Abstract
Streptococcus pneumoniae is a major cause of childhood morbidity and mortality worldwide. A heptavalent polysaccharide-protein conjugate vaccine (PCV) has proven highly effective in preventing pneumococcal disease in industrialized countries. Two higher-valent pneumococcal conjugate vaccines are now widely available, even in the poorest countries. These differ from each other in the number of serotypes and carrier proteins used for their conjugation. Some have assumed that the only meaningful clinical difference between PCV formulations is a function of the number of serotypes each contains. A careful review of recent clinical data with these and several unlicensed PCV formulations points to important similarities but also that some key properties of each vaccine likely differ from one another.
Background
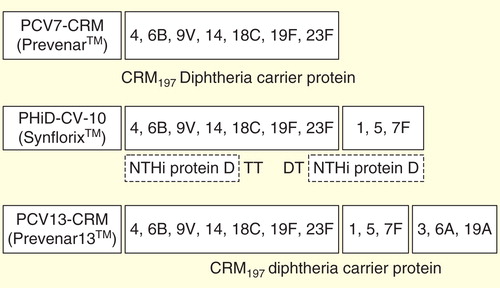
There is little doubt that the heptavalent pneumococcal-protein conjugate vaccine (Pfizer, New York, NY, USA), first licensed 14 years ago, is highly effective in preventing invasive pneumococcal disease (IPD) in children Citation[1], and in providing moderate protection against pneumococcal acute otitis media (AOM) Citation[2] and pneumonia Citation[3]. Even more striking from a public health standpoint has been the herd protection through which routine infant immunization programs have decreased transmission and therefore IPD of each of the 7 vaccine serotypes (VT) in vaccine-ineligible age groups Citation[4,5]. While some increase in IPD caused by serotypes not contained in the vaccine (non-VT) has been observed, in young children this ‘non-VT replacement disease’ has been outweighed in almost all settings by the profound decrease in VT disease Citation[4]. Building on this success, PCV7-CRM (in which the capsular polysaccharides from 7 epidemiologically important serotypes were chemically linked or conjugated to the non-toxic cross-reactive mutant of diphtheria toxin CRM197) was rapidly replaced 5 years ago by two extended valent conjugate vaccines that also contain the globally important serotypes 1 and 5 (among others, ) Citation[6,7]. These are the 10-valent pneumococcal non-typable Haemophilus influenzae (NTHi) protein D conjugate vaccine (PHiD-CV-10; GlaxoSmithKline, Belgium), with 8 polysaccharides conjugated to protein D of H. influenzae, and the other 2 conjugated to tetanus or diphtheria toxoids, and the 13-valent pneumococcal CRM197 conjugate vaccine (PCV13-CRM; Pfizer, New York, NY USA), with all 13 polysaccharides conjugated to CRM197.
These new vaccines were licensed on the basis of immunogenicity and reactogenicity profiles comparable to that of PCV7-CRM, and promptly recommended by WHO and other authorities for broad introduction in developed and developing countries. Given this experience, it is tempting to predict that the disease impact of the new vaccines on pneumococcal disease is solely a function of their serotype content, adjusted for the relative epidemiological importance of the additional types. Accordingly, many publications provide seemingly precise estimates of the local proportion of pneumococcal disease ‘preventable’ by each vaccine formulation. These estimates are fed into health economic models that allow decision-makers to prioritize support for these PCVs in the context of other possible public health interventions, to decide upon the relative value of competing formulations, and for vaccine developers to assess the incremental value of adding even more serotypes. This review argues that there are a number of assumptions about how PCVs ‘behave’ based solely on the PCV7-CRM experience that need to be systematically re-examined in light of what we are learning about other PCVs.
Determining PCV serotype coverage
Calculation of IPD coverage based on post-PCV7-CRM serotype distribution underestimates the impact of any PCV
In countries that have implemented universal infant PCV vaccination, reliance on the most recent IPD serotype distribution data in the target age group (for potential direct impact), or in the non-vaccinated age group (for potential indirect impact) to estimate the future preventive potential of a given PCV can result in misleading conclusions as it discounts the importance of continued prevention of vaccine-type disease. This is because after only a few years, PCV introduction will have already substantially decreased VT IPD, thereby ‘removing’ those serotypes from the vaccine-preventable disease calculations.
However, until complete eradication, these VT continue to circulate and can potentially cause disease, even within the US population where PCV7-CRM was first introduced in a universal mass vaccination (UMV) program 14 years ago Citation[5,8]. Experience with other vaccines (e.g., pertussis, measles and poliomyelitis) has shown that stoppage of those vaccination programs results in a rapid resurgence of disease, indicating that those vaccines continue to prevent disease even in the absence of current cases. By analogy, vaccination against the 7 serotypes (within PHiD-CV-10 or PCV13-CRM) likely still provides significant disease protection value.
The consequences of this approach are illustrated in (‘Method A’) Citation[9], in which the relative coverages of PCV7-CRM, PHiD-CV-10 and PCV13-CRM are depicted based on IPD serotype data from <5 year olds in the UK Citation[10], a post-PCV7 setting where the remaining IPD is overwhelmingly due to non-PCV7-VT. If these relative coverage figures were interpreted as being representative for the future preventive potential of each vaccine formulation, it would suggest that PCV7-CRM has virtually no value in <5 year olds, and that even PCV13-CRM (the combination of PCV7 serotypes, 1, 5, 7F and 3, 6A and 19A) could prevent fewer than half of the cases of IPD in that age group, but still almost 50% more cases than PHiD-CV-10 could prevent.
Figure 2. Number of average adjusted cases of pediatric invasive pneumococcal disease ‘covered’ by different pneumococcal conjugate vaccines in the UK based solely on post-PCV7-CRM serotype information (Method A), solely on pre-PCV7-CRM serotype information (Method B) or on a summation (see text for details) of pre- and post-PCV7-CRM data (Method C). (Note: all calculations assume 6A coverage for PCV7-CRM and PHiD-CV-10 due to 6A/6B cross-protection).
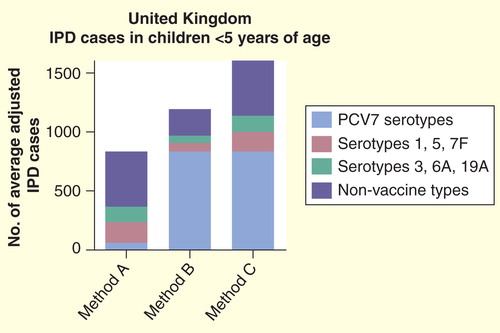
In other words, to rely solely on the traditional serotype coverage method in the post-PCV7-CRM era would substantially underestimate the value of vaccination with any PCV formulation that contains at least the seven types. It would also magnify relatively small differences in vaccine coverage between different formulations. In fact, the logical conclusions of this approach are that there is no longer a need for any PCV to contain the 7 serotypes, and that soon even the higher-valent vaccines will render themselves obsolete and PCV vaccination can stop altogether – something no one would consider.
Calculations of vaccine serotype coverage should take into account those serotypes currently being prevented
One alternative approach would be to use only pre-PCV7-CRM data on serotype distribution and incidence for IPD cases. This has the advantage of relatively large disease and serotype numbers. However, increases in non-VT-PCV7 disease due to secular trends or serotype replacement occurring post-PCV7 implementation are ignored altogether, which is clearly unacceptable (, ‘Method B’).
Another approach would be to add the incidence of PCV7 types in the pre-PCV7 era with the incidence of non-PCV7 types in the post-PCV7-CRM era (, ‘Method C’). The resultant combined figures are artificial, especially since much of the rise in non-PCV7 types may have been a consequence of the disappearance of the PCV7 types. Nonetheless, this approach has the advantage of acknowledging that the serotype epidemiology has changed, as well as indicating that continued prevention of PCV7 types is important. It also suggests that vaccination with any of the formulations would continue to prevent a major proportion of IPD, and puts into perspective the limited incremental value of any higher-valent formulation over PCV7-CRM in this setting. We would recommend that future publications that analyze local serotype data consider including a graph showing calculations using both methods A and C.
