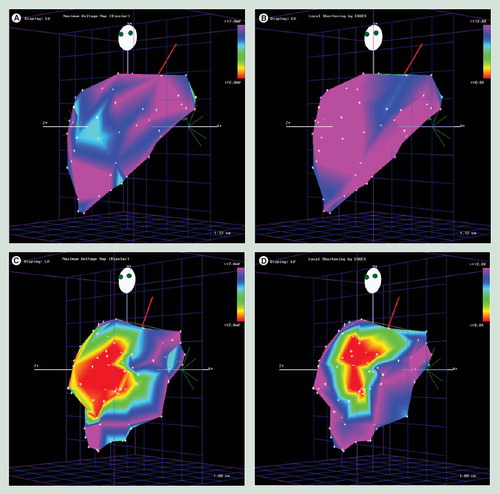
(A) Unipolar voltage map and (B) linear local shortening map of a normal dog left ventricle. (C) After experimental left anterior descending artery occlusion, unipolar voltage map and (D) linear local shortening map were performed on the same animal.
Images courtesy of Biologics Delivery Systems Group Inc.
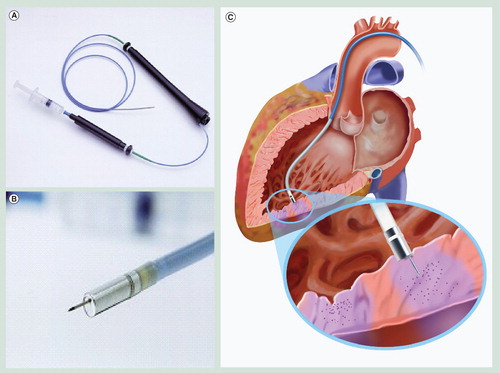
(A) The MyoStar™ injection catheter allows precise delivery of stem cells based on the electromechanical map. (B) A magnified view of the distal tip. (C) Left ventricle illustrating direct stem cell delivery by the MyoStar™ injection catheter.
Images courtesy of Biologics Delivery Systems Group Inc.
Despite improvements in revascularization therapies, access to advanced medical facilities and community awareness of coronary artery disease, heart failure remains one of the most common and costly reasons for hospital admission in the USA. Perhaps a victim of our own success, as patients survive myocardial infarction and live longer, more live with the sequelae of damaged myocardium. As scientific and clinical focus has shifted to regenerative medicine, the promise of stem cell therapy to aid in the improvement of myocardial function has nurtured the investigation of optimal therapeutic strategies. Systemic, intracoronary, intraventricular and direct myocardial delivery of stem cells have produced mixed results. Limitations in available stem cell lines and delivery methods remain challanges to a firm foundation for future clinical success. The advent of sophisticated intracardiac electromechanical mapping (EMM) systems and catheters equipped with injection ports represents a new horizon for stem cell delivery. This article will provide insights into intracardiac EMM for stem cell delivery for damaged myocardium and the future directions with this exciting new therapeutic approach.
Heart failure affects millions of patients worldwide. The socioeconomic burden of this disease is exemplified by the fact that heart failure remains the most common reason for hospital admission in the USA Citation[1] and cases are expected to double in the next 40 years Citation[2]. Associated with this enormous disease burden is the extraordinary cost of treatment, estimated at US$18 billion per year Citation[2]. The increasing prevalence of this disease is probably the result of improved pharmacological regimens and increased survival for patients after myocardial infarction Citation[3]. Heart transplantation remains a viable option for certain patients; however, the limited pool of available donors and the relatively poor long-term survival of patients undergoing transplantation remain areas for improvement. This has spawned the development of novel invasive strategies to treat heart failure patients, including more portable, sustainable ventricular-assist devices and ultrafiltration machines capable of removing large volumes of isotonic fluid through peripheral veins, permitting more advanced outpatient treatment options. Recently, increasing interest has focused on the role of regenerative medicine through the use of stem cell therapy to improve or restore damaged myocardium and enhance cardiac function.
The long-held paradigm that mature cardiac myocytes are incapable of regeneration has recently been confronted by intense research focused on the role of stem cell-based therapy. The hope that postnatal cardiomyogenesis and vasculogenesis will reset or slow the morbidity and mortality burden associated with heart failure fuels this burgeoning area of research.
