 1981 Citation[204]. Malaysia has relied chiefly on high routine coverage to control measles but regional heterogeneity in coverage, and delays in delivery of the second dose until age 7 years resulted in an outbreak in 2011 chiefly affecting <7 year olds. As elsewhere, an increased proportion of cases in WPR now occur among young infants and adults Citation[92,93]. A 2008–2009 outbreak in Vietnam started among unvaccinated university students Citation[92]. Vietnam conducted a campaign targeting 7–20 year olds in key provinces in 2008, and another in 2010 targeting 1–5-year-olds, and case numbers remain very low in 2012 Citation[204]. Laos also implemented wide age range campaigns targeted at 9 month to 19 year olds in 2011. Measles genotyping indicates considerable measles introductions both between countries in the region, but also from outside Citation[93].
The occurrence of outbreaks in countries after years of low incidence is due to the accumulation of susceptible persons. This is attributed to various challenges facing vaccination programmes, as illustrated below.
Current challenges to measles elimination
Inadequate routine coverage
When catch-up campaigns were conducted in the Americas about 20 years ago, WUENIC showed median routine MCV1 coverage of about 84–86% Citation[205]. Impact was sustained by further increasing coverage to over 90% since 1997, with only Haiti having persistently low coverage since then. By contrast, WUENIC of MCV1 in AFR was only 54% in 2001, and remained far below target at 75% in 2010–2011. In 2011, half the countries in AFR had coverage below 80% (including the three largest countries – Nigeria, Ethiopia and DRC). Only a handful of small countries (Botswana, Cape Verde, Eritrea, Mauritius, Rwanda, Seychelles and Swaziland) sustained coverage above 90% from 2007–2011. Worryingly, routine MCV1 coverage tends to be lowest in countries with high birth rates . Over two-thirds of the countries in AFR have birth rates over 40 per 1000 population. This combination of low MCV1 coverage and a rapidly growing population provide ideal conditions for sustaining measles transmission.
In EMR, just over half the population lives in six countries which have multiple challenges to achieving and sustaining high coverage, including civil conflict, low median income/capita, low female literacy and high birth rates; their median WUENIC MCV1 coverage in 2011 was 73.5%. In the European region, 11–16 countries reported less than 90% nationwide MCV1 coverage each year during 2003–2008. All countries in EUR have a routine two-dose schedule, and 4–12 countries and 3–7 countries reported MCV2 coverage <90 and <80%, respectively; each year in this period 11 or more countries did not report MCV2 coverage Citation[81]. In SEAR, regional coverage increased from 62% in 2001 to 79% in 2011. Here, seven out of 11 countries have sustained coverage over 90% but the two largest countries, India and Indonesia, have yet to reach 90% Citation[205]. In WPR, coverage has been high since 2005 in most countries, exceptions being some small island populations, Laos (though it has increased greatly here), Papua New Guinea and in the Philippines (where MCV1 coverage was over 90% from 2005–2010 but only 79% in 2011) Citation[205].
At subnational level, low coverage in certain geographic areas or population groups is important in shaping measles epidemiology. In India, substantial diversity in routine vaccination coverage within the country is mirrored by variable age patterns of infection, seasonality and genotypic diversity Citation[94,95]. In Nigeria, coverage in northern states is about half that of southern states and is lower in rural than urban areas Citation[96]. Migrants, travellers Citation[82–86,97], and certain castes, ethnic or religious groups often have lower coverage Citation[98]. The 2009 outbreak in Bulgaria occurred despite estimates of around 95% MCV1 coverage since 1995 and for MCV2 since 2005 Citation[81]. Most cases (97%) were reported from north-eastern Bulgaria, particularly in Roma populations and in persons who had received less than two doses of MCV Citation[99]. Areas affected by conflict and those receiving refugees are also at risk not only of low coverage but of high mortality when outbreaks occur Citation[100,101].
Variable impact of catch-up campaigns
Implementation and monitoring of catch-up campaigns have varied substantially between countries. In southern Africa, reported coverage ranged from 71% (Lesotho) to 114% (Malawi). Elsewhere in AFR most countries reported well over 90% (and often over 100%) coverage in catch-up campaigns but this has generally not been validated. In EMR, most of the nine countries that reported <90% coverage in their catch-up campaign were affected by conflict, however Egypt only achieved 78% coverage in 2001 Citation[102] and later repeated catch-up campaigns. In the European region, unfounded vaccine safety concerns in 2008 contributed to only 50% campaign coverage in Georgia and led to the suspension of the planned campaign in Ukraine Citation[81]. WPR reports that recent campaigns have had considerable impact on measles incidence, for example China in 2010 (despite parts of the migrant populations eluding specific efforts to target them Citation[103]); Cambodia, in 2011; and Japan Citation[93]. Past campaigns in the Philippines had inadequate coverage (indicated by the age profile of incidence observed after the 2007 campaign). A repeat campaign was done in 2011 with extensive rapid coverage assessments.
The impact of catch-up campaigns on reported incidence is often difficult to quantify. At regional level, the lack of synchronisation of catch-up campaigns between countries may have obscured impact , since resurgences were beginning in some countries before campaigns had been done in others. At country level, other factors complicate interpretation of trends, as illustrated in . In Burkina Faso and Malawi, catch-up campaigns seem to have contributed to lengthening the subsequent inter-epidemic period , although it is difficult to disentangle campaign impact from that of concurrent increases in routine coverage in the former. In others, the campaign coincided with, or immediately followed, a large epidemic , and thus low incidence after the campaign reflects a mixture of vaccine-induced and natural immunity. In DRC, catch-up campaigns had no demonstrable impact on reported measles cases .
Suboptimal or delayed implementation of follow-up campaigns
WHO recommends that follow-up campaigns are repeated regularly to sustain high population immunity until >90–95% immunization coverage has been achieved at the national level for both MCV1 and MCV2 for at least 3 consecutive years, and that data on the degree of heterogeneity of coverage among districts and on the epidemiology of measles should be reviewed by a national committee before stopping campaigns Citation[33]. The higher the birth rate and lower the routine coverage, the more frequently campaigns need to be repeated Citation[33,104–106]. Follow-up campaigns have not been adequate to maintain high rates of immunity in many countries, however. Postponement of planned campaigns has contributed to outbreaks, for example Kenya in 2005–2006 Citation[107]. Outbreaks with a large proportion of cases in children <5 years of age that have occurred in countries such as Angola, Nigeria, the Philippines and Zimbabwe within 1–2 years after a follow-up campaign suggest suboptimal implementation of the campaigns Citation[108]. In Botswana, South Africa, Swaziland and Zimbabwe, the outbreaks were partly related to vaccination refusals from some religious groups. Heterogeneous coverage in campaigns is problematic, as it is for routine coverage Citation[96].
