Abstract
With prolonged duration of Type 2 diabetes mellitus, most patients need a combination of antihyperglycemic drugs to reach their target HbA1c. Evidence shows that single-pill combinations (SPCs) may increase patient satisfaction, adherence, and reduce overall health-care costs. Several SPCs containing metformin and another oral antidiabetic drug (OAD) are available on the market. Although well established in clinical practice, long-term durability and tolerability of traditional OADs can be inadequate. Dipeptidyl peptidase (DPP)-4 inhibitors and sodium glucose cotransporter (SGLT) 2 inhibitors are two newer classes of OADs that are efficacious and are less likely to induce adverse effects such as gastrointestinal reactions, hypoglycemia and weight gain when compared with metformin, sulfonylureas, and thiazolidinediones. This article describes current efficacy and safety data of DPP-4/SGLT2 inhibitor combination therapy. Pharmacokinetics, mechanism-of-action based rationale for the combination and timing of the addition of a SPC to the treatment regimen are discussed.
Many diseases with worldwide prevalence such as cardiovascular disease, stroke, chronic obstructive pulmonary disease and diabetes mellitus are multifactorial. Effective treatments of these diseases often call for combination therapies that target more than one underlying pathway or risk factor. Combination therapies typically involve two or more drugs with complementary mechanisms of action. Single-pill combinations (SPCs) have been used for the treatment of conditions such as hypertension, stroke and human immunodeficiency virus infections Citation[1–3]. Besides additive effects in efficacy, SPCs offer other potential advantages such as higher patient adherence rates and lower cost Citation[4,5].
Combination therapy is commonly used for the treatment of Type 2 diabetes mellitus (T2DM) worldwide, and physicians have several options available when choosing an appropriate combination for patients. International clinical practice recommendations emphasize a patient-centric approach that involves consideration of individual characteristics, tolerability, preferences and cost–effectiveness along with target glycemic control Citation[6].
This review focuses on the potential combination of two drug classes – dipeptidyl peptidase (DPP)-4 inhibitors and sodium glucose cotransporter (SGLT) 2 inhibitors for the treatment of T2DM. DPP-4 inhibitors are a relatively new class of oral antidiabetic drugs – sitagliptin, the first-in-class drug was approved in 2006. SGLT2 inhibitors are the latest class of glucose-lowering agents with first approval in the USA of canagliflozin in 2013. DPP-4 inhibitors (sitagliptin, saxagliptin and linagliptin) and SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin) are included in this review in the context of combination therapy.
Mechanisms of action of DPP-4 & SGLT2 inhibitors
DPP-4 inhibitors
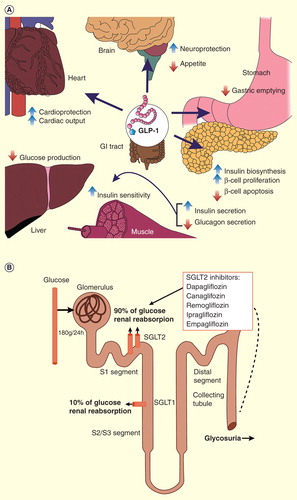
DPP-4 is an enzyme responsible for degradation of the incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide, which play key roles in glucose homeostasis Citation[7]. Postprandially, GLP-1 and glucose-dependent insulinotropic peptide are secreted by intestinal L and K cells, respectively, and stimulate insulin secretion from islet β-cells. Other plausible effects of GLP-1 that contribute to glucose lowering include suppression of glucagon secretion in islet α-cells Citation[8], delayed gastric emptying Citation[9,10] and induction of satiety Citation[7,11]. Endogenous GLP-1 in circulation is short-acting because it is degraded by DPP-4 in 2–3 min Citation[12]. Thus, inhibition of proteolytic activity of DPP-4 has evolved as an approach to increase the half-life of the incretins to improve glycemic control Citation[13].
SGLT2 inhibitors
SGLTs are active carrier proteins responsible for re-absorption of glucose filtered by glomeruli from the lumen back to blood circulation. There are two types of SGLTs. SGLT1 is located in the small intestine and S3 segment of the renal proximal tubule and reabsorbs about 10% of the glucose. SGLT2 is expressed in the renal proximal tubule (segments S1 and S2) and accounts for approximately 90% of glucose re-absorption Citation[14]. In the T2DM state, SGLT2 and glucose transporter 2 (a member of the second family of glucose transporters) are overexpressed, leading to increased glucose re-absorption and blood glucose concentration beyond the normal threshold of 10–11.1 mmol/l. SGLT2 inhibitors block the excess re-absorption, and the surplus glucose is excreted via urine Citation[15,16]. SGLT2 also reabsorbs sodium along with glucose; SGLT2 inhibitors thus promote removal of both glucose and sodium via urine, and potentially confer additional benefits on body weight and blood pressure Citation[17,18].