Basics of electromechanical cardiac mapping
The electromechanical coupling of human cardiomyocytes is a well-understood phenomenon. Normally functioning endomyocardium displays a relatively narrow range of voltage potentials. Although there is some variability among human clinical studies, unipolar endocardial potentials greater than 10 mV and bipolar potentials greater than 2 mV have been recognized as normal Citation[4–7]. Deviations from the mean suggest an abnormality of normal myocardial structure in the form of scar tissue, hibernating myocardium or channelopathy Citation[8–13]. This electrically abnormal tissue can be further categorized by contractile function. By measuring two points on the endocardial surface from end-diastole to end-systole, a value of local linear shortening (LLS) can be described. Studies have shown that normal tissue has an average LLS of greater than 12%, while akinetic myocardial LLS is less than 2% Citation[9,10,12]. Between these values exists hypokinetic myocardium consisting of viable and hibernating muscle. Recent investigation has been directed towards exploiting these voltage discrepancies to further define border zones of normal and abnormal myocardium, better defining attractive targets for regenerative stem cell therapy.
The NOGA™ system (Biosense Webster, Inc.; a Johnson & Johnson company, CA, USA) is a platform designed to take advantage of variable endomyocardial currents to create a voltage map of the cardiac chamber. EMM is performed by incorporating the acquired voltage potentials and myocardial motion (shortening) with a 3D image of the intracardiac chamber acquired in real-time. To do this, NOGA consists of four elements:
• A location pad placed under the patient containing three coils generating ultra-low magnetic field energy (5 × 10-6 to 5 × 10-5 T);
• A fixed-position reference catheter attached to the body surface with a miniature magnetic field sensor;
• A steerable mapping catheter with distal unipolar and bipolar electrodes capable of recording endocardial voltage potentials, as well as a miniature magnetic field sensor;
• A workstation for information processing and 3D reconstruction.
The addition of a biologics-delivery catheter, equipped with a 27-gauge retractable needle permitting small alloquot injections (i.e., stem cells), completes the self-contained system for imaging assessment and treatment.
The mapping catheter is placed in contact with the left ventricular endocardial surface in several locations (points) to generate the physical map. Additional fluoroscopy is then unnecessary as the NOGA system can locate the catheter in real-time within the boundaries of the 3D rendered ventricle. When constructing the 3D representation of the ventricle, multiple contact points are obtained sequentially within the ventricle. Upon this template, a limitless number of voltage recordings can be made, providing ever denser image maps of the endocardial voltage potentials. However, increasing the number of points does not necessarily improve the quality of the map, as dense point clusters can create artifact.
The fixed coils beneath the patient emit different strength magnetic fields that decay as a function of distance from their source. The NOGA catheter triangulates its position based on the intersection of these three field strengths and the time of impulse transit as compared with the static reference. As the mapping catheter moves, it detects changes in the intensity of each field, and can calculate its position and orientation in 6 degrees of freedom (x, y, z, roll, pitch and yaw). It has been shown that the system can be accurate to distances of less than 1 mm Citation[12,14].
Temporal recording is paramount and so data points are ECG gated. However, there are potential sources of error that are harder to correct; of these, respiration has been identified as one of the more challenging Citation[15]. While compensating for the positional shift of the heart during a single cardiac cycle is not necessary, when evaluating multiple cycles over time, the periodicity of movement can affect mechanical analysis Citation[15–17].
Calculating LLS engages a random sampling error due to variable distances between points, myocardial tethering and passive motion of the areas adjacent to actively contracting myocardium, giving falsely elevated values. To overcome this error, NOGA uses a LLS formula that averages the change in distance among the data points compared with an index catheter point, and preferentially weights those within an 8–15 mm radius (this can help to ameliorate clustering artifact) Citation[18]. It is imperative that the catheter maintains contact with the myocardium. When mapping more basal segments from a retrograde approach, the catheter is bent up to 180°, and during systole the apical contraction and cranial shift may cause loss of contact and exacerbate the signal-to-noise ratio.
Validation studies
Echocardiography
Early in the development of EMM, the value of determining healthy, viable and infarcted myocardium became apparent. Unipolar and bipolar voltage thresholds, LLS and triangular local shortening proved reliable surrogates for the determination of myocardial viability. In an experimental canine model of myocardial infarction, myocardial necrosis was associated with reduced endocardial voltage and mechanical activity by EMM Citation[10]. While reduced shortening was present in both irreversible necrosis and hibernating myocardium, it was postulated that the addition of voltage potentials helps to distinguish the two, since hibernating myocardium retained preserved or mildly diminished voltage potentials. This has been validated in both animal and clinical models using transthoracic and transesophageal echocardiography to compare local shortening Citation[10,18–21], as well as echocardiography performed with dobutamine Citation[22,23]. These results are subject to the variable quality of transthoracic echocardiography and transesophageal echocardiography, but all showed significance differences between normal, viable and scarred myocardium, as assessed by echocardiography.