Suboptimal monitoring & surveillance
Monitoring of immunization coverage is one of the weakest links in measles control and elimination Citation[109,110]. For example, Uganda reported 85–90% routine MCV1 coverage from 2004–2007, after its 2003 catch-up campaign reportedly reached 99.5% of children aged 9 months to 14 years Citation[111]. A measles resurgence began in 2006, and most confirmed cases were in under 5-year-olds Citation[111]. Problems with reported routine coverage were shown by data quality self-assessments and by a national survey which estimated only 76% MCV coverage of children aged 12–23 months Citation[205]. The 2009 outbreak in Burkina Faso occurred within 2 years of a follow-up campaign which reported 102% coverage and when WUENIC was 90% for routine coverage; administrative reports of coverage have been only 63% for 2010–2011 Citation[205]. Of the 28 countries in AFR with outbreaks from 2009–2010, 15 had implemented a follow-up campaign within 24 months prior to the outbreak; and all reported ≥90% coverage in their most recent measles campaign Citation[74].
Monitoring of MCV-2 coverage is done less rigorously than that of MCV-1 coverage, and campaign coverage has rarely been validated. Target population (denominator) figures are often projections from old census data and inaccurate Citation[108]. Reports of over 100% coverage of campaigns reflect under-estimates of the target population, vaccination of children outside the target age-group, or inaccurate recording practices. Without reliable coverage figures for all routine doses and campaigns, it is impossible to estimate R accurately and thus to plan appropriate and timely action.
As well as the need for accurate coverage data, high-quality surveillance is essential to be able to detect increases in incidence early and respond appropriately Citation[52,62]. WHO proposes identification of at least two cases of non-measles febrile rash per 100,000 population as an indicator of adequate surveillance. Although an increasing number of countries meet this criteria Citation[91], measles is grossly under-reported, with some regions reporting fewer cases than there are estimated measles deaths . Reporting completeness appears lowest in EMR and SEAR, with little improvement between 2000 and 2010. Insensitive surveillance may be used to evaluate trends if sensitivity remains constant Citation[106] but it is inadequate for planning actions to sustain measles elimination. Surveillance of rubella and CRS is even more limited-even in Europe, Belgium, France and Germany do not have rubella surveillance systems with national coverage.
Serological surveillance is another potentially important but under-utilized tool to indicate problem areas, identify which age groups should be included in campaigns, and evaluate the contribution of campaigns to reducing population susceptibility Citation[112–116]. It contributed to a decision to implement a catch-up measles-mumps-rubella campaign of schoolchildren in England and Wales in 1994 Citation[117], which is believed to have avoided an outbreak Citation[113]. The European region has set age-specific targets for measles antibody prevalence and monitors progress through a co-ordinated European Sero-Epidemiology Network Citation[112,118]. Analysis of data from 1996–2004 showed that seven countries (Belgium, Bulgaria, Cyprus, England and Wales, Ireland, Latvia and Romania) were at risk of epidemics due to low antibody prevalence in children and, in some, in adults Citation[118]. Had these findings been acted on more quickly, some of the recent outbreaks in these countries might have been avoided.
The need for early & effective outbreak response
Until 2009, the WHO-recommended response to measles outbreaks focused on case management rather than outbreak response vaccination (ORV). In 2009, based on epidemiological analysis and evaluations of ORV Citation[119], the recommendations changed to include ORV campaigns when specific criteria are met Citation[206]. ORV campaigns have been conducted in several African countries Citation[77,108,119–121]. The impact of ORV on reducing the likely magnitude or duration of the outbreak depends on how early ORV is implemented in the course of the outbreak, epidemiologic and demographic factors Citation[119] and whether resources allow vaccination of a wide enough age group according to the local epidemiology Citation[77].
Measles outbreak investigations provide an important opportunity to identify problems with a country’s measles control activities. The distribution of measles cases by age, vaccination status and location may reveal populations that are missed by routine vaccination and campaigns, reduced vaccine effectiveness, and the accumulation of susceptibility in older age groups Citation[77,122]. Such problems must be addressed to avoid a repeated resurgence of measles which may otherwise follow the post-outbreak honeymoon period.
Implications for rubella control & elimination
To date, rubella control or elimination programmes have mostly been restricted to countries with low or moderate birth rates , in which routine infant vaccine coverage levels of 80% or less are predicted to be adequate to interrupt transmission Citation[123]. Two exceptions are Guatemala (in the Americas which has a regional rubella elimination goal) and Iraq (in EMR). Funding from GAVI is now available for MR catch-up campaigns in eligible countries (which mostly have high birth rates), with the aim of interrupting transmission, following which routine vaccination is expected to sustain low incidence levels and indirectly protect any remaining susceptible adult women Citation[207]. To apply, countries must demonstrate that they can achieve and maintain routine coverage of 80% or greater. WHO advises that countries introducing RCVs implement strategies to vaccinate women of childbearing age Citation[51,124]. This was done in Latin America through campaigns and in North America and Europe through routine adolescent and/or post-partum vaccination, but so far GAVI has not proposed to fund such strategies. This situation will need close monitoring because 80% coverage may not be adequate to sustain rubella elimination in countries where birth rates are over 30/1000 Citation[123], especially if coverage of catch-up campaigns and routine vaccination shows substantial heterogeneity, as has been seen for measles.
In Costa Rica, RCV was introduced for infants and over 80% coverage achieved from 1984 onwards. A catch-up campaign of MMR was conducted reaching 93% of children aged 9 months to 14 years in 1993 and follow-up campaigns in 1994 and 1997. The total burden of CRS was probably reduced over the course of vaccination (the pre-vaccination burden is not known) but in a large rubella outbreak in 1998–1999, incidence rates were highest among those aged 25–34 years. In response, a campaign was conducted targeting adolescent and young adult women in affected districts and subsequently a national MR campaign reached 98% of all adults in 2001 Citation[125].
