Response to: Raccah D. Efficacy and safety of lixisenatide in the treatment of Type 2 diabetes mellitus: a review of Phase III clinical data. Expert Rev. Endocrinol. Metab. 8(2), 105–121 (2013).
We welcome the recent review by Raccah of the clinical efficacy and safety of lixisenatide, a new glucagon-like peptide (GLP)-1 receptor agonist Citation[1]. Lixisenatide is characterized by a short elimination half-life (2–4 h) Citation[2], and the addition of this agent to the class may broaden treatment options Citation[3,4].
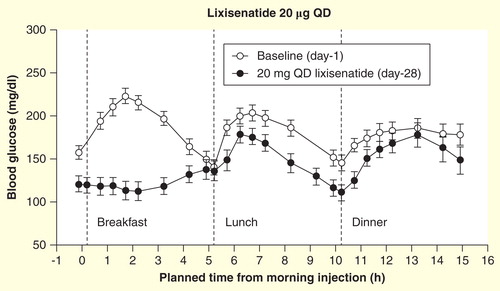
We would like, however, to point out a miscommunication in the review, where it is suggested that the ability to dose short-acting lixisenatide once daily is a reflection of its high affinity for the GLP-1 receptor. For a given half-life, duration of action is a function of administered dose and threshold for metabolic effect. Thus, a highly potent receptor agonist may remain effective for several half-lives if a sufficiently high dose is administered, but given that a 24-h dosing period is at least six times the elimination half-life of lixisenatide, approximately 12.5–25% of the administered dose is likely to be found in the circulation at dinner and less than 2% would remain at the end of the dosing period. Such amounts are unlikely to provide the full pharmacodynamic effect at dinner and would be expected to provide little or no effect by the end of the 24-h dosing period. This supposition is confirmed by the pharmacodynamics data published by Lorenz and colleagues Citation[5]. The 16-h blood glucose profile of lixisenatide 20 µg once daily showed that, while substantial postprandial glucose control was achieved after breakfast (the meal proximal to dosing), the effectiveness was markedly reduced after lunch and marginal after dinner, when compared with baseline. While the effect of lixisenatide on postprandial glucose was statistically significant when compared with placebo at each of the three meals, the profile at breakfast was markedly different compared with lunch and dinner, most likely reflecting that very little efficacy of lixisenatide is present at these meals.
Figure 1. Sixteen-hour blood glucose concentrations in response to standardized meals at breakfast, lunch and dinner at baseline and day 28 in patients (n = 21) with Type 2 diabetes after administration of lixisenatide. Data are mean ± standard error.
Data from a pharmacodynamic comparison of lixisenatide and liraglutide have recently been published by Kapitza and colleagues Citation[6]. Examination of these data shows that lixisenatide leads to lower blood glucose levels than liraglutide during the postprandial period immediately following administration, but that glucose levels remain lower with liraglutide than with lixisenatide throughout all other periods of the 24-h measurement reflecting differing activity profiles of these two agents. While the effect of lixisenatide on blood glucose levels was confined to the post-breakfast period, reductions in breakfast postprandial glucose may contribute to more prolonged metabolic effects.
Following consideration of the peaks in plasma glucose levels, it could be inferred that participants in the Kapitza study were provided with a large breakfast followed by a smaller lunchtime meal. As previously mentioned, the Lorenz study data demonstrated that the effectiveness of lixisenatide decreased throughout the day. Consequently, the provision of a smaller meal in the middle of the day in the Kapitza study may mitigate any diminished effect of lixisenatide at this time.