Nuclear imaging
The detection of hibernating and thus viable myocardium by radionuclide assessment has been well established Citation[24–33]. EMM has also been compared with radionuclide perfusion imaging. Data from EMM were plotted into nine regions specifically correlated with radionuclide imaging, and comparison of perfusion images to EMM was performed for segments with normal, reversible or fixed perfusion defects. Voltage potentials and local endocardial shortening (LS) were reduced in patients with reversible (voltage potential: 12.0 ± 2.8 mV; LS: 10.3 ± 3.7%) and fixed (voltage potential: 7.5 ± 3.4 mV; LS: 3.4 ± 3.4%) defects compared with normal perfusion (voltage potential: 14.0 ± 2.0 mV; LS: 12.5 ± 2.8%) Citation[34].
The use of EMM for scar determination has been demonstrated in a porcine model of chronic anterior myocardial infarction. Lower voltage potentials (1.2 ± 0.5 mV, 2.8 ± 0.9 mV and 5.1 ± 2.1 mV) were observed in infarct zones when compared with border and remote areas. Good correlation to pathological analysis of infarct was observed when areas of lower voltage potential were grouped (r2 = 0.96). Mapping was similarly effective in locating infarct and border zone geometry Citation[6]. Discrimination between viable and infarcted myocardium in humans has been validated in numerous studies Citation[11,35–37], and represents one of the most important features of NOGA to guide stem cell therapy.
Magnetic resonance imaging
Currently, MRI is the gold standard for distinguishing normal myocardium from viable or hibernating myocardium and scarred tissue. Confirmation studies to assess whether EMM voltage potentials could determine myocardial viability showed that unipolar potentials could reliably distinguish normal from abnormal tissue Citation[38]. Viability was represented by unipolar voltages ranging from 6.4 to 7.5 mV, with transmural scar identified by values less than 4.6 mV. The receiver operating characteristic curve evaluating the ability of EMM to assess normal from viable myocardium showed 80% sensitivity and specificity when using a cut-off value of 7.9 mV. Separating normal from scar was 93% sensitive and 88% specific with a threshold of 6.5 mV Citation[38]. The distance traversed from infarcted scar to normal muscle includes a watershed area of hibernation; being able to distinguish this border zone is clinically relevant for stem cell delivery. Although dependent upon the density of mapping points, the real-time identification of border zone myocardium is what separates EMM from currently available viability assessment tools.
Direct intramyocardial therapeutic delivery
The accurate electromechanical assessment of the endocardial surface allowed further exploration of novel therapies to target ischemic or border zone myocardium. Technological advances, specifically the development of a specialized injection catheter capable of EMM – the MyoStar™ injection catheter (Biosense–Webster, Waterloo, Belgium) – allowed the exploration of delivering molecules and cells directly to ischemic or border zone myocardium. Initial studies evaluating the use of an EMM injection catheter were performed in swine. After standard EMM of the left ventricle, six discrete sites were injected with methylene blue and location at necropsy correlated with sites indicated by the in vivo endocardial map. No epicardial staining, sustained atrial or ventricular arrhythmia, or sustained injury pattern by endocardial electrogram was observed Citation[39]. Further studies reproduced these findings Citation[8,13]. Investigations then confirmed the feasibility of an endocardial approach by evaluating the integrity of molecules and cells injected through the MyoStar catheter. Successful gene transfection has been observed after adenoviral vectors containing LacZ transgene as well as a gene encoding adenovirus vascular endothelial growth factor Citation[8]. Myogenic murine cells injected in tissue culture via the MyoStar catheter compared with a standard needle showed identical proliferative properties Citation[40]. Soon thereafter, the first in-human study to investigate safety and feasibility of catheter-based myocardial gene transfer was performed Citation[41]. Patients with multivessel coronary artery disease, medically refractory angina and evidence of reversible ischemia were randomized to receive naked DNA encoding VEGF-2 by MyoStar versus mock procedures. As previously observed in animal studies, injection caused no sustained ventricular arrhythmia, electrographic evidence of infarction or ventricular perforation.