A perceived benefit of rubella vaccine introduction efforts may be reinforcement of existing measles programmes Citation[125]. It will be vital to ensure that this indeed occurs. The persistence of subpopulations with very low coverage and extinction–recolonization dynamics Citation[126] can reinforce inequities in coverage, with poorly served districts experiencing a higher burden of CRS than they would have done if no vaccination had occurred, while other districts reap the benefits Citation[127]. Past profiles of immunization coverage can be used to design catch-up strategies Citation[128] but this will require improving data on the quality and coverage of campaigns and routine vaccination, and the ability to act on data Citation[129].
Conclusion
Measles mortality reduction is highly cost-effective and substantial progress has been made. Estimated mortality in 2010 was <7% of that in the pre-vaccine era. Most of the estimated reduction was due to increases in routine coverage with at least one dose of measles vaccine Citation[130]. CRS is a major preventable cause of severe disability and there should be low marginal costs to including rubella in measles elimination activities. The MRI plan aims to achieve measles and rubella elimination in at least five WHO regions by 2020 Citation[208]. The strategy has five components, including achieving and maintaining high coverage with two doses of measles and rubella–containing vaccines, establishing effective surveillance and outbreak response, building public confidence in and demand for vaccination, and conducting operational research. To date, however, most funding has been allocated for campaigns, and GAVI funding appears to reinforce this tendency.
In the Americas, the routine programme spearheaded measles and rubella elimination and emphasis has consistently been placed on improving routine immunization to reach all communities and monitoring the equity of coverage in routine and campaign strategies Citation[7,53,131]. Regional and national advisors have invested time and effort in improving program monitoring and in taking corrective action when problems are identified Citation[132,133]. In the western Pacific, the announcement of an ambitious hepatitis B control goal concurrently with the measles elimination goal helped to ensure that countries strengthen integrated services Citation[134] and both goals appear to be within reach Citation[209].
By contrast, specific funding has not been provided to enable low income countries to strengthen routine MR vaccination programs and their monitoring. This is particularly worrisome given recent analyses which suggest that broad-scale vaccine coverage goals are unlikely to have the same impact on the interruption of measles transmission in all demographic settings and that target vaccine coverage should be scaled positively with either population size or the size of the birth cohort Citation[135]. Routine MCV coverage in many low income countries, and in particular those with large birth cohorts such as DR Congo, Ethiopia, parts of India, and Nigeria, has stalled at levels far below those needed for measles elimination and the estimates of coverage are probably inflated in many countries Citation[110,136]. This, together with the lack of externally validated data on the quality and coverage of campaigns and low completeness of measles surveillance makes it very difficult to plan appropriate strategies to sustain low transmission. The MR laboratory network has expanded remarkably over the last decade Citation[137] but this needs to be accompanied by strengthened field investigations and timely data-driven action.
Follow-up campaigns are expected to compensate for low routine coverage but the degree to which they reach children missed by the routine programme varies substantially between and within countries. Follow-up campaigns in the Congo reported 82% coverage in 2010 and 78% in 2011 (routine coverage is estimated at 90%), Equatorial Guinea reported 50% in 2011 (routine 51%); Senegal reported 81% in 2010 (routine 81%); Somalia reported 70% coverage in a national campaign in 2010 and 36% in a subnational campaign 2011 Citation[210] and a survey in Karachi reported that only 17% of children had received measles vaccine in the 2011 campaign Citation[138]. Elsewhere campaigns reported unfeasibly high coverage rates which were belied by subsequent outbreaks involving unvaccinated children in the cohorts eligible for the campaign. If measles and rubella are to be eliminated, there needs to be a drastic improvement in program implementation and monitoring.
The recent measles resurgence has been accompanied by a shift in age distribution to older children and adults, who were missed by vaccination efforts in earlier decades, and in infants too young to be vaccinated. This raises several concerns. First, the assumption that catch-up MR campaigns will interrupt rubella transmission is optimistic based on past experience with measles in AFR and EMR. Second, no funding is earmarked for vaccination of those in most need of protection like women of childbearing age, who may continue to be exposed to infection through migration to, or importations from, countries which do not use RCV. Third, unless routine coverage improves dramatically and campaigns are conducted to much higher standards, susceptibles will accumulate again after the catch-up campaign and resurgences of rubella involving older persons and cases of CRS will occur in the future Citation[123]. Since there are no robust data on trends in CRS incidence pre-vaccination in low-income countries, outbreaks could lead health workers and communities to believe that vaccination has been ineffective or worse, whether or not overall incidence has truly increased Citation[139]. Fourth, sustaining elimination in countries and regions which have eliminated measles and rubella is expensive in the face of frequent importations Citation[140,141] and new strategies are needed to reduce importations.
Although measles and rubella elimination is biologically feasible, its attainment will require a dramatic shift in political will and commitment. Despite initial optimism about eradicating major infections after the success of smallpox eradication, the failure to achieve the dracunculiasis and poliomyelitis elimination goals set 25 or more years ago highlights the extent of the challenges to elimination of other infections Citation[142]. The diversity of obstacles to achieving and sustaining high routine coverage even in high income countries, the delays in implementation of campaigns due to political and economic barriers, the inability of some countries to reach all children even via campaigns, the paucity of reliable data on coverage and the political and social turmoil in many countries raise major challenges to achieving the required population immunity. Routine immunization has been the backbone of MR elimination in the Americas and surveillance is a vital component of effective disease control Citation[7]. Investment and intensive action to strengthen immunization systems and their monitoring are urgent if the goals are to be achieved in all regions.
Expert commentary
Success in the Americas and some countries outside that region proves that measles and rubella transmission can be interrupted for long periods of time. Coverage of at least one dose of measles vaccine, delivered through routine services and mass campaigns, has been increasing in the last decade across most of the rest of the globe, resulting in considerable reductions in childhood morbidity and mortality. However, measles outbreaks continue to occur in much of the world, either due to constitutively low coverage (e.g., in conflict affected countries, or areas with weak health care systems), suboptimal routine two dose coverage (e.g., many countries in Europe), or reliance on campaigns that have not achieved adequate coverage, combined with inadequate monitoring of immune status (e.g., many countries in Africa). When outbreaks occur after a long period of low incidence, there is often a shift in the age distribution of cases towards older or very young individuals, with concomitant challenges for surveillance and control. Where rubella-containing vaccine has been introduced (mostly in low or moderate birth rate settings), late age rubella outbreaks have also been observed, of considerable concern for the burden of congenital rubella syndrome (CRS). Achieving and sustaining low or absent transmission of measles and rubella requires especially high coverage in countries with large birth cohorts and high birth rates which lead to large numbers of new susceptibles entering the population each year. While the experience in the Americas proves that elimination is possible, coordinated and sustained efforts by all countries and their global partners will be required to reach these goals.