All of these approaches estimate vaccine impact on IPD, where a specific serotype has been identified from a sterile site by culture or molecular methods. However, this in itself will underestimate the impact of any PCV, as it has recently been shown that pneumococcal conjugate vaccination can prevent a substantially larger number of clinically identical ‘suspected IPD’ cases lacking definitive pneumococcal detection Citation[11].
The above calculations assume that the impact of any PCV is purely a function of which serotypes are included. However, a closer look at the clinical evidence amassed with PCV7-CRM, with other conjugate formulations developed but never licensed, as well as emerging evidence with the two newer PCVs Citation[12], indicates a more nuanced picture in which individual serotypes and each vaccine formulation need to be considered separately to formulate accurate predictions. In the remainder of this review, we attempt to contrast what we thought we ‘knew’ from the PCV7 experience with what we have learned so far from the experience with the higher-valent vaccines. The story becomes more challenging, but also more interesting.
Inclusion of a serotype may not automatically translate into disease protection in the vaccinees
Although the overall efficacy against VT IPD is very high for PCV7-CRM, slightly lower efficacy against 19F disease, compared with other serotypes, was observed in some of the IPD and AOM efficacy and effectiveness studies Citation[10,13–15]; the latter may be reflected in a lingering, low level of 19F IPD seen in several post-PCV7-CRM licensure studies Citation[5,16]. This highlights the possibility that there may be lower protection against some of the serotypes included in the higher-valent formulations.
Direct IPD protection: serotypes 5, 6A, 7F & 19 A
Nevertheless, licensure of both PHiD-CV-10 and PCV13-CRM followed WHO guidance, whereby (presumably protective) immune responses for each of the new serotypes were elicited in proportions of children similar to those seen with serotypes included in PCV7-CRM, and these antibodies were functional (opsonophagocytic) Citation[17,18]. In particular, serotype 7F in both vaccines, and the 6A and 19A conjugates in PCV13-CRM elicit immune responses (geometric mean concentrations and geometric mean titers) as robust as those seen for the heptavalent types Citation[17,18]. Furthermore, 6A, 7F and 19A appear epidemiologically similar to the 7 serotypes in PCV7-CRM in that they infect people of all ages but with highest disease incidence in the youngest children, and are detected frequently in nasopharyngeal (NP) carriage studies. Thus, there is no reason to a priori predict that direct protection against 6A, 7F and 19A IPD would be much different than that seen against the 7-valent types, and indeed initial post-marketing surveillance already indicates a high level of protection against 7F and 19A Citation[19–21]. With regard to serotype 5, although it also has relatively low immunogenicity, the African studies with 9-valent PCV-CRM nonetheless demonstrated excellent serotype 5-specific IPD efficacy Citation[22,23].
Questions about direct protection against serotypes 1 & 3
In contrast, the evidence is less robust for 1 and 3 and there may be reasons to be cautious. Epidemiologically, serotype 1 is quite different: it is rarely detected in healthy carriers and often occurs in outbreaks Citation[24,25], characteristics shared with serotype 5. In addition, serotype 1 causes IPD at similar rates in younger and older children and even adults, which suggests that age-associated immunity against this type is not as readily acquired as with the heptavalent types Citation[26]. In fact, when taken together, the two African efficacy studies failed to provide conclusive evidence of serotype 1 efficacy, although the total number of serotype 1 cases was small Citation[22,23].
Yet serotype 1 disease appears inherently preventable by vaccination. In the pre-antibiotic era, treatment of serotype 1 pneumonias with serotype 1-specific immune sera appeared successful Citation[27]. Pneumococcal polysaccharide vaccines provided effectiveness point estimates against serotype 1 IPD in adults higher than those seen for other VTs Citation[28,29]. The observation that conjugate immunogenicity against serotype 1 is relatively low, and that the breakthrough cases of serotype 1 disease in the African studies occurred in the 2nd and 3rd year of life prompted the suggestion that the duration of protection in the absence of a booster dose was less than optimal (the vaccine was only given at 6, 10 and 14 weeks) Citation[30]. Indeed, preliminary calculations for PCV13-CRM given in a 2 + 1 schedule suggest high effectiveness against serotype 1 IPD 15 and 42 months after introduction Citation[21,31].
The situation with serotype 3 is even less clear. In the pre-antibiotic era, treatment of serotype 3 pneumonias with serotype 3-specific immune sera was singularly unsuccessful Citation[27], in contrast to the experience with antisera of several other types. Pneumococcal polysaccharide vaccine studies in older children and adults have yielded mixed results; two suggested some effectiveness against serotype 3 IPD Citation[28,29] but with lower point estimates than those seen with other types, while a third reported a tendency for more serotype 3 cases in vaccinated compared to unvaccinated adults Citation[32].
Furthermore, the immunogenicity of serotype 3 conjugates in infants is relatively low, and in contrast to other serotypes, there is no clear evidence of toddler boosting by several different serotype 3 conjugates as measured by ELISA assays Citation[17,33–35]. Assessment of functional antibody responses is difficult as serotype 3’s large, macroscopically distinguishable mucoidal polysaccharide capsule prevents easy lysis in a laboratory opsonophagocytosis assay (OPA) Citation[36]. Nonetheless, OPA assays constructed with sensitive, possibly clinically atypical strains provide some evidence of serotype 3 toddler boosting after, for example, PCV11-PD, the serotype 3-containing all protein D conjugate precursor of PHiD-CV-10 Citation[36].
Figure 3. Serotype-specific antibody concentrations after administration of different serotype 3-containing pneumococcal conjugated vaccine formulations according to 3 + 1 infant schedules, as measured by ELISA. (A) PCV11-PD, Czech Republic and Slovakia; (B) PCV13-CRM, Germany; (C) PCV11-TT-DT, The Philippines; (D) PCV8-DT, Finland and (E) PCV8-TT, Finland.
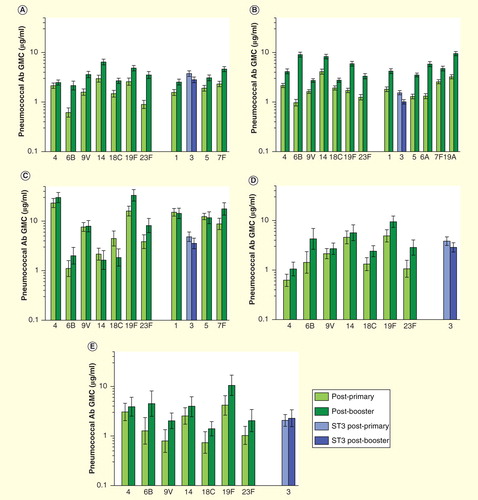
The only direct assessment of the clinical efficacy of a serotype 3 conjugate comes from a double-blind randomized controlled trial (RCT) that revealed that PCV11-PD was completely unable to prevent serotype 3 AOM and even showed a trend toward increased serotype 3 cases post-booster Citation[33]. A RCT with PCV13-CRM provided no evidence that PCV13-CRM can decrease serotype 3 NP carriage Citation[37]. A recent serotype-specific effectiveness analysis undertaken 3.5 years after UMV introduction of PCV13-CRM, showed no significant protection against serotype 3 IPD, and suggested that markedly higher serum antibody levels would be needed for this serotype compared with the others Citation[21]. While there are sometimes suggestive findings from case–control studies or ecological analyses Citation[38], small numbers of cases and temporal variations Citation[39] make it difficult to draw firm conclusions. To date, there is no conclusive evidence that PCV13-CRM prevents serotype 3 IPD, AOM or provides any serotype 3 herd protection Citation[38–41].
Direct protection against mucosal disease: difficult to predict serotype-specific efficacy against AOM based on immunogenicity
It has been postulated Citation[42] that antibody thresholds to prevent IPD are lower than those for mucosal disease or carriage. This raises the possibility that vaccines with distinct immunogenicity profiles, although similarly effective against VT IPD, could nonetheless differ in impact on VT AOM.