Rationale for DDP-4/SGLT2-inhibitor combination
From a drug-tolerability perspective, a DPP-4/SGLT2-inhibitor combination appears to be a rational option for a range of patients with T2DM. Patients who need dual or triple therapy are likely to have advanced T2DM and related disorders. In this vulnerable population, risk of adverse events (AEs) resulting from polypharmacy is heightened. Hypoglycemia and weight gain are potentially undesirable side effects. Both DPP-4 inhibitors and SGLT2 inhibitors pose low mechanism-based risk of hypoglycemia – clinical evidence has shown that neither drug class increases the incidence of hypoglycemia when background therapy does not include sulfonylureas or insulin. DPP-4 inhibitors have been shown to be body-weight neutral, whereas SGLT2 inhibitors have been shown to reduce body weight and systolic blood pressure (SBP) Citation[19,20]. Most patients with long-standing T2DM have comorbidities and/or risk factors for cardiovascular and kidney disease. Long-term cardiovascular and renal outcome trials of drugs from both classes are in progress Citation[21–23].
From a mechanism-of-action perspective, the combination of DPP-4/SGLT2 inhibitors offers potentially additive effects. Recent studies have shown that in patients with T2DM, SGLT2 inhibitors increase plasma glucagon levels, leading to an increase in endogenous glucose production (EGP). For example, in a cohort study, empagliflozin 25 mg/day treatment for 4 weeks in patients with T2DM (n = 66) led to improved glycemic control versus baseline (mean [standard deviation; SD] fasting plasma glucose [FPG], 7.6 ± 1.1 mmol/l vs 8.7 ± 1.6 mmol/l; glycated hemoglobin [HbA1c], 6.8 ± 0.8% vs 7.2 ± 1.0%; both p < 0.0001), with a concurrent increase in fasting EGP relative to baseline (median [interquartile range; IQR], 17.5 [4.1] vs 13.8 [5.2] mmol.KgFFM-1.min-1), respectively. Postprandial EGP and plasma glucagon also increased after 4-week empagliflozin treatment. Similar increases in plasma glucagon levels and EGP were observed after a single dose of empagliflozin 25 mg in the same study. These increases appeared to be attenuated after 4 weeks compared with the single dose Citation[24]. In a 2-week study in patients with T2DM (n = 18), dapagliflozin 10 mg/day improved insulin sensitivity compared with placebo (measured by insulin-mediated glucose disposal, 5.7 ± 0.4 mg/kg/min with dapagliflozin vs 4.2 ± 0.5 mg/kg/min with placebo; p < 0.05); however, those receiving dapagliflozin also had increased EGP relative to placebo (2.55 ± 0.20 mg/kg/min and 2.11 ± 0.10 mg/kg/min, respectively; p < 0.05) Citation[25]. Furthermore, the study showed increased EGP with dapagliflozin offset the net urine glucose excretion and was accompanied by an increase in fasting plasma glucagon concentration (77 ± 6 pg/ml on day 14 vs 64 ± 4 pg/ml at baseline, p < 0.05).
Glucagon is a potent stimulator of hepatic glucose production in response to hypoglycemia; however, in individuals who are glucose intolerant or have T2DM, glucagon secretion is dysregulated, contributing to hyperglycemia Citation[26]. In the dapagliflozin study, the investigators noted a 23% increase in plasma glucagon concentration with dapagliflozin that paralleled the increase in EGP Citation[25]. Based on this observation, and the effect of incretin mimetics on glucagon suppression Citation[27], the investigators hypothesized that a concomitant incretin-based therapy would counteract the increase in EGP and enhance glycemic efficacy of a SGLT2 inhibitor Citation[25]. Other studies investigating glucose-lowering effects of DPP-4 inhibitors have demonstrated their glucagon suppression effects Citation[28–30].
Pharmacokinetics/pharmacodynamics
Clinical studies to date have shown no significant drug–drug interactions between DPP-4 inhibitors and SGLT2 inhibitors. For example, when empagliflozin and sitagliptin were co-administered in healthy volunteers, the area under concentration-time curve (AUCT, SS) and maximum concentration (Cmax, SS) for both agents were within the standard bioequivalence range of 80–125% Citation[31]. Similarly, time from last dosing to maximum concentration (tmax, SS) and terminal half-life (t1/2, SS) of both drugs alone (empagliflozin, 2.5 and 8.5 h; sitagliptin, 3.0 and 12.7 h) were also not affected when co-dosed .