Five-year view
Prospects for measles and rubella elimination over the next 5 years differ markedly at a global scale. For Europe, challenges to be addressed include disruptions to health services through reorganisations and the economic downturn, low priority given to measles vaccination by many health professionals and parents, difficulties in reaching travelling groups and vaccine refusal in others, and pockets of susceptibility in wide age groups including adults. In the Americas, sustaining elimination will require continued high coverage and rapid response to importations of the infections, and public education in the presence of changing attitudes about measles vaccination among those who have never experienced the disease. Outside of Europe and the Americas, the key is strengthening routine infant immunization and improving the use of mass vaccination campaigns to reach those unreached by routine services; clearly this will be particularly difficult in countries affected by conflict. After over a decade of mass vaccination campaigns and increasing routine coverage leading to reductions in measles transmission, individuals have reached older ages before being exposed to measles. The immunity profile of populations in different settings is often difficult to predict due to past deficiencies in accurate monitoring of coverage and disease incidence and assumptions about the distribution of susceptibility that may no longer hold. Serosurveys and serosurveillance may play a greater role in informing policy. For countries where rubella continues to circulate, the concerning signature of wider age-ranges of incidence for measles highlight the importance of improving strategies and emphasizing surveillance and use of data for action after the introduction of rubella-containing vaccines.
If the polio endgame is achieved, it is likely that energies will turn toward measles and rubella. Innovations in how campaigns are effectively planned and deployed, perhaps in combination with a mixture of case-based and serological surveillance, changes in funding toward improved monitoring and strengthening of routine vaccination, and taking action to ensure adequate immunity in all age groups will be essential to make this a success.
Table 1. Ratio of estimated measles deaths to reported measles cases by region, 2010 and 2000.
Key issues
• Measles and rubella have been eliminated from the Americas, and four of the five other WHO regions have established measles elimination goals.
• Despite impressive progress globally in reducing measles mortality via vaccination, large outbreaks have recently affected multiple countries around the world, often involving wide age groups including adults.
• Challenges to reaching and sustaining high enough routine vaccination coverage include weak or conflict-affected health systems, especially in some large countries with high birth rates, and barriers to reaching certain population groups even in high income countries.
• Mass vaccination campaigns have been used extensively in the last decade but their monitoring has been inadequate and impact often uncertain.
• Few low-income countries have introduced rubella vaccine because of the unrecognised disease burden, higher cost of the combined vaccine and concerns about a paradoxical increase in congenital rubella syndrome if adequate coverage is not ensured. Now that GAVI funding is available for measles-rubella vaccine catch-up campaigns, more countries are introducing rubella vaccine.
• Well-implemented campaigns may interrupt rubella transmission but women of childbearing age may continue to be exposed to infection through migration to, or importations from, countries which do not vaccinate against rubella.
• Countries and regions aiming to eliminate measles and control rubella urgently need to address past deficiencies in the implementation and monitoring of both routine and campaign strategies and in the use of data for action.
Financial & competing interests disclosure
This work was funded in part by the Bill and Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Clements CJ, Strassburg M, Cutts FT, Torel C. The epidemiology of measles. World Health Statistics Quarterly. Rap. Trimestriel de Statistiques Sanitaires Mondiales 45(2–3), 285 (1992).
- World Health Organization. Measles – Progress towards global control and regional elimination, 1990–1998. Wkly. Epidemiol. Rec. 73, 389–396 (1998).
- Foster A, Sommer A. Corneal ulceration, measles, and childhood blindness in Tanzania. Br. J. Ophthalmol. 71, 331–343 (1987).
- Moss WJ, Griffin DE. Measles. Lancet 379, 153–164 (2012).
- Simons E, Ferrari M, Fricks J et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet 379(9832), 2173–2178 (2012).
- Moss WJ, Strebel P. Biological feasibility of measles eradication. J. Infect. Dis. 204(Suppl. 1), S47–S53 (2011).
- De Quadros CA, Andrus JK, Danovaro-Holliday MC, Castillo-Solórzano C. Feasibility of global measles eradication after interruption of transmission in the Americas. Expert Revi. Vaccines 7(3), 355–362 (2008).
- Cooper LZ. The history and medical consequences of rubella. Rev. Infect. Dis. 7, S2–S10 (1985).
- Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 320(8302), 781–784 (1982).
- Gregg NM. Congenital cataract following German measles in the mother. Transact. Ophthalmol. Soc. Australia 3, 31–46 (1941).
- Cutts FT, Vynnycky E. Modelling the incidence of congenital rubella syndrome in developing countries. Int. J. Epidemiol. 28, 1176–1184 (1999).
- Vynnycky E, Adams EJ, Cutts FT et al. Using seroprevalence and immunisation coverage data to estimate the global burden of Congenital Rubella Syndrome, 1996–2010. (Submitted).
- Plotkin SA. The history of rubella and rubella vaccination leading to elimination. Clin. Infect. Dis. 43(Suppl. 3), S164–S168 (2006).
- Omer SB, Hiremath GS, Halsey NA. Respiratory administration of measles vaccine. Lancet 375, 706–708 (2010).
- Díaz-Ortega JL, Bennett JV, Castañeda D, Martinez D, De Castro JF. Antibody persistence in young adults 1 year after MMR immunization by aerosol or by subcutaneous route. Vaccine 28, 7228–7232 (2010).
- Simon JK, Ramirez K, Cuberos L et al. Mucosal IgA responses in healthy adult volunteers following intranasal spray delivery of a live attenuated measles vaccine. Clin. Vaccine Immunol. 18, 355–361 (2011).
- Cutts FT, Clements CJ, Bennett JV. Alternative routes of measles immunization: a review. Biologicals 25, 323–338 (1997).