Intramyocardial delivery of stem cells
Exploring the role of endothelial progenitor cells (EPC) for postnatal vasculogenesis led to discovery of novel methods for isolation of EPCs from peripheral blood Citation[42]. These cells, when injected intravenously into animal models of ischemia, reach sites of active angiogenesis where they differentiate into mature endothelial cells Citation[43]. To assess the ability of cultured EPCs to incorporate into ischemic myocardium, human-derived EPCs were injected intravenously into an athymic nude rat model of induced myocardial ischemia Citation[44]. These cells accumulated into areas of ischemia at foci of neovascularization. After 28 days, rats receiving human EPCs had increased capillary density compared with the control (290.1 ± 21.5 vs 191.1 ± 17.8/mm2; p = 0.0009). Significant improvements in both systolic and diastolic left ventricular dimensions and fractional shortening were observed for the rats receiving EPCs. In a similar rat model of induced myocardial ischemia, intravenous injection of uncultured EPCs after left anterior artery ligation resulted in marked, sustained improvement in left ventricular ejection fraction (LVEF) and neoangiogenesis. These improvements were only observed in the rats receiving EPCs Citation[45]. Similar concentrations of peripherally obtained EPCs used in these trials would be unrealistic for human trials due to the enormous quantity of peripheral blood required for purification. However, using direct endocardial injection of human-derived EPCs in a nude rat model of myocardial ischemia, Kawamoto et al. showed that concentrations of approximately 20-times less than previously reported demonstrated significantly increased capillary density and decreased areas of fibrosis compared with controls Citation[46]. In the same study, the authors directly injected autologous EPCs into a swine model of myocardial ischemia. After the induction of ischemia, EMM was performed to target the areas of viable myocardium for EPC injection. A significant reduction in the zone of ischemia (from 27.3 ± 8.5% to 12.3 ± 6.3% at 4 weeks; p = 0.0034) and increase in both LVEF and capillary density were seen as compared with controls Citation[46]. This study showed that autologous cells could be used in an immunocompetent host and that direct endocardial injection required fewer EPCs compared with intravenous delivery. This sets the stage for human studies to establish the role of EMM as well as direct endocardial injection of stem cells for the treatment of the damaged myocardium.
Role of local delivery
Four nonsurgical routes of administration for stem cell treatment of myocardial ischemia have been reported: intravenous, intraventricular, intracoronary and intramyocardial. Although no serious adverse effects have been seen with any of these methods, we believe that each has its advantages and disadvantages. These methods are described in order to illustrate the need for improved delivery techniques and to establish the role of direct endocardial injection as the leading candidate for future studies.
Intravenous & intraventricular administration
As described earlier, intravenously injected stem cells have the potential to collect in areas of myocardial ischemia Citation[43,44,47]. Neovascularization and improvements in LVEF have been observed in models of myocardial infarction following intravenously transplanted stem cells Citation[44,45,48,49]. However, these stem cells are often trapped in the pulmonary circulation as well as the liver, kidney and spleen Citation[50,51]. The delivery of stem cells to nontarget tissues is particularly important with larger cell lineages such as mesenchymal stem cells, which are prone to this ‘entrapment’ phenomenon due to their larger size. Although the number of stem cells observed in ischemic myocardium increased when injected within the left ventricle, significant quantities of cells were again lost to other tissue beds Citation[47,50]. This lack of efficiency also creates potential for unanticipated systemic effects and makes intravenous and intraventricular administration less desirable compared with more potent localized injection techniques.
Intracoronary administration
Selective intracoronary administration of stem cells promotes migration and incorporation of cells into the myocardium Citation[44,49,52,53]. The selective delivery of a large concentration of cells to the local area of ischemia has been utilized in numerous human trials Citation[54–57]. The method proved safe and many trials reported increased ejection fraction, reduction in infarct size and myocardial perfusion to the ischemic zone. The major limitation to this method is delivery of the cells to the tissue bed. The site of myocardial ischemia may be downstream from a chronic total occlusion. Antegrade injection, even with proximal balloon occlusion while delivering the cells with an over-the-wire balloon catheter may fail to allow delivery of the cells to the target tissue. Local flow dynamics preferentially direct blood and similar fluids or cells to areas of less resistance, steering these constituents away from the targeted tissue bed. Other potential risks of intracoronary injection include coronary microcirculation embolism of the cell mass and trauma to the parent vessel. However, in spite of these important limitations, intracoronary stem cell delivery has been applied in multiple centers because it does not require highly specialized training or skills.
Direct myocardial injection
Direct epicardial and endocardial stem cell injection has yielded similar clinical results to those modalities described previously, including improvement in LVEF and neovascularization in addition to exercise improvement compared with controls Citation[58–63]. Data assessing delivery methods showed increased engraftment and reduced systemic delivery of mesenchymal cells via intracoronary and endocardial administration compared with intravenous routes Citation[64]. Minimally invasive surgical techniques allow adequate access to the ischemic myocardium via minithoracotomy or subdiaphragmatic approach Citation[65], although the common adverse events complicating cardiac surgery, such as atrial arrhythmias, have been reported Citation[66]. The tolerability of percutaneous endocardial injection has been favorable Citation[58] and thus the morbidity associated with any surgery, including minimally invasive procedures, may be avoided with percutaneous endocardial cell delivery. This technique allows maximization of stem cell delivery to the target tissue while minimizing risk of systemic toxicity.