Yet, at present, there is no evidence that the differences in immunogenicity between PCV7-CRM, PCV7-OMP (7 capsular polysaccharides conjugated to the outer membrane protein of Neisseria meningitidis), PCV11-PD and PHiD-CV-10 have translated into differences in efficacy against VT AOM as assessed through tympanocentesis within three double-blind RCTs Citation[14,33,43,44]. As assessment of PCV7-CRM and PCV7-OMP occurred in the same study, it is possible to compare vaccine-type AOM efficacy values with immunogenicity for each of the 7 serotypes . In several cases, one or the other vaccine elicited three- to fivefold higher antibody levels for a given serotype, but there was no obvious relationship with the corresponding vaccine efficacy (VE) point estimates.
Table 1. Efficacy of vaccination with four pneumococcal conjugate vaccine or vaccine candidates in infants and young children against vaccine type and serogroup 6 acute otitis media as assessed in tympanocentesis-based double-blind randomized controlled studies.
Table 2. Lack of obvious relationship between serotype-specific immunogenicity and efficacy against vaccine type acute otitis media.
The above findings are consistent with the observation that attempts to determine population-based ELISA antibody correlates of protection against AOM and NP carriage have, to date, proven elusive for more than an occasional serotype Citation[42,45]. In other words, this immune parameter simply may not reflect a key protective mechanism for these mucosal end points.
Interpretation of results from AOM efficacy & effectiveness studies of PCVs is not straightforward
PCV7-CRM and PHiD-CV-10 each demonstrated statistically significant protection against overall AOM in separate double-blind randomized controlled efficacy trials, with point estimates (and 95% CI) of 6.4% (3.9–8.7) Citation[2] and 19.0% (4.4, 31.4) Citation[43], respectively, in intent-to-treat analyses (there are no AOM efficacy data with PCV13-CRM). The difference in efficacy point estimates is consistent with those derived from other efficacy studies of PCV7-CRM Citation[2,14] and PCV11-PD Citation[33], but differences in study site baseline epidemiology and other confounding variables make it difficult to directly compare these values or use them as the basis for predictions of vaccine impact Citation[46].
Unfortunately, the magnitude of the effectiveness of PCV7-CRM in infant immunization programs is even more unclear, as it has been reported in North American settings to range from −7 to 48% Citation[2]. A recent review Citation[2] highlighted the approximately 20% decreases in AOM visits observed in almost all of these studies in the years prior to PCV7-CRM introduction, likely attributable to several factors including decreasing exposure of children to second-hand tobacco smoke Citation[47]. The authors concluded that a time-series analytic methodology that adjusts for changes in baseline and other confounders is crucial for any analysis of PCV impact on AOM Citation[2].
No evidence that differences in PCV serotype composition significantly affect direct protection against pneumonia
For pneumonia, results from six separate RCTs with PCVs have been analyzed using the WHO standardized alveolar consolidated end point designed to facilitate inter-trial comparisons. In five of these ‘vaccine-probe’ studies, 7-, 9-, 10- and 11-valent conjugates (there are no efficacy data with PCV-CRM-13) prevented 20–37% of alveolar consolidated pneumonias conducted in very different settings Citation[43,48]. (The sixth study was conducted with PCV7-CRM in a Native American population but unlike the others was randomized by cluster, rather than by individual, examined only in-patient pneumonia, and failed to provide any evidence of efficacy Citation[49]). While the lack of definitive etiological information on pneumonia makes it difficult to predict the impact of a specific vaccine formulation even on VT pneumococcal pneumonia, these results from the five studies nonetheless indicated that in each setting at least 20–37% of those pneumonias must have been caused by VTs and/or vaccine-related types. In fact, the proportion due to VT could be much greater, depending on the actual VE against VT pneumococcus and the proportion of consolidated pneumonias actually due to pneumococcus Citation[50].
A more perplexing finding is that, based on the point estimates, there is no indication of a trend for greater protection with higher valence vaccines. This might be explained if only a few serotypes common to each formulation are primarily responsible for the portion of consolidated pneumonia caused by vaccine types. After all, a limited number of serotypes (most notably serotypes 1 and 3) predominated as principal causes of pediatric complicated pneumonia and empyema worldwide in the pre-PCV7 era Citation[26]. It is also possible that setting- or study-specific factors, such as patient selection and degree of access to care, antibiotic treatment patterns or local differences in serotypes or pathogens, are simply greater determinants of overall VE than small differences in serotype coverage in the different vaccine formulations. Differences in patient access might alone Citation[51] explain the trend for greater efficacy (point estimate 37%) in the rural Gambia Citation[22] than in urban South Africa (point estimate 20%) Citation[23], as both trials used the same vaccine (PCV9-CRM).
Regardless of the explanation, the available pneumonia efficacy data provide no support to the notion that one can easily predict the pneumonia impact of a PCV by relying on the number of serotypes or even that increasing numbers of serotypes beyond seven will result in a detectable increase in protection against pneumonia.
Some vaccine formulations may offer cross-protection, but this cannot be assumed
The importance of cross-protection (or rather, the lack thereof) was poignantly highlighted in the pre-antibiotic era, when pneumonia patients were routinely treated with serotype-specific antisera. Prince Valdemar of Denmark was diagnosed in 1939 with pneumonia and treated with serogroup 9 antisera. Only after the failure of treatment (with what later was determined to be 9N antisera) was it realized that there was more than one serotype within serogroup 9 and that cross-protection did not exist in this case. The newly identified serotype was christened 9V in his honor posthumously Citation[52].
From a public health standpoint, understanding the extent of cross-protection is especially important for serotypes 6A and 19A, as these have long been recognized to be epidemiologically significant in both children and adults Citation[7,53]. Neither one is included in PHiD-CV-10, while both are in PCV13-CRM.
6B-6A cross-protection: not all 6B-conjugates are capable of it, even though ‘high’ antibody levels are probably not needed
There is extensive structural similarity of 6A and 6B. In fact, due to the excellent anti-6A immunological response to the 6B moiety, the 23-valent polysaccharide vaccine does not include 6A Citation[53]. Regarding conjugates, 6B-containing PCV7-CRM is highly effective against 6A IPD, resulting in 50–75% decreases in vaccine-eligible cohorts within 3 years of UMV with both 3 + 1 and 2 + 1 schedules Citation[54–56].
One important lesson from this experience (which may also hold for 19A – see below) is that this excellent clinical cross-protection was observed even though the levels of antibody elicited were well below those seen for ‘true’ vaccine types. Thus, post-primary PCV7-CRM elicited much lower anti-6A geometric mean concentration levels (0.23 µg/ml) than it did against true vaccine types (range 1.44–4.61 µg/ml) or, for that matter, than those elicited against 6A by 6A-containing PCV13-CRM (1.33 µg/ml) Citation[17]. In contrast, the percentages of children with functional (opsonophagocytic) antibodies over the 1:8 threshold against 6A were closer: 72% for PCV7-CRM against 6A and 96–100% against true vaccine types and 96% for PCV13-CRM against 6A Citation[17]. This observation of substantial clinical effectiveness is actually thus consistent with WHO licensure criteria, which as noted earlier relies on immunological comparisons of the proportions of children above a certain threshold, since those (and not GMCs) are what correlated with clinical protection Citation[57].
Despite these low antibody levels, PCV7-CRM also eventually led to dramatically decreased 6A NP carriage Citation[8] and significant herd protection against 6A IPD Citation[55,56]. Interestingly, not every study could detect a clear impact of this vaccine at every time point on 6A NP carriage Citation[58,59] using culture-detection techniques, suggesting it may manifest as relatively subtle changes in 6A carriage density. Alternatively, it is theoretically possible that a true decrease in 6A was masked in these early studies by a concurrent and equivalent rise in (then unknown) 6C, but some subsequent studies that did distinguish 6A from 6C also failed to detect any decrease in the first few years Citation[60,61]. These findings are consistent with the observation that the population-level declines in 6A NP carriage and IPD following PCV7-CRM UMV were slower to manifest than those seen with 6B Citation[55,56]. These are also consistent with the slightly lower 6A IPD vaccine effectiveness point estimate calculated in the US case–control study (76%) compared to those seen for the true VTs (89–100%) Citation[15], as well as the somewhat less potent effect of the vaccine on 6A versus 6B NP carriage Citation[58].