- Low N, Kraemer S, Schneider M, Restrepo AM. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine 26, 383–398 (2008).
- Sabin AB. Immunization against measles by aerosol. Rev. Infect. Dis. 5, 514–523 (1983).
- Dilraj A, Cutts FT, De Castro JF et al. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet 355, 798–803 (2000).
- Dilraj A, Sukhoo R, Cutts FT, Bennett JV. Aerosol and subcutaneous measles vaccine: measles antibody responses 6 years after re-vaccination. Vaccine 25, 4170–4174 (2007).
- Bennett JV, Fernandez De Castro J, Valdespino-Gomez JL. Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: randomized trials in Mexican schoolchildren. Bull. World Health Organ. 80, 806–812 (2002).
- Wong-Chew RM, García-León ML, Espinosa-Torres Torrija B et al. Increasing the time of exposure to aerosol measles vaccine elicits an immune response equivalent to that seen in 9-month-old Mexican children given the same dose subcutaneously. J. Infect. Dis. 204, 426–432 (2011).
- Garrison LPJ, Bauch CT, Bresnahan BW, Hazlet TK, Kadiyala S, Veenstra DL. Using cost–effectiveness analysis to support research and development portfolio prioritization for product innovations in measles vaccination. J. Infect. Dis. 204, S124–S132 (2011).
- Higginson D, Theodoratou E, Nair H et al. An evaluation of respiratory administration of measles vaccine for prevention of acute lower respiratory infections in children. BMC Public Health 11(Suppl. 3), S31 (2011).
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, November 2012 – conclusions and recommendations. Wkly. Epidemiol. Rec. 88, 1–16 (2013).
- World Health Organization. Mumps virus vaccines. WHO position paper. Wkly. Epidemiol. Rec. 82, 49–60 (2007).
- Moss WJ, Scott S. The immunological basis for immunization series: module 7: measles – Update 2009. World Health Organization (2009).
- Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J. Infect. Dis. 204(Suppl. 1), S133–S149 (2011).
- Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 25(34), 6296–6304 (2007).
- Orenstein W, Markowitz L, Preblud S, Hinman A, Tomasi A, Bart KJ. Appropriate age for measles vaccination in the United States. Dev. Biol. Stand. 65, 13 (1986).
- De Serres G, Boulianne N, Meyer F, Ward BJ. Measles vaccine efficacy during an outbreak in a highly vaccinated population: incremental increase in protection with age at vaccination up to 18 months. Epidemiol. Infect. 115, 315–323 (1995).
- World Health Organization. Measles vaccines: WHO position paper. Wkly. Epidemiol. Rec. 84(35), 349–360 (2009).
- World Health Organization. Expanded Programme on Immunization. Measles immunization. Wkly. Epidemiol. Rec. 54, 337–339 (1979).
- Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin. Infect. Dis. 29(1), 106–112 (1999).
- Helfand RF, Witte D, Fowlkes A et al. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J. Infect. Dis. 198(10), 1457–1465 (2008).
- Fowlkes A, Witte D, Beeler J et al. Persistence of vaccine-induced measles antibody beyond age 12 months: a comparison of response to one and two doses of Edmonston-Zagreb measles vaccine among HIV-infected and uninfected children in Malawi. J. Infect. Dis. 204(Suppl. 1), S149–S157 (2011).
- Helfand RF, Moss WJ, Harpaz R, Scott S, Cutts F. Evaluating the impact of the HIV pandemic on measles control and elimination. Bull. World Health Organ. 83(5), 329–337 (2005).
- Scott S, Mossong J, Moss WJ, Cutts FT, Cousens S. Predicted impact of the HIV-1 epidemic on measles in developing countries: results from a dynamic age-structured model. Int. J. Epidemiol. 37(2), 356–367 (2008).
- Rainwater-Lovett K, Moss WJ. Immunologic basis for revaccination of HIV-infected children receiving HAART. Fut. Virol. 6(1), 59–71 (2011).
- Robertson S, Cutts F, Samuel R, Diaz-Ortega J. Control of rubella and congenital rubella syndrome (CRS) in developing countries, Part 2: Vaccination against rubella. Bull. World Health Organ. 75(1), 69 (1997).
- Reef SE, Plotkin SA. Rubella vaccine. In: Vaccines (6th edition). Plotkin SA, Orenstein WA, Offit PA. (Eds). Elsevier Saunders 688–717 (2012).
- Markowitz LE, Preblud SR, Fine PEM, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediat. Infect. Dis. J. 9(2), 101–110 (1990).
- Boué A, Nicolas A, Montagnon B. Reinfection with rubella in pregnant women. Lancet 297(7712), 1251–1253 (1971).
- Morgan Capner P, Rodeck C, Nicolaides K, Cradock Watson J. Prenatal detection of rubella specific IgM in fetal sera. Prenat. Diagn. 5(1), 21–26 (1985).
- Hinman AR, Irons B, Lewis M, Kandoloa K. Economic analyses of rubella and rubella vaccines: a global review. Bull. World Health Organ. 80(4), 264–270 (2002).
- Cutts F, Robertson S, Diaz-Ortega J, Samuel R. Control of rubella and congenital rubella syndrome (CRS) in developing countries, Part 1: Burden of disease from CRS. Bull. World Health Organ. 75(1), 55 (1997).
- Lawn JE, Reef S, Baffoe-Bonnie B, Adadevoh S, Caul EO, Griffin GE. Unseen blindness, unheard deafness, and unrecorded death and disability: congenital rubella in Kumasi, Ghana. Am. J. Public Health 90, 1555–1561 (2000).
- Strebel PM, Gacic-Dobo M, Reef S, Cochi SL. Global use of rubella vaccines, 1980–2009. J. Infect. Dis. 204, S579–S584 (2011).
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2011 – conclusions and recommendations. Wkly. Epidemiol. Rec. 86, 205–220 (2011).
- World Health Organization. Rubella vaccines: WHO position paper. Wkly. Epidemiol. Rec. 86, 301–316 (2011).
- De Quadros C, Andrus J, Danovaro C, Castillo-Solorzano C. Is global measles eradication feasible: five years after the interruption of endemic measles transmission in the Americas. Expert Rev. Vaccines 7(3), 355–362 (2008).