Based on image analysis of the data presented by Kapitza et al., we have estimated that the reduction in 24-h glucose levels (AUC0–24h) was approximately twofold greater with the longer-acting liraglutide than with lixisenatide (−48 h.mmol/l vs −25 h.mmol/l, respectively). No substantial effect on glucose levels was apparent with lixisenatide by 6.5 h after dosing when compared with baseline. It appears, therefore, that circulating levels of lixisenatide may not be sufficient to provide therapeutic effect for a full 24 h following a single daily dose, the observed effect on postprandial glucose notwithstanding. Heart rate data seem to support this observation. In healthy volunteers, therapeutic doses of lixisenatide were associated with an increased heart rate (7.3 bpm) up to 10 h following drug administration Citation[7], an effect seen with other agents in this class. However, in the subsequent Kapitza study, in which supine heart rate was measured 24 h after last study dose administration, lixisenatide was associated with a decrease in heart rate from baseline. Liraglutide-treated patients, in contrast, demonstrated an increased heart rate. These data support that the effect of lixisenatide is no longer present 24 h after dosing. There were no reports of increased heart rate in the lixisenatide Phase III trials; however, this is most likely due to the time lapse between drug administration and heart rate measurement.
Whether 24-h glucose control, or management of postprandial glucose excursions, is the key goal of therapy remains a matter of debate. The current American Diabetes Association/European Association for the Study of Diabetes guidelines Citation[8,9], drawing on evidence from a corpus of well-conducted trials, focus on HbA1c, a composite measure of glycemic control reflecting glucose levels both in the postprandial and in the preprandial/fasting states. Consideration should be given to the effect of antidiabetic agents on each of these glycemic targets.
The choice of dosing lixisenatide once daily is not one of pharmacokinetics or pharmacodynamics, but of clinical application, and may be appropriate in individuals who require improved prandial glucose control at a single meal during the day. As patients generally consume multiple meals per day, we would however question whether, as suggested by Raccah, a choice needs to be made between a short-acting and a long-acting GLP-1 receptor agonist in order to control postprandial glucose excursions and fasting glucose; a long-acting therapy, with a duration of action of ≥24 h, would be capable of controlling postprandial glucose levels across all three main meals of the day in addition to significant effects on fasting glucose levels. The Kapitza data demonstrated that once-daily administration of liraglutide resulted in reduced postprandial and fasting glucose levels over a 24-h period, suggesting that liraglutide therapy may be the most appropriate choice in patients with Type 2 diabetes requiring 24-h blood glucose control.
Financial & competing interests disclosure
M Donsmark and LB Knudsen are both full-time employees of Novo Nordisk A/S, which markets liraglutide for the treatment of diabetes. The authors hold minor stock portions as part of an employee offering program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors thank Watermeadow Medical, funded by Novo Nordisk A/S, for writing assistance.
Notes
References
- Raccah D. Efficacy and safety of lixisenatide in the treatment of Type 2 diabetes mellitus: a review of Phase III clinical data. Expert Rev Endocrinol Metab 2013;8(2):105-21
- Distiller LA, Ruus P; on behalf of the ACT6011 Study Group. Pharmacokinetics and pharmacodynamics of a new GLP-1 agonist AVE0010 in type 2 diabetes patients. Poster 520-P. Presented at the American Diabetes Association 68th Scientific Sessions; 2008. Available from: http://professional.diabetes.org/Content/Posters/2008/p520-P.pdf [Last accessed 12 August 2013]
- Thorkildsen C, Neve S, Larsen BD, et al. Glucagon-like peptide 1 receptor agonist ZP10A increases insulin mRNA expression and prevents diabetic progression in db/db mice. J Pharmacol Exp Ther 2003;307(2):490-6
- Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of Type 2 diabetes. Regul Pept 2010;164(2–3):58-64
- Lorenz M, Pfeiffer C, Steinsträßer A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes – Relationship to postprandial glycemia. Regul Pept 2013;185(10):1-8
- Kapitza C, Coester HV, Poitiers F, et al. Pharmacodynamic characteristics of lixisenatide once daily vs liraglutide once daily in patients with T2DM inadequately controlled with metformin. Diabetes Obes Metab 2013;15(7):642-9
- Sanofi-Aventis Lyxumia EMA submission. Available fom www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002445/WC500140449.pdf [Last accessed 12 August 2013]
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013;36(Suppl 1):S11-36
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35(6):1364-79