Taken together, the ultimately impressive impact of PCV7-CRM following its widespread introduction on 6A carriage and disease led to the commonly held assumption that any 6B-containing vaccine would be cross-protective against 6A disease. Yet even this is probably not correct. As shown in , while all four conjugate formulations tested within gold standard double-blind RCTs showed high efficacy against 6B AOM, the CRM and protein D-conjugates (PCV7-CRM, PCV11-PD and PHiD-CV-10), but not the OMP conjugate (PCV7-OMP), provided evidence of 6A efficacy. Consistent with these efficacy differences is the lower functional anti-6A antibody response to PCV7-OMP compared with PCV7-CRM Citation[62], although the immunological parameter underlying AOM protection has not yet been established.
The observation that PCV7-CRM and PHiD-CV-10 elicit similar proportions of children with anti-6A OPA >1:8 might suggest similar levels of IPD cross-protection Citation[18,63]. However, direct evidence for PHiD-CV-10 is limited because those countries with previous experience with PCV7-CRM had little 6A disease remaining. Chile was the only country with at least 10 6A IPD cases annually in children <2 years of age in each of the 4 years prior to PHiD-CV-10 introduction in 2011, averaging 18 cases per year, but had only 5 in 2012 Citation[64].
Questions about cross-protection against newly identified serotypes 6C and 6D have also been raised. 6C appears to be responsible for only a few percent of the remaining IPD in post-PCV7 populations in children Citation[65,66], but may represent a significant target in adults Citation[31,55]. 6A-CRM (but not 6B-CRM) conjugates elicit functional antibodies against 6C in a significant proportion of children Citation[67], suggesting this may translate into cross-protection for 6C disease from PCV13-CRM Citation[55,65]. No data are available about whether 6B-containing PHiD-CV-10 offers any protection against 6C or 6D.
19 F-19A cross-protection can exist
Despite suggestive observations Citation[68] that 19F polysaccharides may provide some protection against 19A disease, PCV7-CRM, in particular, does not appear to elicit clinically meaningful cross-protection against 19A IPD Citation[19,69], especially in the face of high 19A carriage levels and antibiotic selection pressure (favoring antibiotic non-susceptible serotypes such as 19A). However, this does not preclude the possibility that other 19F-containing vaccines may offer some cross-protection.
Indeed, the OMP, CRM and protein D conjugates differ in their respective abilities to elicit opsonophagocytic antibodies to the cross-reactive 19A, with the protein D conjugates showing significantly higher proportions of children with OPA >1:8 Citation[70,71]. These conjugate-specific differences in cross-reactivity may seem surprising, given the common polysaccharide origins for each of the vaccines. However, the precise way in which the polysaccharides are derived from the purified capsules, the specific oxidation and conjugation steps involved in conjugate development, as well as the identity of the carrier proteins differ by vaccine. Any of these could influence whether cross-reactive epitopes are destroyed or, conversely, unmasked in the final conjugates. In fact, there is some evidence that the different conjugation methods used for the CRM conjugates (oxidation and reductive amination) versus the 19F-DT conjugate in PHiD-CV-10 (cyanylation) can result in antigenically-distinct alterations to the 19F carbohydrate moiety Citation[70].
In line with these immunogenicity results, some cross-protection against 19A from the 19F contained in PHiD-CV-10 is suggested by the pattern of decreases in 19A IPD in vaccine-eligible cohorts consistently observed 1–2 years after UMV with PHiD-CV-10 in various settings Citation[20,64,71–76]. This was true even in a ‘high’ 19A setting, Quebec, where the incidence of 19A IPD in children <2 years of age immediately prior to PHiD-CV-10 introduction in 2008 was 28/100,000, and comprised 48% of the remaining IPD Citation[72,76].
Table 3. Trend in 19A cases: comparison pre- versus post-PHiD-CV-10 implementation in vaccine-eligible children (countries reporting at least 5 cases of 19A annually pre-PHiD-CV-10).
Furthermore, two recent case–control analyses have pointed to 67–83% protection by PHiD-CV-10 against 19A IPD Citation[20,77]; in Quebec, which uses a 2 + 1 schedule, the estimated vaccine effectiveness was similar to that subsequently estimated with PCV13-CRM Citation[78].
In conclusion, there is evidence from both RCTs and post-marketing surveillance that PCVs containing the same serotypes can nonetheless differ in the extent of cross-protection they provide.
Herd (indirect) protection
One of the major surprises with PCV7-CRM was the rapidity and magnitude of the herd protection it elicited, which was documented for each vaccine serotype in different UMV settings Citation[4]. One question is whether there is any intrinsic reason to believe that the higher valency PCVs will elicit more or less herd protection against serotypes 1, 3, 5, 6A, 7F or 19A compared to what was seen with PCV7 types and PCV7-CRM.
For serotypes 6A, 7F and 19A, there does not seem any a priori reason to believe that conjugate vaccines containing those types will not provide substantial herd protection. Decreases in NP colonization in each have already been observed with PCV13-CRM Citation[34]. Consistent with this, evidence of IPD herd effect for 7F and 19A is already available from early US CDC surveillance data with PCV13-CRM Citation[79].
In contrast, the lack of a detectable impact of PCV13-CRM on serotype 3 NP carriage Citation[37] would lead one to expect little herd protection Citation[38,40].
Serotype-specific epidemiological differences suggest herd protection against 1 & 5 disease should not be taken for granted
It is sometimes questioned whether conjugate vaccines could provide herd protection against serotypes 1 and 5 because they are rarely detected in NP carriage studies. Low detection is likely a consequence of both low density as well as short duration of carriage. However, as long as NP carriage is believed to be a necessary prerequisite for disease Citation[80], vaccine-induced decreases in carriage regardless of its precise level should commensurately decrease transmission from vaccinees. Indeed, substantial herd protection from PCV7-CRM has been observed for serotype 4 Citation[5], another serotype that is rarely detected in carriage studies. And there is preliminary evidence of PCV13-CRM impact on serotype 1 carriage, although numbers are small Citation[37].
A more compelling reason why PCVs might not induce appreciable herd protection against serotype 1 and 5 disease relates to directionality of their transmission. The similarity of serotype 1 IPD rates in children and adults Citation[24], and the much greater detection in ill versus healthy individuals, suggest that sick individuals of any age may represent the major source of serotype 1 transmission Citation[81]. In other words, the reservoir of serotype 1 carriage may not be restricted to young healthy children. Thus, infant immunization alone may be insufficient to control serotype 1 (or serotype 5) disease in adults. In both cases, due to outbreak nature of disease caused by those serotypes Citation[24] several years will be needed to assess this.
PCV-specific differences in VT carriage impact & herd protection
To date, only two published studies have compared the NP carriage impact of different licensed formulations. One compared PCV7-CRM with PHiD-CV-10 in a setting of decreased PCV7-type transmission (several years following routine infant PCV7-CRM immunization), and it failed to detect any significant difference in impact on vaccine- or vaccine-related type carriage Citation[82]. Three placebo-controlled NP carriage studies conducted in an environment where VTs were still circulating at high levels suggested that PHiD-CV-10’s impact on VTs may be 25–50%, appreciable but perhaps slightly lower than the 40–60% generally seen in PCV7-CRM studies Citation[71].
A second study compared PCV13-CRM and PCV7-CRM, and it showed that the former had a greater impact on 19A and 6A carriage than the latter. Somewhat surprisingly, 19F carriage was also reduced in PCV13-CRM vaccinees relative to the PCV7-CRM children. The authors speculated that this may be due to altered manufacturing process of 19F-CRM in PCV13-CRM versus PCV7-CRM Citation[37]. Although PCV7-CRM has markedly reduced 6A and 19F IPD in older age groups (in the case of 6A through cross-protection against carriage provided by 6B), these findings may suggest that PCV13-CRM might induce herd protection against those types in a vaccine-naïve population at a faster rate than PCV7-CRM.
While it is recognized that that some study to study variability exists in the magnitude of individual serotype carriage effects, these results support the notion that different vaccines containing the same serotypes may nonetheless have distinct impacts on colonization. The more important question is whether these quantitative differences could translate into long-lasting, meaningful differences in herd protection against disease. One mathematical model that examined this has suggested that UMV of infants with a vaccine that decreased VT colonization by 30% would ultimately lead to near elimination of VT IPD only a year later than a vaccine with 60% impact on VT colonization Citation[83].