- Andrus JK, De Quadros CA, Castillo Solórzano C, Roses Periago M, Henderson D. Measles and rubella eradication in the Americas. Vaccine S4, D91–D96 (2011).
- Burki T. GAVI alliance to roll out rubella vaccine. Lancet Infect. Dis. 12, 15–16 (2012).
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J. Infect. Dis. 189, S27 (2004).
- Grais RF, Ferrari M, Dubray C et al. Estimating transmission intensity for a measles epidemic in Niamey, Niger: lessons for intervention. Trans. R. Soc. Trop. Med. Hyg. 100(9), 867–873 (2006).
- Fine PEM. Herd immunity: history, theory, practice. Epidemiol. Rev. 15(2), 265–302 (1993).
- Cutts FT, Abebe A, Messele T et al. Sero-epidemiology of rubella in the urban population of Addis Ababa, Ethiopia. Epidemiol. Infect. 124, 467–479 (2000).
- Farrington C, Whitaker H. Estimation of effective reproduction numbers for infectious diseases using serological survey data. Biostatistics 4(4), 621–632 (2003).
- Mclean AR, Anderson RM. Measles in developing countries. Part I: epidemiological parameters and patterns. Epidemiol. Infect. 100, 111–133 (1988).
- Chen RT, Weierbach R, Bisoffi Z et al. A ‘post-honeymoon period’ measles outbreak in Muyinga sector, Burundi. Int. J. Epidemiol. 23(1), 185–193 (1994).
- World Health Organization. Monitoring progress towards measles elimination. Wkly. Epidemiol. Rec. 85, 489–496 (2010).
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J. Infect. Dis. 189(Suppl. 1), S1–S3 (2004).
- Gustafson TL, Lievens AW, Brunell PA, Moellenberg RG, Buttery C, Sehulster LM. Measles outbreak in a fully immunized secondary-school population. N. Engl. J. Med. 316(13), 771 (1987).
- Markowitz LE, Preblud SR, Orenstein WA et al. Patterns of transmission in measles outbreaks in the United States, 1985–1986. N. Engl. J. Med. 320(2), 75–81 (1989).
- Robertson SE, Markowitz LE, Berry DA, Dini EF, Orenstein WA. A million dollar measles outbreak: epidemiology, risk factors, and a selective revaccination strategy. Public Health Rep. 107(1), 24 (1992).
- Centers for Disease Control and Prevention. Measles prevention: recommendations of the Immunization Practices Advisory Committee. MMWR. 38(S9), 1–13 (1989).
- Centers for Disease Control and Prevention. Achievements in Public Health: Elimination of Rubella and Congenital Rubella Syndrome – United States, 1969–2004. MMWR. 54, 279–282 (2005).
- Gindler J, Tinker S, Markowitz L, Atkinson W, Dales L, Papania M. Acute measles mortality in the United States, 1987–2002. J. Infect. Dis. 189(Suppl. 1), S69–S77 (2004).
- National Vaccine Advisory Committee. The measles epidemic: the problem, barriers and recommendations. JAMA. 266, 1547–1552 (1991).
- Centers for Disease Control and Prevention. Status report on the Childhood Immunization Initiative: national, state, and urban area vaccination coverage levels among children aged 19–35 months – United States, 1996. MMWR. 46, 657–664 (1997).
- Biellik R, Madema S, Taole A et al. First 5 years of measles elimination in southern Africa: 1996–2000. Lancet 359(9317), 1564–1568 (2002).
- Baliraine FN, Bwogi J, Bukenya H et al. Possible interruption of measles virus transmission, Uganda, 2006–2009. Emerg. Infect. Dis. 17(1), 110 (2011).
- World Health Organization. Measles outbreaks and progress towards meeting measles pre-elimination goals: WHO African Region, 2009–2010. Wkly. Epidemiol. Rec. 86, 129–140 (2011).
- Cutts FT, Henderson RH, Clements CJ, Chen RT, Patriarca PA. Principles of measles control. Bull World Health Organ. 69(1), 1–7 (1991).
- Goodson JL, Masresha BG, Wannemuehler K, Uzicanin A, Cochi S. Changing epidemiology of measles in Africa. J Infect Dis. 204(Suppl. 1), S205–214 (2011).
- Minetti A, Kagoli M, Katsulukuta A et al. Lessons and challenges for measles control from unexpected large outbreak, Malawi. Emerg. Infect. Dis. 19(2), 202 (2013).
- Naouri B, Ahmed H, Bekhit R, Teleb N, Mohsni E, Alexander Jr JP. Progress toward measles elimination in the eastern Mediterranean region. J. Infect. Dis. 204(Suppl. 1), S289–S298 (2011).
- World Health Organization. Measles – Horn of Africa, 2010–2011. Wly Epidemiol. Rec. 87, 329–336 (2012).
- Peltola H, Jokinen S, Paunio M, Hovi T, Davidkin I. Measles, mumps, and rubella in Finland: 25 years of a nationwide elimination programme. Lancet Infect. Dis. 8(12), 796–803 (2008).
- Martin R, Wassilak S, Emiroglu N et al. What will it take to achieve measles elimination in the World Health Organization European Region: progress from 2003–2009 and essential accelerated actions. J. Infect. Dis. 204(Suppl. 1), S325–S334 (2011).
- Georgakopoulou T, Grylli C, Kalamara E, Katerelos P, Spala G, Panagiotopoulos T. Current measles outbreak in Greece. Euro Surveill 11(2), E060223 (2006).
- Filia A, Curtale F, Kreidl P et al. Cluster of measles cases in the Roma/Sinti population, Italy, June–September 2006. Eur. Surveill. 11(10), E061012 (2006).
- Seguliev Z, Duric P, Petrovic V et al. Current measles outbreak in Serbia: a preliminary report. Euor Surveill. 12(11), 3155 (2007).
- Muscat M, Bang H, Wohlfahrt J, Glismann S, Mølbak K. Measles in Europe: an epidemiological assessment. Lancet 373(9661), 383–389 (2009).
- Muscat M. Who gets measles in Europe? J. Infect. Dis. 204(Suppl. 1), S353–S365 (2011).
- Ghebrehewet S, Hayhurst G, Keenan A, Moore H. Outbreak of measles in central and eastern Cheshire, UK, October 2008–February 2009. Epidemiol. Infect. 1(1), 1–8 (2012).