Candidate stem cell lines
Despite mounting data on the use of various cell lineages for the treatment of myocardial ischemia, there is no consensus regarding the optimal lineage. Furthermore, there is no supporting evidence that any bone marrow-derived stem cells can substantially differentiate in vivo into cardiomyocytes. Skeletal myoblasts, initially found to be a safe and feasible option for cardiomyogenesis Citation[67–70] are arrhythmogenic following transplantation Citation[71]; consequently their use in clinical studies has diminished. Mesenchymal cells, although less immunogenic than other cell lines Citation[63], differentiate at very low rates into myocytes in vivoCitation[63,72]. Transplantation into murine myocardium was accompanied by bone formation in one study, Citation[73] highlighting the potential risk of utilizing this cell line. Currently EPCs, one of the most promising candidate cell lines found in both bone marrow and circulating blood, have been shown to differentiate into epithelial cells in vivo and may promote angiogenesis Citation[74]. Additionally, the ability to obtain and purify autologous EPCs from peripheral blood Citation[75] and potentially expand them ex vivo makes this lineage a good candidate for future studies.
NOGA™ & stem cell delivery
There are currently over 15 Phase I and II studies worldwide using NOGA-based stem cell injection (target population > 1500 patients) for the treatment of myocardial ischemia or heart failure. Various progenitor lineages, including mesenchymal stem cells, skeletal myoblasts, EPCs and bone marrow-derived mononuclear cells, are being investigated for patients with chronic myocardial ischemia, heart failure, refractory angina and acute myocardial infarction. This year, the first US use of NOGA-assisted stem cell delivery for patients with acute myocardial infarction, a previously considered relative contraindication for this approach, began enrollment. Cell delivery will be performed with a NogaStar™ Mapping Catheter with the MyoStar Left Ventricular Injection Catheter (Biologics Delivery Systems Group). A total of 15–20 transendocardial injections of 0.2 ml (not to exceed a total of 4.0 ml) each will be performed over 30–60 s each, targeting ischemic viable myocardium at the border zone of the infarction.
The target myocardial region (scar vs border zone vs normal myocardium) may vary depending on the cell type and/or underlying disease. For example, in the A Multicenter Study to Assess the Safety and Cardiovascular Effects of Myocell™ Implantation by a Catheter Delivery System in Congestive Heart Failure Patients Post Myocardial Infarction (MARVEL) study, skeletal myoblasts will be delivered to the center of the scar myocardium (as opposed to the border zone viable myocardium) in patients with congestive heart failure. In this application, the goal is to improve cardiac function and myogenesis and decrease the risk of arrhythmias. The ability to determine target tissue location and character to guide stem cell delivery represents one of the main advantages of a 3D EMM navigation system.
Future applications
Current NOGA systems are based on Windows XP platform, which improves tactile sensation, real-time image visualization and catheter response compared with previous NOGA generations. The XP platform has also been shown to be a more stable algorithm with easier user interface which decreases mapping time. In the near future, improvements in image augmentation and manipulation will be coupled with stereotactic technology creating more precise tissue examination and therapeutic delivery Citation[76]. A new class of magnetically steerable catheters allows the operator to control catheters remotely by computer: reducing radiation exposure to the physician and patient, providing more reproducible 3D localization, and improved safety for the procedure. This technology is already available in its nascent stage with NOGA’s sister platform for electrophysiologic applications (the CARTO system) now coupled with a Stereotaxis NIOBE® Magnetic Navigation System (Stereotaxis, MO, USA). The incorporation of intracardiac echocardiography catheters allows additional noncontrasted, real-time and nonfluoroscopic imaging of the heart and chambers. This will provide superior overlapping data on contractility to define border zones, improve procedural safety with better and direct visualization of intracardiac structures and rapid identification of potential complications, including pericardial effusions. Next generation NOGA systems will also be able to incorporate previously obtained MRI images, permitting data overlay and integration, ultimately yielding improved accuracy and precision of viability, pathology or anatomic variation. Software enhancements, including algorithms to compensate for patient movement and cardiac cycle length variability, will likely improve loop stability. These additions will augment EMM’s ability to define wall motion abnormality and improve diagnosis of viable myocardium, reduce procedure time and hopefully improve outcomes and safety.
The advantages of utilizing EMM to guide and deliver stem cell therapy make this technology a potent tool in this new frontier of regenerative medicine. The ability to use an online, nonfluoroscopic imaging modality within the cardiac catheterization laboratory permits the diagnosis and treatment of diseased myocardium within one procedure. Continual feedback data based on voltage potentials, the electrocardiogram and loop stability allow the operator to assess the depth of endomyocardial injection, reducing the likelihood of undue trauma or perforation during the procedure. The role of NOGA and similar EMM systems tailored to specific stem cell lines and diseases will likely prove to be the vehicle to delivery angiogenesis therapy to the heart. Future technological improvements, including the incorporation of adjuvant imaging modalities, which will improve viable tissue characterization and localization, will further improve geographic delivery of therapeutics.
Key issues
• Electromechanical mapping incorporates electrophysiologic and motion data to create a 3D representation of the heart in vivo. By assessing voltage potentials and linear shortening, it is possible to differentiate normal from viable and irreversibly scarred myocardium. Coupling the mapping catheter with an injection port enables combined diagnostic and therapeutic procedures to target viable myocardium for stem cell delivery.