To date, only one study has demonstrated herd protection of PCVs in a ‘gold standard’ double-blind controlled trial. This was a cluster-randomized study in Finland, comparing PHiD-CV-10 with non-PCV control vaccines, and which directly demonstrated Citation[73] herd protection in non-vaccinated individuals due to PCVs.
There are no head-to-head data comparing the magnitude of herd protection generated by the different vaccines. Any attempts to compare results in different settings with different vaccines are easily confounded by country-specific differences in degree of vaccination coverage, dosage schedules, secular trends as well as societal mixing patterns (‘the contact matrix’). A major determinant is likely the existence, or not, of catch-up vaccination in older children. Importantly, both PCV13-CRM and PHiD-CV-10 have provided evidence of herd protection against at least some of the VTs Citation[38,73,84], but whether there are long-lasting quantitative differences remains to be seen.
Herd protection for cross-reactive serotypes
It has been previously mentioned that PCV7-CRM provided excellent herd protection against 6A IPD Citation[55,56]. With regard to the newer vaccines, one NP colonization study showed that PHiD-CV-10 significantly decreased 19A carriage Citation[85], but no Citation[71] or only small, statistically insignificant Citation[82] effects were observed in the other two. Clear evidence of PHiD-CV-10 decrease of 6A carriage within clinical studies has not yet been reported, perhaps akin to the situation with PCV7-CRM noted earlier Citation[58,59].
Possible PCV-specific differences in serotype replacement
It is generally accepted that PCV7-CRM can induce serotype replacement at the level of the nasopharynx and in disease Citation[4,86]. This does not preclude synergism of the vaccine effect on non-VT with that due to other factors, such as antibiotic selection pressure. For example, the rise in 19A IPD seen after PCV7-CRM in <2 year olds in the USA was almost entirely due to increases in antibiotic non-susceptible 19A IPD, with only a small rise in antibiotic-susceptible 19A IPD detected Citation[83], an observation difficult to explain as a vaccine impact alone.
Randomized controlled studies with PCV7-CRM have consistently shown rapid and virtually complete replacement of VT at the nasopharynx by non-VT Citation[26,87]. In almost all settings after PCV7-CRM introduction into UMV, overall carriage prevalence of pneumococcus has accordingly stayed nearly the same Citation[33,87–92], although recent studies from the Gambia Citation[93] and South Africa Citation[94] suggest that replacement carriage in older age groups may take longer to manifest than the decrease in VT carriage. The one published head-to-head study of PCV7-CRM and PCV13-CRM detected no difference in overall NP carriage between the two vaccines Citation[37], consistent with similar increases in non-VT carriage elicited by each.
Interestingly, some other data from RCTs raise the possibility that not all PCVs may behave as PCV7-CRM in this regard. In placebo-controlled studies, PCV7-OMP failed to reveal significant non-VT NP carriage replacement Citation[95,96]. The three placebo-controlled studies of protein D vaccines in environments with high VT carriage, which as described earlier showed only moderate effects on VT carriage, also suggested limited non-VT replacement that in all cases appeared delayed compared with the decrease in VT. In the largest such study even by the latest time point (18–22 months), the net pneumococcal carriage was still 25% below controls Citation[33,85,97].
However, it should be noted that the previously mentioned head-to-head colonization study with PHiD-CV-10 and PCV7-CRM, conducted in a post-PCV7-CRM environment with likely minimal VT circulation (there was no placebo control group), failed to reveal any vaccine-specific differences in non-VT NP carriage Citation[82].
Non-VT replacement observed at the level of the nasopharynx does not necessarily directly translate into non-VT disease: children
Despite geographic variability in the magnitude of serotype replacement in IPD, in almost all sites (with the notable exception of the Alaskan Natives) Citation[4,86], the decrease in VT IPD seen after PCV7-CRM introduction was greater than the increase in non-VT IPD in children, thereby leading to a net decrease in overall IPD among children. After 7 years of PCV7-CRM, the net decrease totaled approximately 50% in a recent meta-analysis of 21 surveillance sites worldwide Citation[4].
Why does this discrepancy in replacement magnitude exist for children? It has been known for a long time that the polysaccharide capsule of the pneumococcus can affect its ability to become invasive. As a general principle, the thicker the capsule the better the serotype will be at colonizing and the worse it will be at invading once it colonizes the nasopharynx Citation[86]. Therefore, the propensity of a serotype to cause IPD will be a product of these two properties: the ability to colonize and the ability to invade. PCV7-CRM targeted the most prevalent IPD serotypes in the USA, some of which were effective colonizers (e.g., 6B, 23F), some invasive (e.g., 4) and some both (e.g., 14). For the most part, the non-VT serotypes involved in replacement carriage are less invasive in children than the 7 serotypes in PCV7-CRM, thereby leading to the halving of overall IPD in children Citation[98]. Predictions of the reduction in overall IPD in children <5 years after PCV7-CRM based solely on the pre-PCV7-CRM carriage prevalence and the established invasiveness of a serotype were, on average, close to the observed reductions Citation[99]. Using similar considerations, a more elaborate population-wide mathematical model has recently estimated the potential impact of higher-valent vaccines, although under the assumption that cross-protection does not exist Citation[100].
Non-vaccine type carriage replacement may more readily translate into disease in older children & adults
In contrast with young children, in older children and adults there has remained considerable geographic variability in the degree of non-VT replacement disease. For example, after 5 years of UMV with PCV7-CRM in Quebec and Norway, the number of cases of VT IPD in >5 year olds each decreased by approximately 75–80%. However, in Quebec the rise in non-VT IPD totally offset this benefit, while in Norway the rise was more limited, resulting in a net decrease of approximately 25% in all IPD Citation[20,38]. Accordingly, the same meta-analysis mentioned above found that when all PCV7-CRM UMV data sets are taken together, the non-VT IPD replacement completely offset the decrease in VT IPD in the oldest age groups Citation[4]. It has long been established that non-VT cause a greater proportion of IPD in older children and adults than in young children Citation[7], and since adults with IPD tend to have more underlying illnesses this might make them more susceptible to disease from the less invasive serotypes Citation[101,102]. In line with this thinking, perhaps population differences in proportions of adults with underlying illnesses, or who are smokers, might explain some of the geographical variability.
Other reasons why higher valency PCVs might lead to less serotype replacement disease than PCV7-CRM, at least in the short term
First, the two extended conjugate formulations already appear to provide direct or cross-protection against two of the most common replacement serotypes seen with PCV7-CRM, 7F and 19A, as well as against two other important highly invasive causes of IPD, serotypes 1 and 5.
Second, there are intriguing observations from the three randomized AOM efficacy trials. As noted earlier, each vaccine showed similar efficacies against VT AOM (57–69%). In the FinOM trial, PCV7-CRM and PCV7-OMP each revealed approximately 30% increases in non-VT AOM in the vaccinated group that bordered on statistical significance Citation[14,44]. In contrast, the two studies assessing protein D-containing conjugate proteins (PCV11-PD and PHiD-CV-10) failed to provide any suggestion of replacement AOM by non-VT Citation[33,98]. Small case numbers, differences in trial design and setting and case ascertainment make it impossible to draw definitive conclusions. Unfortunately, no RCT has yet assessed PCV13-CRM efficacy against AOM.
Finally, even if the protein D vaccines elicit less non-VT replacement in AOM and carriage during the time course of the study, it is unknown whether such effects would persist, especially in a UMV population, as the dynamics of serotype replacement take multiple years to play out after vaccine introduction. Indeed, the virtual elimination of VT has been seen in cross-sectional NP studies following extensive use of PCV7-CRM Citation[89], even though in RCTs the decrease in VT was only approximately 50%. These observations point to the need to carefully monitor non-VT NP carriage and IPD rates for multiple years following introduction of each of the new vaccines.