- Kremer JR, Brown KE, Jin L et al. High genetic diversity of measles virus, World Health Organization European region, 2005–2006. Emerg. Infect. Dis. 14(1), 107 (2008).
- Cortés JMM, Morilla EP, García VG et al. Measles outbreak in Andalusia, Spain, January to August 2011. Euro Surveill 17(42), 15 pii:20300 (2012).
- Velicko I, Müller LL, Pebody R et al. Nationwide measles epidemic in Ukraine: the effect of low vaccine effectiveness. Vaccine 26, 6980–6985 (2008).
- World Health Organization. Progress in global control and regional elimination of measles, 2000–2011. Wkly. Epidemiol. Rec. 88, 29–36 (2013).
- Sniadack DH, Mendoza-Aldana J, Huyen DTT et al. Epidemiology of a measles epidemic in Vietnam 2008–2010. J. Infect. Dis. 204(Suppl. 1), S476–S482 (2011).
- Sniadack DH, Mendoza-Aldana J, Jee Y, Bayutas B, Lorenzo-Mariano KM. Progress and challenges for measles elimination by 2012 in the Western Pacific Region. J. Infect. Dis. 204(Suppl. 1), S439–S446 (2011).
- Wairagkar N, Chowdhury D, Vaidya S et al. Molecular epidemiology of measles in India, 2005–2010. J. Infect. Dis. 204(Suppl. 1), S403–S413 (2011).
- Murhekar MV, Hutin YJ, Ramakrishnan R et al. The heterogeneity of measles epidemiology in India: implications for improving control measures. J. Infect. Dis. 204(Suppl. 1), S421–S426 (2011).
- Weldegebriel GG, Gasasira A, Harvey P et al. Measles resurgence following a nationwide measles vaccination campaign, 2005–2008. J. Infect. Dis. 204(Suppl. 1), S226–S231 (2011).
- Yaméogo KR, Perry RT, Yaméogo A et al. Migration as a risk factor for measles after a mass vaccination campaign, Burkina Faso, 2002. Int. J. Epidemiol. 34(3), 556–564 (2005).
- Ettarh RR, Mutua MK, Kyobutungi C. Ethnicity and delay in measles vaccination in a nairobi slum. Trop. Med. Health 40(2), 59 (2012).
- Marinova L, Muscat M, Kojouharova M, Mihneva Z, Kojouharova M. An update on an ongoing measles outbreak in Bulgaria, April–Nov 2009. Euro Surveillance 14(50)(2009).
- Toole MJ, Waldman RJ. The public health aspects of complex emergencies and refugee situations. Ann. Rev. Public Health 18(1), 283–312 (1997).
- Guerrier G, Zounoun M, Delarosa O et al. Malnutrition and mortality patterns among internally displaced and non-displaced population living in a camp, a village or a town in Eastern Chad. PloS One 4(11), e8077 (2009).
- World Health Organization. Progress towards reducing measles mortality and eliminating measles, WHO eastern Mediterranean Region, 1997–2007. Wkly. Epidemiol. Rec. 83, 97–104 (2008).
- Hu X, Xiao S, Chen B, Sa Z. Gaps in the 2010 measles SIA coverage among migrant children in Beijing: evidence from a parental survey. Vaccine 30, 5721–5725 (2012).
- World Health Organization. Global reductions in measles mortality 2000–2008 and the risk of measles resurgence. Wkly Epidemiol. Rec. 49, 505–516 (2009).
- Lessler J, Lowther SA, Moss WJ, Cummings DA. Maintaining high rates of measles immunization in Zambia. Epidemiol. Infect. 5, 1–11 (2010).
- Bauch CT, Szusz E, Garrison LP. Scheduling of measles vaccination in low-income countries: projections of a dynamic model. Vaccine 27, 4090–4098 (2009).
- World Health Organization. Progress in measles control: Kenya, 2002–2007. Wly Epidemiol. Rec. 82, 329–336 (2007).
- Masresha BG, Fall A, Eshetu M et al. Measles mortality reduction and pre-elimination in the African region, 2001–2009. J. Infect. Dis. 204, S198–S204 (2011).
- Cutts FT, Zell ER, Mason D, Bernier RH, Dini EF, Orenstein WA. Monitoring progress toward US preschool immunization goals. JAMA 267(14), 1952–1955 (1992).
- Cutts FT, Izurieta H, Rhoda D. Measuring coverage in maternal and child health: design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PloS Med. 10(5), e1001404 (2013).
- Mbabazi WB, Nanyunja M, Makumbi I et al. Achieving measles control: lessons from the 2002–06 measles control strategy for Uganda. Health policy and planning 24(4), 261–269 (2009).
- Osborne K, Weinberg J, Miller E. The European Sero-Epidemiology Network. Euro Surveillance 2(4), 29–31 (1997).
- Gay N, Ramsay M, Cohen B et al. The epidemiology of measles in England and Wales since the 1994 vaccination campaign. Commun. Dis. Rep. CDR Rev. 7(2), R17 (1997).
- Lowther SA, Curriero FC, Kalish BT, Shields TM, Monze M, Moss WJ. Population immunity to measles virus and the effect of HIV-1 infection after a mass measles vaccination campaign in Lusaka, Zambia: a cross-sectional survey. Lancet 373(9668), 1025–1032 (2009).
- Nigatu W, Samuel D, Cohen B et al. Evaluation of a measles vaccine campaign in Ethiopia using oral-fluid antibody surveys. Vaccine 26(37), 4769–4774 (2008).
- Ohuma E, Okiro E, Bett A et al. Evaluation of a measles vaccine campaign by oral-fluid surveys in a rural Kenyan district: interpretation of antibody prevalence data using mixture models. Epidemiol. Infect. 137(2), 227–233 (2009).
- Babad H, Nokes DJ, Gay N, Miller E, Morgan-Capner P, Anderson R. Predicting the impact of measles vaccination in England and Wales: model validation and analysis of policy options. Epidemiol. Infect. 114(2), 319–344 (1995).
- Andrews N, Tischer A, Siedler A et al. Towards elimination: measles susceptibility in Australia and 17 European countries. Bull. World Health Organ. 86(3), 197–204 (2008).