• The optimal strategy for delivering stem cells to viable myocardium has been debated. Direct endomyocardial delivery of stem cells has proven to be associated with increased engraftment and decreased systemic delivery and, as such, would appear to be the superior method for regenerative cardiac interventions.
• The role of electromechanical mapping for stem cell delivery has expanded due to the efficacy of the procedure as well as increasing insight into appropriate stem cell lineages for cardiomyogenesis and vasculogenesis. Future technological enhancements with incorporation of other imaging modalities to improve tissue localization will improve clinical safety and improve precise delivery of therapeutics.
Financial & competing interests disclosure
Marco Costa serves as an advisor to Biologic Delivery Systems and has recieved honoraria in the past. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J. Am. Geriatr. Soc.45(8), 968–974 (1997).
- Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am. Heart J.137(2), 352–360 (1999).
- Maier B, Thimme W, Schoeller R, Fried A, Behrens S, Theres H. Improved therapy and outcome for patients with acute myocardial infarction – Data of the Berlin Myocardial Infarction Registry from 1999 to 2004. Int. J. Cardiol. DOI 10.1016/j.ijcard.2007.08.043 (2007) (Epub ahead of print).
- Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation101(11), 1288–1296 (2000).
- Boulos M, Lashevsky I, Reisner S, Gepstein L. Electroanatomic mapping of arrhythmogenic right ventricular dysplasia. J. Am. Coll. Cardiol.38(7), 2020–2027 (2001).
- Callans DJ, Ren JF, Michele J, Marchlinski FE, Dillon SM. Electroanatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction. Correlation with intracardiac echocardiography and pathological analysis. Circulation100(16), 1744–1750 (1999).
- Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation108(6), 704–710 (2003).
- Kornowski R, Leon MB, Fuchs S et al. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy. Results in normal and ischemic porcine models. J. Am. Coll. Cardiol.35(4), 1031–1039 (2000).
- Gepstein L, Hayam G, Shpun S, Ben-Haim SA. Hemodynamic evaluation of the heart with a nonfluoroscopic electromechanical mapping technique. Circulation96(10), 3672–3680 (1997).
- Kornowski R, Hong MK, Gepstein L et al. Preliminary animal and clinical experiences using an electromechanical endocardial mapping procedure to distinguish infarcted from healthy myocardium. Circulation98(11), 1116–1124 (1998).
- Gyongyosi M, Sochor H, Khorsand A, Gepstein L, Glogar D. Online myocardial viability assessment in the catheterization laboratory via NOGA electroanatomic mapping: quantitative comparison with thallium-201 uptake. Circulation104(9), 1005–1011 (2001).
- Gepstein L, Hayam G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitroand in vivo accuracy results. Circulation95(6), 1611–1622 (1997).
- Kornowski R, Fuchs S, Tio FO, Pierre A, Epstein SE, Leon MB. Evaluation of the acute and chronic safety of the biosense injection catheter system in porcine hearts. Catheter Cardiovasc. Interv.48(4), 447–453; discussion 454–445 (1999).
- Ben-Haim SA, Osadchy D, Schuster I, Gepstein L, Hayam G, Josephson ME. Nonfluoroscopic, in vivo navigation and mapping technology. Nat. Med.2(12), 1393–1395 (1996).
- Klemm HU, Franzen O, Ventura R, Willems S. Catheter based simultaneous mapping of cardiac activation and motion: a review. Indian Pacing Electrophysiol. J.7(3), 148–159 (2007).
- Klemm HU, Steven D, Johnsen C et al. Catheter motion during atrial ablation due to the beating heart and respiration: impact on accuracy and spatial referencing in three-dimensional mapping. Heart Rhythm4(5), 587–592 (2007).
- Noseworthy PA, Malchano ZJ, Ahmed J, Holmvang G, Ruskin JN, Reddy VY. The impact of respiration on left atrial and pulmonary venous anatomy: implications for image-guided intervention. Heart Rhythm2(11), 1173–1178 (2005).
- Kornowski R, Fuchs S, Shiran A et al. Catheter-based electromechanical mapping to assess regional myocardial function: a comparative analysis with transthoracic echocardiography. Catheter Cardiovasc. Interv.52(3), 342–347 (2001).
- Lessick J, Smeets JL, Reisner SA, Ben-Haim SA. Electromechanical mapping of regional left ventricular function in humans: comparison with echocardiography. Catheter Cardiovasc. Interv.50(1), 10–18 (2000).
- Kornowski R, Hong MK, Shiran A et al. Electromechanical characterization of acute experimental myocardial infarction. J. Invasive Cardiol.11(6), 329–336 (1999).