PCV-specific protection against disease caused by other pathogens
Indirect protection
RCTs in South Africa and Latin America have shown that PCV9-CRM and PHiD-CV-10 decreased virus-associated pneumonia cases, with the authors arguing that the finding supports the concept that some viral pneumonia may actually be superinfected with the pneumococcus [Tregnaghi MW, Pers. Comm.] Citation[103]. Limited evidence has suggested that PCV7-CRM may increase Staphylococcus aureus carriage, although this has not been observed in all studies Citation[104].
It has also been proposed that any vaccine effective against pneumococcal AOM could indirectly prevent secondary episodes caused by NTHi, and this effect should thus be most apparent in 2nd, 3rd or 4th episodes of AOM Citation[105]. However, this hypothesis was not borne out in a recent re-analysis of the randomized controlled PCV7-CRM AOM efficacy trial Citation[106]. This re-analysis reported an increased number of NTHi AOM cases in the PCV7-CRM vaccinated group compared to the control group (which received hepatitis B vaccine), with no tendency for any protection regardless of the episode number.
Direct protection against a 2nd pathogen
New PCVs may differ in this regard. Like its 11-valent precursor, PHiD-CV-10 incorporates a carrier protein derived from NTHi, itself a major cause of AOM, and a RCT with PCV11-PD had revealed modest (32.7% [0.77–54.3]) but statistically significant efficacy against H. influenzae AOM Citation[31]. A subsequent RCT with PHiD-CV-10 itself produced a lower VE estimate (21.5% [−43.4–57.0]) that did not achieve statistical significance Citation[43].
Evidence of a protein D-containing vaccine impact on NTHi NP carriage is also inconclusive: two RCTs suggested a small, transient effect on NTHi colonization, especially apparent after the booster dose Citation[107], but two larger studies Citation[82,85] failed to detect any impact. It should be noted, however, that the relationship of overall NTHi carriage levels with NTHi otitis media (possibly caused by a subset of virulent NTHi) has not yet been determined.
In conclusion, to date there is no conclusive evidence that vaccine impact on other pathogens differs between the licensed pneumococcal vaccine formulations, but it would be premature to conclude that such differences do not exist.
Conclusion
The overall impact of a given PCV formulation on IPD, AOM or pneumonia cannot be predicted solely on the basis of its serotype content Citation[108], as there can be vaccine-specific differences in serotype efficacy, in cross-protection and perhaps even herd protection and serotype replacement, not to mention other pathogens. Remaining areas of uncertainty include whether serotype 3-containing vaccines induce clinically significant direct or indirect protection against that serotype, the extent of herd-protection against vaccine-related types and the degree of non-vaccine serotype replacement elicited by each of the new vaccines. Apparent vaccine-specific differences in the rate of herd protection or replacement occurring in the first few years following implementation of UMV programs may disappear in the longer term (‘steady state’). In any event, the higher-valent vaccines, specifically PCV13-CRM and PHiD-CV-10, are not simply line extensions of PCV7-CRM whose value is proportionate to the incremental number of serotypes. As a consequence, well-designed effectiveness and impact studies of these newer PCVs are crucial to understand their true impact and health economic value.
Given the existence of two highly effective higher-valent conjugate vaccines with acceptable safety profiles, the key determinant of population-level disease protection is probably immunization coverage, rather than the potential slight differences that one formulation may have over the other. From a public health standpoint, this is the ideal situation, since healthy competition can only make the vaccines more affordable and thereby allow countries to maximize their efforts to increase coverage. Until a long-term pneumococcal vaccine solution is devised, one based on common antigens potentially covering all serotypes, the present challenge remains getting these two PCVs out to those who need them most.
Expert commentary
Introduction of PCV7-CRM in industrialized countries led to a substantial reduction in the burden of IPD in vaccinated children and also in unvaccinated children and adults due to herd protection. New vaccine formulations have increased serotype coverage (PHiD-CV-10 and PCV13-CRM) but differ in polysaccharide dose, carrier proteins and conjugation methods. Data from early post-licensure effectiveness evaluations indicate that both vaccines are effective in combating IPD, pneumonia and AOM. They also indicate that the number of pneumococcal serotypes in these vaccines (10 vs 13) is an insufficient basis upon which to predict their overall disease impact, because not all serotypes may elicit a protective immune response, cross-protection may cover vaccine-related serotypes and because herd protection and replacement are too complex to evaluate and predict. New approaches are required to adequately assess the long term effects of higher-valent PCVs.
Five-year view
Long-term effects of PCVs will depend on the extent to which the vaccines can induce herd protection and not create a niche for new colonization and replacement disease by lesser (but still) virulent non-VTs and other respiratory pathogens. Based on current trends, in 5 years continuing surveillance across vaccine-introducing countries will reveal some non-VT replacement disease but an overall net effect in children. In other words, current PCVs will likely remove most of the more virulent strains from the nasopharynx, and leave the niche for colonization by strains less virulent in causing disease. The increasing availability of effectiveness data will further stimulate and help sustain the global demand for PCVs.
While pediatric PCV formulations will also have some value when given to adults, there will be diminishing returns in light of increasing herd protection from infant programs. Specific conjugate formulations targeting non-VTs more prevalent in adults may prove to be an attractive approach. Once proof of concept is established, there will be increased interest in common antigen pneumococcal vaccines for all ages with the potential for across-the-serotypes protection. Protein and PCV combination formulations may represent the future approach for pneumococcal vaccination. However, it will be increasingly debated whether the goal should be complete elimination of the pneumococcus from the nasopharynx. The pneumococcus will likely remain an important cause of disease for some time to come.
Trademark statement
Synflorix™ is a registered trademark of the GlaxoSmithKline group of companies.
Prevenar™/Prevnar™ and Prevenar13®/Prevnar13 ® are registered trademarks of Pfizer/Wyeth LLC.
Two higher-valent pneumococcal conjugate vaccines are recommended by WHO for routine use globally.
Both were licensed based on comparison of their immunogenicity (ELISA, opsonophagocytosis assay and boostability) with protein conjugate vaccine PCV7-CRM.
A careful review of clinical data accumulated in the past few years with these and several unlicensed PCV formulations reveals that not all serotypes are equally preventable.
Furthermore, some key properties of each vaccine may differ from the other, such as differing degrees of cross-protection against vaccine-related types, acute otitis media, or perhaps even differences in net herd effect.
Accordingly, the overall net effect of each vaccine is not directly predictable based only on serotype content, and thus these higher-valent vaccines are not simply ‘line-extensions’ of the heptavalent conjugate.
There is currently little evidence to indicate that the levels of effectiveness of both vaccines in routine use are significantly different.
It is expected that this will allow healthcare providers as well as recommending bodies and tendering authorities to have a choice of highly effective PCVs for prevention of pneumococcal disease and deaths.
Financial & competing interests disclosure
This work was supported by GlaxoSmithKline (GSK) Biologicals SA. The authors are employed by GSK and own stock in GSK. WP Hausdorff is co-inventor of the 13-valent pneumococcal conjugate vaccine (but receives no royalties) developed and produced by Wyeth/Pfizer and described in the article. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was provided by XPE Pharma and Science on behalf of GlaxoSmithKline Vaccines through Vasile Coman who provided Editorial Support and Marie-Line Seret and Bram Blomme who provided publication coordination and management.