- Cairns KL, Perry RT, Ryman TK, Nandy RK, Grais RF. outbreak response immunization be recommended for measles outbreaks in middle-and low-income countries? An update. J. Infect. Dis. 204(Suppl. 1), S35–S46 (2011).
- Luquero FJ, Pham-Orsetti H, Cummings DA et al. A long-lasting measles epidemic in Maroua, Cameroon 2008–2009: mass vaccination as response to the epidemic. J. Infect. Dis. 204, S243–S251 (2011).
- Goodson JL, Wiesen E, Perry RT et al. Impact of measles outbreak response vaccination campaign in Dar es Salaam, Tanzania. Vaccine 27(42), 5870–5874 (2009).
- World Health Organization. Progress in global measles control, 2000–2010. Wkly. Epidemiol. Rec. 87(5), 45–52 (2012).
- Metcalf CJE, Lessler J, Klepac P, Cutts FT, Grenfell BT. Minimum levels of coverage needed for rubella vaccination: impact of local demography, seasonality and population heterogeneity. Epidemiology and Infection 16, 1–12 (2012).
- World Health Organization. Rubella Vaccine: WHO position paper. Wkly. Epidemiol. Rec. 75, 161–172 (2000).
- Morice A, Carvajal X, Leon M et al. Accelerated rubella control and congenital rubella syndrome prevention strengthen measles eradiction: the Costa Rican experience. J. Infect. Dis. 187, S158–S163 (2003).
- Metcalf CJE, Munayco CV, Chowell G, Grenfell BT, Bjørnstad ON. Rubella meta-population dynamics and importance of spatial coupling to the risk of congenital rubella syndrome in Peru. J. R. Soc. Interface 8, 369–376 (2011).
- Metcalf CJ, Cohen C, Lessler J et al. Implications of spatially heterogeneous vaccination coverage for the risk of congenital rubella syndrome in South Africa. J. R. Soc. Interface 10(78), 20120756 (2013).
- Zimmerman LA, Muscat M, Jankovic D et al. Status of Rubella and Congenital Rubella Syndrome Surveillance, 2005–2009, the World Health Organization European Region. J. Infect. Dis. 204(Suppl. 1), S381–S388 (2011).
- Muscat M, Zimmerman L, Bacci S et al.; Toward rubella elimination in Europe: an epidemiological assessment. Vaccine 30, 1999–2007 (2012).
- Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, Mcfarland JW, Hersh BS. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet 369, 191–200 (2007).
- De Quadros C, Hersh B, Nogueira A, Carrasco P, Da Silveira CM. Measles eradication: experience in the Americas. Bull. World Health Organ. 76(Suppl. 2), 47 (1998).
- Izurieta H, Venczel L, Dietz V et al. Monitoring measles eradication in the region of the Americas: critical activities and tools. J. Infect. Dis. 187(Suppl. 1), S133–S139 (2003).
- Dietz V, Venczel L, Izurieta H et al. Assessing and monitoring vaccination coverage levels: lessons from the Americas. Rev. Panam. Salud Publica 16(6), 432–442 (2004).
- Rani M, Yang B, Nesbit R. Hepatitis B control by 2012 in the WHO Western Pacific Region: rationale and implications. Bull. World Health Organ. 87, 707–713 (2009).
- Ferrari MJ, Grenfell BT, Strebel PM. Think globally, act locally: the role of local demographics and vaccination coverage in the dynamic response of measles infection to control. Philos. Trans. R. Soc. London B doi:10.1098/rstb.2012.0141 (2013) (Epub ahead of print).
- Lim SS, Stein DB, Charrow A, Murray CJL. Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet 372(9655), 2031–2046 (2008).
- Featherstone DA, Rota PA, Icenogle J et al. Expansion of the global measles and rubella laboratory network 2005–09. J. Infect. Dis. 204(Suppl. 1), S491–S498 (2011).
- Khowaja AR, Sheikh S, Saleem AF, Zaidi AKM. awareness and coverage of mass measles vaccination drive 2011 cross-sectional survey in the metropolitan city of Karachi, Pakistan. Asia Pac. J. Public Health (2012).
- Panagiotopoulos T, Antoniadou I, Valassi-Adam E. Increase in congenital rubella occurrence after immunisation in Greece: retrospective survey and systematic review. Br. Med. J. 319, 1462–1467 (1999).
- Sosa N, Guerra I, Abrego L et al. Successful public health response to four cases of imported measles in Panama. J. Infect. Dev. Ctries. 6(8), 605–610 (2012).
- De Serres G, Markowski F, Toth E et al. The largest measles epidemic in North America in a decade – Quebec, Canada, 2011: contribution of susceptibility, serendipity and super-spreading events. J. Infect. Dis. 207(6), 990–998 (2013).
- Hopkins DR. Disease eradication. N. Engl. J. Med. 368, 54–63 (2013).
Websites
- Gavi. GAVI Alliance: what we do. www.gavialliance.org/support/apply/countries-eligible-for-support (Accessed 16 April 2013)
- World Health Organization. Global measles and rubella strategic plan, 2012–2020 (2012). http://whqlibdoc.who.int/publications/2012/9789241503396_eng.pdf (Accessed April 2013)
- Measles Initiative. www.measlesrubellainitiative.org (Accessed January 2013)
- World Health Organization. Reported measles cases. http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidencemeasles.html (Accessed July 2013)
- WHO/UNICEF. WHO-UNICEF estimates of MCV coverage. http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tswucoveragemcv.html (Accessed 18 January 2013)
- World Health Organization. Response to measles outbreaks in mortality reduction settings. (2009) www.who.int/immunization/documents/diseases/measles_who_ivb_09_03/en
- GAVI Alliance. Measles-rubella vaccine support. www.gavialliance.org/support/nvs/measles-rubella (Accessed April 2013)
- World Health Organization. Measles. Fact sheet N°286 www.who.int/mediacentre/factsheets/fs286/en (Accessed April 2013)
- World Health Organization. Western Pacific Region nearing 2012 milestone in hepatitis B control. www.wpro.who.int/mediacentre/releases/2012/20120927d/en/index.html (Accessed 24 January 2013)
- World Health Organization. Retrospective measles data on Supplementary Immunization Activities 2000–2011. www.who.int/immunization_monitoring/data/data_subject/en/index.html (Accessed April 2013)