- Fuchs S, Kornowski R, Shiran A, Pierre A, Ellahham S, Leon MB. Electromechanical characterization of myocardial hibernation in a pig model. Coron. Artery Dis.10(3), 195–198 (1999).
- Lessick J, Hayam G, Zaretsky A, Reisner SA, Schwartz Y, Ben-Haim SA. Evaluation of inotropic changes in ventricular function by NOGA mapping: comparison with echocardiography. J. Appl. Physiol.93(2), 418–426 (2002).
- Poppas A, Sheehan FH, Reisman M, Harms V, Kornowski R. Validation of viability assessment by electromechanical mapping by three-dimensional reconstruction with dobutamine stress echocardiography in patients with coronary artery disease. Am. J. Cardiol.93(9), 1097–1101 (2004).
- Barrington SF, Chambers J, Hallett WA, O’Doherty MJ, Roxburgh JC, Nunan TO. Comparison of sestamibi, thallium, echocardiography and PET for the detection of hibernating myocardium. Eur. J. Nucl. Med. Mol. Imaging31(3), 355–361 (2004).
- Bonow RO, Dilsizian V. Assessing viable myocardium with thallium-201. Am. J. Cardiol.70(14), E10–E17 (1992).
- Bonow RO, Dilsizian V. Thallium-201 and technetium-99m-sestamibi for assessing viable myocardium. J. Nucl. Med.33(5), 815–818 (1992).
- Fernandes VB, Ben Freedman S, Allman KC et al. Detection of myocardial viability in stunned or hibernating myocardium by delayed emptying on radionuclide ventriculography. Am. J. Cardiol.67(6), 529–532 (1991).
- Haque T, Furukawa T, Takahashi M, Kinoshita M. Identification of hibernating myocardium by dobutamine stress echocardiography: comparison with thallium-201 reinjection imaging. Am. Heart J.130(3 Pt 1), 553–563 (1995).
- Hoeflin F, Roesler H, Ledermann H, Romanello S, Weinreich R. Detection of non-perfused, viable myocardium with 18F-FDG using a specially designed gamma camera. A simple method to detect hibernating myocardium. Acta Radiol. Suppl.376, 133–134 (1991).
- Kuikka JT, Mussalo H, Hietakorpi S, Vanninen E, Lansimies E. Evaluation of myocardial viability with technetium-99m hexakis-2-methoxyisobutyl isonitrile and iodine-123 phenylpentadecanoic acid and single photon emission tomography. Eur. J. Nucl. Med.19(10), 882–889 (1992).
- Marwick TH, MacIntyre WJ, Salcedo EE, Go RT, Saha G, Beachler A. Identification of ischemic and hibernating myocardium: feasibility of post-exercise F-18 deoxyglucose positron emission tomography. Cathet. Cardiovasc. Diagn.22(2), 100–106 (1991).
- Matsuo H, Watanabe S, Nishida Y et al. Identification of asynergic but viable myocardium in patients with chronic coronary artery disease by gated blood pool scintigraphy during isosorbide dinitrate and low-dose dobutamine infusion: comparison with thallium-201 scintigraphy with reinjection. Ann. Nucl. Med.8(4), 283–293 (1994).
- Parodi O, De Maria R, Testa R et al. Super-normal 201Tl retention in hibernating myocardium: an ex-vivo study using the failing human heart. Cardiovasc. Res.38(3), 727–735 (1998).
- Kornowski R, Hong MK, Leon MB. Comparison between left ventricular electromechanical mapping and radionuclide perfusion imaging for detection of myocardial viability. Circulation98(18), 1837–1841 (1998).
- Gyongyosi M, Khorsand A, Sochor H et al. Characterization of hibernating myocardium with NOGA electroanatomic endocardial mapping. Am. J. Cardiol.95(6), 722–728 (2005).
- Koch KC, vom Dahl J, Wenderdel M et al. Myocardial viability assessment by endocardial electroanatomic mapping: comparison with metabolic imaging and functional recovery after coronary revascularization. J. Am. Coll. Cardiol.38(1), 91–98 (2001).
- Wiggers H, Botker HE, Sogaard P et al. Electromechanical mapping versus positron emission tomography and single photon emission computed tomography for the detection of myocardial viability in patients with ischemic cardiomyopathy. J. Am. Coll. Cardiol.41(5), 843–848 (2003).
- Perin EC, Silva GV, Sarmento-Leite R et al. Assessing myocardial viability and infarct transmurality with left ventricular electromechanical mapping in patients with stable coronary artery disease: validation by delayed-enhancement magnetic resonance imaging. Circulation106(8), 957–961 (2002).
- Vale PR, Losordo DW, Tkebuchava T, Chen D, Milliken CE, Isner JM. Catheter-based myocardial gene transfer utilizing nonfluoroscopic electromechanical left ventricular mapping. J. Am. Coll. Cardiol.34(1), 246–254 (1999).