Table 1. Pharmacokinetic characteristics of DPP-4 inhibitors and SGLT2 inhibitors administered together.
In another combination study, co-administration of empagliflozin and linagliptin did not affect the pharmacokinetic parameters of each other Citation[32]. The steady-state mean (SD) urinary glucose excretion over 24 h with empagliflozin alone and in combination was 54.8 g (11.2) and 67.2 g (14.6), respectively; linagliptin alone and as add-on to empagliflozin caused similar trough DPP-4 inhibition (83.7 and 83.9%) Citation[32]. A study (NCT01189201) investigating bioavailability of empagliflozin/linagliptin single-pill combination (SPC) in comparison with the mono-components, and with a second formulation of the SPC is completed, but the results have not been published Citation[33]. The study also evaluated the influence of food on the bioavailability of the linagliptin/empagliflozin SPC.
Pharmacokinetic/pharmacodynamic data of dapagliflozin given in combination with sitagliptin and saxagliptin are available. Kasichayanula et al. showed that dapagliflozin and sitagliptin given together did not alter the exposure and Cmax of either drug Citation[34]. A single-dose, open-label, three-treatment-period study in healthy participants with saxagliptin 5 mg in combination with dapagliflozin 10 mg revealed that the AUCT, SS and Cmax, SS of either drug were comparable when given in combination or alone Citation[35]. Dapagliflozin had identical tmax with or without saxagliptin; saxagliptin median tmax (0.5 [0.5–2.0] h) was slightly lower in mono-treatment arm than in the combination arm. The half-lives of both drugs in combination were comparable to those in respective mono-treatments (mean [SD], dapagliflozin, 15.9 [7.3] h; saxagliptin, 5.9 [2.2] h).
Efficacy
Pre-clinical as well as clinical efficacy data on DPP-4/SGLT2-inhibitor combination treatment are emerging.
Animal studies
In db/db mice, linagliptin and BI-38335 (an investigatory SGLT2 inhibitor) combination showed greater improvements in glycemic parameters, glucose tolerance, islet cell function and morphology, β-cell volume and islet and peripheral tissue inflammation than the individual treatments Citation[36]. For example, mean ± standard error HbA1c at 8 weeks were 7.25 ± 0.30% in the vehicle group and 6.15 ± 0.27% (p < 0.01), 5.90 ± 0.20% (p < 0.001), and 5.45 ± 0.53% (p < 0.001) in the linagliptin, BI-38335, and combination groups, respectively. In glucose tolerance test, AUC0-2 h was reduced by 25, 40 and 50% with linagliptin, BI-38335 and the combination, respectively, as compared with vehicle control group. The combination elicited higher glucose-stimulated insulin secretion (2.57 ± 0.35 μg/islet/h) than linagliptin, (1.23 ± 0.42 μg/islet/h, p < 0.01) or BI-38335 (1.34 ± 0.31 μg/islet/h, p < 0.05) alone.
In another study investigating insulin sensitivity in db/db mice, whole body glucose-disposal rates improved with 8-week treatment with empagliflozin 10 mg/kg/d (5.9 mg/kg/min; p < 0.001) and linagliptin 3 mg/kg/d (3.4 mg/kg/min; p < 0.01) given as monotherapies versus vehicle-treated animals (1.9 mg/kg/min). Notably, empagliflozin + linagliptin combination treatment achieved greater increase in glucose-disposal rate (7.8 mg/kg/min, p < 0.001) relative to the vehicle group Citation[37]. Glucose uptake in the liver and kidney were significantly higher with both monotherapies (both p < 0.05) and the combination therapy (p < 0.01) compared with the vehicle, but muscle and adipose tissue glucose uptake did not significantly alter with any treatment relative to the vehicle Citation[37].
Human studies
In placebo-controlled individual clinical trials of ≥12-week duration, the HbA1c and FPG changes with DPP-4 inhibitors generally ranged from −0.5 to −0.9% and −11 to −25 mg/dl, respectively; the greatest improvements were observed when a DPP-4 inhibitor was used as initial combination with metformin (HbA1c change −1.7 to −2.1% and FPG change −60 to −70 mg/dl) Citation[38]. In a large meta-analysis of 55 randomized controlled trials of 12–52 weeks' duration, DPP-4 inhibitors elicited a significant change in HbA1c (weighted mean difference [WMD]: 95% CI: −0.65% [−0.71 to −0.60]) relative to placebo Citation[39]. The same analysis revealed no significant change in HbA1c relative to an active comparator (WMD: 95% CI: −0.04% [−0.09 to −0.16]); of note, this subgroup analysis was associated with high heterogeneity.