Notes
References
- Klugman KP , Cutts F , Adegbola RA , et al. Meta-analysis of the efficacy of conjugate vaccines against invasive pneumococcal disease. In: Siber GR , Klugman KP , Mäkelä PH , editors. Pneumococcal vaccines: the impact of conjugate vaccine. ASM Press; Washington, DC, USA: 2008
- Taylor S , Marchisio P , Vergison A , et al. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin Infect Dis 2012;54(12):1765-73
- Fitzwater SP , Chandran A , Santosham M , Johnson HL . The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2012;31(5):501-8
- Feikin DR , Kagucia EW , Loo JD , et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013;10(9):e1001517
- Pilishvili T , Lexau C , Farley MM , et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201(1):32-41
- Pneumococcal vaccines WHO position paper –. 2012. Wkly Epidemiol Rec 2012;87(14):129-44
- Hausdorff WP , Bryant J , Paradiso PR , Siber GR . Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000;30(1):100-21
- Wroe PC , Lee GM , Finkelstein JA , et al. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr Infect Dis J 2012;31(3):249-54
- Hausdorff WP , Fierens F , Hoet B . Efficacy and effectiveness of PHiD-CV against invasive pneumococcal disease and pneumonia: recent data. Presented at Nordic Vaccine Meeting; 5 – 7 September 2012; Copenhagen, Denmark
- Andrews N , Waight PA , Borrow R , et al. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS One 2011;6(12):e28435
- Palmu AA , Jokinen J , Nieminen H , et al. Vaccine effectiveness of the pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against clinically suspected invasive pneumococcal disease: a cluster-randomised trial. Lancet Resp Med 2014; doi: 10.1016/S2213-2600(14)70139-0. [Epub ahead of print]
- Lee H , Choi EH , Lee HJ . Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines. Korean J Pediatr 2014;57(2):55-66
- Black SB , Shinefield HR , Ling S , et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002;21(9):810-15
- Eskola J , Kilpi T , Palmu A , et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344(6):403-9
- Whitney CG , Pilishvili T , Farley MM , et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006;368(9546):1495-502
- Bettinger JA , Scheifele DW , Kellner JD , et al. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000-2007. Vaccine 2010;28(9):2130-6
- Kieninger DM , Kueper K , Steul K , et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine 2010;28(25):4192-203
- Vesikari T , Wysocki J , Chevallier B , et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009;28(4 Suppl):S66-76
- Miller E , Andrews NJ , Waight PA , et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011;11(10):760-8
- De Wals P , Lefebvre B , Markowski F , et al. Impact of 2+1 pneumococcal conjugate vaccine program in the province of Quebec, Canada. Vaccine 2014;32(13):1501-6
- Andrews NJ , Waight PA , Burbidge P , et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014;14(9):839-46
- Cutts FT , Zaman SM , Enwere G , et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005;365(9465):1139-46
- Klugman KP , Madhi SA , Huebner RE , et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003;349(14):1341-8
- Hausdorff WP , Feikin DR , Klugman KP . Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005;5(2):83-93
- Yaro S , Lourd M , Traore Y , et al. Epidemiological and molecular characteristics of a highly lethal pneumococcal meningitis epidemic in Burkina Faso. Clin Infect Dis 2006;43(6):693-700
- Lipsitch M , Whitney CG , Zell E , et al. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med 2005;2(1):e15
- Heffron R . Pneumonia: with special reference to Pneumococcus lobar pneumonia. Commonwealth Fund; Cambridge, London, UK: 1939
- Butler JC , Breiman RF , Campbell JF , et al. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA 1993;270(15):1826-31
- Singleton RJ , Butler JC , Bulkow LR , et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine 2007;25(12):2288-95
- Klugman KP , Madhi SA , Adegbola RA , et al. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine 2011;29(18):3372-3
- Miller E , Andrews NJ , Waight PA , et al. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011;29(49):9127-31
- Andrews NJ , Waight PA , George RC , et al. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012;30(48):6802-8
- Prymula R , Peeters P , Chrobok V , et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006;367(9512):740-8
- Puumalainen T , Dagan R , Wuorimaa T , et al. Greater antibody responses to an eleven valent mixed carrier diphtheria- or tetanus-conjugated pneumococcal vaccine in Filipino than in Finnish or Israeli infants. Pediatr Infect Dis J 2003;22(2):141-9
- Togashi T , Yamaji M , Thompson A , et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in healthy infants in Japan. Pediatr Infect Dis J 2013;32(9):984-9
- Poolman J , Kriz P , Feron C , et al. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 2009;27(24):3213-22
- Dagan R , Patterson S , Juergens C , et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013;57(7):952-62
- Steens A , Bergsaker MA , Aaberge IS , et al. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013;31(52):6232-8
- Harboe ZB , Dalby T , Weinberger DM , et al. Impact of 13-valent conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014. doi: 10.1093/cid/ciu524. [Epub ahead of print]
- Demczuk WH , Martin I , Griffith A , et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010-2012. Can J Microbiol 2013;59(12):778-88
- JCVI pneumococcal sub-committee meeting. Available from: http://media.dh.gov.uk/network/261/files/2012/07/JCVI-minutes-Pneumococcal-sub-committee-meeting-held-on-30-May-2012.pdf [Last accessed 7 March 2014]
- Millar EV , O’Brien KL , Bronsdon MA , et al. Anticapsular serum antibody concentration and protection against pneumococcal colonization among children vaccinated with 7-valent pneumococcal conjugate vaccine. Clin Infect Dis 2007;44(9):1173-9
- Tregnaghi MW , Saez-Llorens X , Lopez P , et al. for the COMPAS Group . Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med 2014;11(6):e1001657
- Kilpi T , Ahman H , Jokinen J , et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis 2003;37(9):1155-64
- Jokinen JT , Ahman H , Kilpi TM , et al. Concentration of antipneumococcal antibodies as a serological correlate of protection: an application to acute otitis media. J Infect Dis 2004;190(3):545-50
- De Wals P , Erickson L , Poirier B , et al. How to compare the efficacy of conjugate vaccines to prevent acute otitis media? Vaccine 2009;27(21):2877-83
- Alpert HR , Behm I , Connolly GN , Kabir Z . Smoke-free households with children and decreasing rates of paediatric clinical encounters for otitis media in the United States. Tob Control 2011;20(3):207-11
- Lucero MG , Dulalia VE , Nillos LT , et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev 2009(4):CD004977
- Loo JD , Conklin L , Fleming-Dutra KE , et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J 2014;33(Suppl 2):S140-51
- Hausdorff WP , Dagan R . Serotypes and pathogens in paediatric pneumonia. Vaccine 2008;26 Suppl 2:B19-23
- Root ED , Lucero M , Nohynek H , et al. Distance to health services affects local-level vaccine efficacy for pneumococcal conjugate vaccine (PCV) among rural Filipino children. Proc Natl Acad Sci USA 2014;111(9):3520-5
- Henrichsen J . Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol 1995;33(10):2759-62
- Robbins JB , Austrian R , Lee CJ , et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis 1983;148(6):1136-59
- Foster D , Walker AS , Paul J , et al. Reduction in invasive pneumococcal disease following implementation of the conjugate vaccine in the Oxfordshire region, England. J Med Microbiol 2011;60(Pt 1):91-7
- Park IH , Moore MR , Treanor JJ , et al. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis 2008;198(12):1818-22
- Richter SS , Heilmann KP , Dohrn CL , et al. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011. Emerg Infect Dis 2013;19(7):1074-83
- Siber GR , Chang I , Baker S , et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007;25(19):3816-26
- Dagan R , Givon-Lavi N , Porat N , Greenberg D . The effect of an alternative reduced-dose infant schedule and a second year catch-up schedule with 7-valent pneumococcal conjugate vaccine on pneumococcal carriage: a randomized controlled trial. Vaccine 2012;30(34):5132-40
- O’Brien KL , Millar EV , Zell ER , et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 2007;196(8):1211-20
- Grivea IN , Tsantouli AG , Michoula AN , Syrogiannopoulos GA . Dynamics of Streptococcus pneumoniae nasopharyngeal carriage with high heptavalent pneumococcal conjugate vaccine coverage in Central Greece. Vaccine 2011;29(48):8882-7