- Oron U, Halevy O, Yaakobi T et al. Technical delivery of myogenic cells through an endocardial injection catheter for myocardial cell implantation. Int. J. Cardiovasc. Intervent.3(4), 227–230 (2000).
- Vale PR, Losordo DW, Milliken CE et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation103(17), 2138–2143 (2001).
- Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science275(5302), 964–967 (1997).
- Asahara T, Masuda H, Takahashi T et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res.85(3), 221–228 (1999).
- Kawamoto A, Gwon HC, Iwaguro H et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation103(5), 634–637 (2001).
- Kocher AA, Schuster MD, Szabolcs MJ et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med.7(4), 430–436 (2001).
- Kawamoto A, Tkebuchava T, Yamaguchi J et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation107(3), 461–468 (2003).
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res.95(1), 9–20 (2004).
- Orlic D, Kajstura J, Chimenti S et al. Bone marrow cells regenerate infarcted myocardium. Nature410(6829), 701–705 (2001).
- Orlic D, Kajstura J, Chimenti S et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl Acad. Sci. USA98(18), 10344–10349 (2001).
- Aicher A, Brenner W, Zuhayra M et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation107(16), 2134–2139 (2003).
- Barbash IM, Chouraqui P, Baron J et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation108(7), 863–868 (2003).
- Bittner RE, Schofer C, Weipoltshammer K et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat. Embryol. (Berl.)199(5), 391–396 (1999).
- Robinson SW, Cho PW, Levitsky HI et al. Arterial delivery of genetically labelled skeletal myoblasts to the murine heart: long-term survival and phenotypic modification of implanted myoblasts. Cell Transplant5(1), 77–91 (1996).
- Schachinger V, Assmus B, Britten MB et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J. Am. Coll. Cardiol.44(8), 1690–1699 (2004).
- Strauer BE, Brehm M, Zeus T et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation106(15), 1913–1918 (2002).
- Wollert KC, Meyer GP, Lotz J et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet364(9429), 141–148 (2004).
- Erbs S, Linke A, Adams V et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circ. Res.97(8), 756–762 (2005).
- Fuchs S, Satler LF, Kornowski R et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J. Am. Coll. Cardiol.41(10), 1721–1724 (2003).
- Perin EC, Dohmann HF, Borojevic R et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation107(18), 2294–2302 (2003).
- Perin EC, Dohmann HF, Borojevic R et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation110(11 Suppl.1), II213–II218 (2004).
- Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet361(9351), 47–49 (2003).
- Patel AN, Geffner L, Vina RF et al. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J. Thorac. Cardiovasc. Surg.130(6), 1631–1638 (2005).
- Amado LC, Saliaris AP, Schuleri KH et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl Acad. Sci. USA102(32), 11474–11479 (2005).
- Freyman T, Polin G, Osman H et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J.27(9), 1114–1122 (2006).
- Pompilio G, Steinhoff G, Liebold A et al. Direct minimally invasive intramyocardial injection of bone marrow-derived AC133+ stem cells in patients with refractory ischemia: preliminary results. Thorac. Cardiovasc. Surg.56(2), 71–76 (2008).
- Pompilio G, Cannata A, Peccatori F et al. Autologous peripheral blood stem cell transplantation for myocardial regeneration: a novel strategy for cell collection and surgical injection. Ann. Thorac. Surg.78(5), 1808–1812 (2004).
- Herreros J, Prosper F, Perez A et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur. Heart J.24(22), 2012–2020 (2003).
- Menasche P, Hagege AA, Scorsin M et al. Myoblast transplantation for heart failure. Lancet357(9252), 279–280 (2001).
- Menasche P, Hagege AA, Vilquin JT et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol.41(7), 1078–1083 (2003).
- Siminiak T, Kalawski R, Fiszer D et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase I clinical study with 12 months of follow-up. Am. Heart J.148(3), 531–537 (2004).
- Makkar RR, Lill M, Chen PS. Stem cell therapy for myocardial repair: is it arrhythmogenic? J. Am. Coll. Cardiol.42(12), 2070–2072 (2003).
- Miyahara Y, Nagaya N, Kataoka M et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med.12(4), 459–465 (2006).
- Breitbach M, Bostani T, Roell W et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood110(4), 1362–1369 (2007).
- Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog. Cardiovasc. Dis.49(6), 421–429 (2007).
- Iwasaki H, Kawamoto A, Ishikawa M et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation113(10), 1311–1325 (2006).
- Patterson M, Duckers E, Ramacharitar S et al. Magnetically supported procedures and cardiac regeneration. Eurointervention2(Suppl. B), B42–B46 (2007).