Glycemic efficacy of SGLT2 inhibitors parallels that of DPP-4 inhibitors with monotherapy leading to mean changes in HbA1c ranging from −0.4 to −1.1% in randomized controlled trials lasting ≥12 weeks. A systematic review of 58 trials revealed that SGLT2 inhibitors caused a greater decrease in HbA1c compared with placebo (WMD: 95% CI: monotherapy −0.79% [−0.96 to −0.62]; combination therapy −0.61% [−0.69 to −0.53]) Citation[40]; however, compared with other antidiabetic therapies, SGLT2 inhibitors administered alone or in combination achieved similar glycemic control. In the same analysis, SGLT2 treatment also lowered mean body weight (WMD: 95% CI: −1.74 kg [−2.03 to −1.45] vs placebo and −1.80 kg [−3.50 to −0.11 kg] vs other antidiabetic therapies) and SBP (WMD: 95% CI: −3.77 mmHg [−4.65 to −2.90] vs placebo and −4.45 mmHg [−5.73 to −3.18] vs other antidiabetic treatments).
Versus placebo
The CANagliflozin cardioVascular Assessment Study (CANVAS) is designed to evaluate cardiovascular outcomes in at-risk patients Citation[41]. In a subgroup analysis of CANVAS, (n = 361; mean age, 63 years; body mass index [BMI], 37.4 kg/m2), canagliflozin 100 or 300 mg added to a DPP-4 inhibitor significantly reduced HbA1c and body weight compared with placebo after 18 weeks Citation[42]. Addition of dapagliflozin 10 mg to sitagliptin 100 mg with or without metformin background for 24 weeks (mean [SD] age 54.8 [10.4] years, and body weight, 91.0 [21.6] kg) achieved statistically significant reductions in HbA1c, FPG and body weight Citation[43]). In this study, SBP at week 8 did not change significantly with the combination relative to placebo in patients with baseline seated SBP ≥130 mmHg. However, patients receiving the combination experienced small mean increases in total cholesterol, high-density lipoprotein and low-density lipoprotein (placebo-corrected differences 95% CI: 3.6% [0.6 to 6.7]; 4.6% [1.8 to 7.4] and 3.6% [−1.3 to 8.8], respectively). After 24 weeks, the combination treatment also resulted in a 24.9% increase in β-cell function from baseline (as measured by a Homeostasis Model Assessment [HOMA]-2 analysis) versus a 5.2% increase with placebo Citation[43].
Table 2. Efficacy of DPP-4 inhibitors and SGLT2 inhibitors given as SPC or LPC.
Versus individual treatments
Recent clinical evidence also indicates that combining DPP-4/SGLT2 inhibitors may result in greater glycemic control than would be achieved by individual treatments. In a 24-week study, adding dapagliflozin 10 mg and saxagliptin 5 mg to metformin in patients with sub-optimally controlled hyperglycemia significantly reduced HbA1c versus the individual agents alone ; data on changes in FPG, body weight or blood pressure were not reported in the published abstract Citation[44]. The baseline HbA1c in the dapagliflozin + saxagliptin study was higher (mean [SD], 8.9 [1.2]) than that reported in the other studies with DPP-4/SGLT2-inhibitor combinations .
Linagliptin + empagliflozin and saxagliptin + dapagliflozin SPCs are under review by the US FDA. In a Phase III study in treatment-naïve patients (mean [SD] age, 54.6 [10.2] years; BMI, 31.6 [5.6] kg/m2), SPCs of empagliflozin 25 mg/linagliptin 5 mg and empagliflozin 10 mg/linagliptin 5 mg significantly reduced HbA1c, FPG and body weight relative to linagliptin 5 mg monotherapy at week 24 Citation[45]. A significant reduction in HbA1c was also noted with empagliflozin 10 mg/linagliptin 5 mg versus empagliflozin 10 mg monotherapy Citation[45]. In patients pre-treated with metformin, the same SPCs significantly reduced HbA1c and FPG relative to linagliptin and empagliflozin monotreatments Citation[46]. In this population, body-weight reduction with the SPCs was significant only against linagliptin monotreatment. Glucose-lowering effect of both the fixed-dose combinations was sustained up to 52 weeks Citation[47,48].
Several other trials investigating the efficacy and safety of a DPP-4/SGLT2-inhibitor combination with background metformin therapy are currently underway .