- Ho PL , Chiu SS , Chan MY , et al. Changes in nasopharyngeal carriage and serotype distribution of antibiotic-resistant Streptococcus pneumoniae before and after the introduction of 7-valent pneumococcal conjugate vaccine in Hong Kong. Diagn Microbiol Infect Dis 2011;71(4):327-34
- Ekström N , Haikala R , Lehtonen H , et al. Functional activity of antibodies to pneumococcal serotypes 6A and 19A in the Finnish Otitis Media (FinOM) Vaccine Trial. Presented at 4th International Symposium on Pneumococci and Pneumococcal Diseases; 9 – 13 May 2004; Helsinki, Finland
- Wysocki J , Tejedor JC , Grunert D , et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different Neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009;28(4 Suppl):S77-88
- SIREVA II . Available from: http://www.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3609&Itemid=3953&lang=pt [Last accessed 7 March 2014]
- McEllistrem MC , Nahm MH . Novel pneumococcal serotypes 6C and 6D: anomaly or harbinger. Clin Infect Dis 2012;55(10):1379-86
- Kuch A , Sadowy E , Skoczynska A , Hryniewicz W . First report of Streptococcus pneumoniae serotype 6D isolates from invasive infections. Vaccine 2010;28(39):6406-7
- Cooper D , Yu X , Sidhu M , et al. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011;29(41):7207-11
- Hausdorff WP , Hoet B , Schuerman L . Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr 2010;10:4
- Hicks LA , Harrison LH , Flannery B , et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis 2007;196(9):1346-54
- Poolman J , Frasch C , Nurkka A , et al. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin Vaccine Immunol 2011;18(2):327-36
- Mrkvan T , Hoet B , Adegbola RA , et al. Serotype 19A and the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV): lessons learned to date. Presented at 31st Meeting of the European Society for Paediatric Infectious Diseases; 28 May – 1 June 2013; Milan, Italy
- de Wals P , Lefebvre B , Defay F , et al. Invasive pneumococcal diseases in birth cohorts vaccinated with PCV-7 and/or PHiD-CV in the province of Quebec, Canada. Vaccine 2012;30(45):6416-20
- Kilpi T , Palmu AA , Ruokokoski E , et al. Indirect impact of pneumococcal Haemophilus influenzae protein-D conjugate vaccine (PHID-CV10) on invasive pneumococcal disease (IPD) in a clinical trial. Presented at 8th World Congress on Pediatric Infectious Diseases; 19 – 22 November 2013; Cape Town, South Africa
- Invasive pneumococcal disease reports in New Zealand. Available from: https://surv.esr.cri.nz/surveillance/IPD.php [Last accessed 7 March 2014]
- Impact of 10-valent pneumococcal conjugate vaccine (PCV10) against invasive pneumococcal disease (IPD) among vaccine-eligible children in Finland. Available from: http://www.slideshare.net/THLfi/ip-ddirect-nvpwspid2013finland [Last accessed March7 2014]
- Quebec: INSPQ Annual surveillance report 2010. Available from: http://www.inspq.qc.ca/pdf/publications/1383_ProgSurvPneumocoque2010.pdf [Last accessed 7 March 2014]
- Domingues CMAS , Verani JR , Montenegro ER , et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study Lancet Resp Med. 2014; in press
- de Wals P . Invasive pneumococcal disease in two birth cohorts vaccinated, respectively, with 2+1 PCV-7 or PHiD-CV-10 doses. Presented at 8th International Symposium on Pneumococci and Pneumococcal Diseases; 11 – 15 March 2012; Iguaçu Falls, Brazil
- Moore M , Taylor T , Pondo T , et al. Impact of 13-valent pneumococcal conjugate vaccine (PCV13) against invasive pneumococcal disease (IPD) among children <5 years old in the U.S. Pneumonia 2014;3:149
- Simell B , Auranen K , Kayhty H , et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 2012;11(7):841-55
- Hausdorff WP , Brueggemann AB , Hackell JG , Scott JA . Pneumococcal serotype epidemiology. In: Siber GR , Klugman KP , Mäkelä PH , editors. Pneumococcal Vaccines: the impact of conjugate vaccine. ASM Press; Washington, DC, USA: 2008
- van den Bergh MR , Spijkerman J , Swinnen KM , et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis 2013;56(3):e30-9
- Van Effelterre T , Moore MR , Fierens F , et al. A dynamic model of pneumococcal infection in the United States: implications for prevention through vaccination. Vaccine 2010;28(21):3650-60
- Brandileone MCC , Almeida SCG , Zanella RC , et al. Effect of PCV10 vaccination on pneumococcal serotypes in Brazil using the national pneumococcal laboratory network surveillance. Pneumonia 2014;3:158
- Vesikari T , Forstén A , Seppä I , et al. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage in Finnish children: a cluster-randomised controlled trial. Presented at 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 27 – 30 April 2013; Berlin, Germany
- Weinberger DM , Malley R , Lipsitch M . Serotype replacement in disease after pneumococcal vaccination. Lancet 2011;378(9807):1962-73
- van Gils EJ , Veenhoven RH , Hak E , et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA 2009;302(2):159-67
- Cohen R , Levy C , Bonnet E , et al. Dynamic of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 introduction in France. Vaccine 2010;28(37):6114-21
- Huang SS , Hinrichsen VL , Stevenson AE , et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 2009;124(1):e1-11
- Vestrheim DF , Hoiby EA , Aaberge IS , Caugant DA . Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol 2010;17(3):325-34
- Prymula R , Hanovcova I , Splino M , et al. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae Protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine 2011;29(10):1959-67
- Russell FM , Carapetis JR , Satzke C , et al. Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster. Clin Vaccine Immunol 2010;17(12):1970-6
- Roca A , Hill PC , Townend J , et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med 2011;8(10):e1001107
- Nzenze SA , Shiri T , Nunes MC , et al. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr Infect Dis J 2013;32(11):1270-8
- Yeh SH , Zangwill KM , Lee H , et al. Heptavalent pneumococcal vaccine conjugated to outer membrane protein of Neisseria meningitidis serogroup b and nasopharyngeal carriage of Streptococcus pneumoniae in infants. Vaccine 2003;21(19-20):2627-31
- Dagan R , Melamed R , Muallem M , et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis 1996;174(6):1271-8
- Sáez-Llorens X , Wong D , Rodríguez M , et al. Effect of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccines (PHiD-CV) on nasopharyngeal bacterial carriage in Panamanian children. Presented at 7th World Congress of the World Society for Pediatric Infectious Diseases; 16 – 19 November 2011; Melbourne, Australia
- Weinberger DM , Harboe ZB , Flasche S , et al. Prediction of serotypes causing invasive pneumococcal disease in unvaccinated and vaccinated populations. Epidemiology 2011;22(2):199-207
- Weinberger DM , Bruden DT , Grant LR , et al. Using pneumococcal carriage data to monitor postvaccination changes in invasive disease. Am J Epidemiol 2013;178(9):1488-95
- Nurhonen M , Auranen K . Optimal serotype compositions for Pneumococcal conjugate vaccination under serotype replacement. PLoS Comput Biol 2014;10(2):e1003477
- Hsu HE , Shutt KA , Moore MR , et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 2009;360(3):244-56
- Cohen AL , Harrison LH , Farley MM , et al. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS 2010;24(14):2253-62
- Madhi SA , Klugman KP . A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004;10(8):811-13
- Dunne EM , Smith-Vaughan HC , Robins-Browne RM , et al. Nasopharyngeal microbial interactions in the era of pneumococcal conjugate vaccination. Vaccine 2013;31(19):2333-42
- Barkai G , Leibovitz E , Givon-Lavi N , Dagan R . Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr Infect Dis J 2009;28(6):466-71
- Jokinen J , Palmu AA , Kilpi T . Acute otitis media replacement and recurrence in the Finnish otitis media vaccine trial. Clin Infect Dis 2012;55(12):1673-6
- Borys D , Saéz-Llorens X , Prymula R , et al. 10- and 11-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccines (PHiD-CV and 11PN-PD) on nasopharyngeal bacterial carriage. Presented at 8th International Symposium on Pneumococci and Pneumococcal Diseases; 11 – 15 March 2012; Iguaçu Falls, Brazil
- Deceuninck G , De Wals P . Effectiveness of three pneumococcal conjugate vaccines (PCVs) to prevent invasive pneumococcal disease (IPD) in Quebec, Canada. Pneumonia 2014;3:163
- Nurkka A , Ahman H , Yaich M , et al. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine 2002;20(1-2):194-201
- Prince Valdemar of Denmark, Wikipedia. Available from: http://en.wikipedia.org/wiki/Prince_Valdemar_of_Denmark
- Bacterial Meningitis in the Netherlands: annual Reports for 2010 and 2012. Available from: www.amc.nl/web/Research/Departments/Overview/Medical-Microbiology/Medical-Microbiology/Current-research/Reference-Laboratory-for-Bacterial-Meningitis.htm?print=true [Last accessed 7 March 2014]