Table 3. Ongoing clinical trials of DPP-4/SGLT2-inhibitor combination.
Safety
In clinical trials, DPP-4 inhibitors and SGLT2 inhibitors individually have shown favorable safety profiles with no excess rates of gastrointestinal reactions, weight gain or hypoglycemia relative to placebo or an active comparator; higher rates of hypoglycemia were observed with the two drug classes only with sulfonylurea or insulin background Citation[19,20,49]. Common side effects associated with SGLT2 inhibitors include genital mycotic infections, urinary tract infections (UTIs), AEs related to osmotic diuresis and volume depletion (e.g., polyuria and hypotension) Citation[40,50]. For example, in a meta-analysis (58 studies), SGLT2 inhibitors were more likely to cause genital tract infections and UTIs compared with placebo (odds ratios [ORs]: 95% CI: 3.50 [2.46 to 4.99] and 1.34 [1.03 to 1.74]) or another antidiabetic drug (ORs: 95% CI: 5.06 [3.44 to 7.45] and 1.42 [1.06 to 1.90]) Citation[40]. Generally, both genital and UTIs have been mild to moderate in nature, treatable with standard therapies, occurred during initial months of treatment with typically low rates of recurrence (<3%) and have led to <1% discontinuation rates Citation[18,51,52]. Neither of the two drug classes have been associated with increased overall risk of composite cardiovascular events Citation[40,49]; however, in a cardiovascular outcomes study with saxagliptin in diabetes patients with a history of or increased risk of cardiovascular disease, saxagliptin treatment resulted in an increased rate of hospitalization due to heart failure relative to placebo (3.5 vs 2.8%; hazard ratio: 95% CI: 1.27 [1.07 to 1.51], p = 0.007) Citation[53]. A follow-up study, which further analyzed the increased risk of hospitalization due to heart failure with saxagliptin, showed that individuals at greatest risk had prior heart failure, impaired renal function or higher baseline levels of the heart failure marker N-terminal of the pro-hormone B-type natriuretic peptide Citation[54].
A widely expressed concern related to SPC therapies is that they can potentially cause higher incidences of AEs; however, AE rates likely depend on the individual component drugs in the combination. In clinical studies, DPP-4/SGLT2-inhibitor combinations were generally well tolerated. Overall safety data with combinations were limited to abstract publications at the time this review was prepared; profiles are shown in . In the post hoc analysis of CANVAS, at 18 weeks, overall AE rates were higher in the low-dose and high-dose canagliflozin plus a DPP-4 inhibitor groups than in the placebo group Citation[42]; similarly, documented hypoglycemia frequencies in patients on insulin, sulfonylurea or meglitinide background therapy were higher with both canagliflozin groups versus the placebo group. More patients in the high-dose canagliflozin group discontinued treatment due to AEs (6/111, 5.4%) than patients in the low-dose canagliflozin (1/103, 1%) and placebo groups (1/102, 1%). A higher proportion of patients in the canagliflozin 300 and 100 mg groups experienced drug-related AEs (29/111, [26.1%] and 21/103, [20.4]) than in the placebo group (14/102, [13.7%]) Citation[42].
Table 4. Safety and tolerability of DPP-4 inhibitors and SGLT2 inhibitors given as SPC or LPC.
In the study investigating effects of dapagliflozin added on to sitagliptin, 48-week treatment rates of AEs with dapagliflozin 10 mg plus sitagliptin 100 mg were slightly higher relative to placebo plus sitagliptin ; however, serious AEs (6.7 vs 8%), AEs leading to discontinuation (3.1% both groups) and hypoglycemia (5.3 vs 6.2%) occurred at comparable rates between groups Citation[43]. More patients in the dapagliflozin group than in the placebo group suffered from signs, symptoms and events suggestive of genital infections (22/225 [9.8%] vs 1/226 [0.4%]); infections were non-serious, and most patients responded to one course of treatment. One patient from the dapagliflozin group withdrew from the study due to vulvovaginal mycotic infection Citation[43]. Approximately 75% of the subjects who experienced ≥1 event suggestive of or an AE of genital infection were female. At 48 weeks, signs, symptoms and events suggestive of UTI were comparable between groups (15/225 [6.7%] with dapagliflozin; 14/226 [6.2%] with placebo), but a higher proportion of patients taking dapagliflozin than those taking placebo had diagnosed events of UTI (13/225 [5.8%], and 8/226 [3.5%]). Dapagliflozin combined with sitagliptin numerically increased AEs of renal impairment (RI; 8/225 [3.6%]) compared with placebo (4/226 [1.8%]), which was attributed to decreased renal creatinine clearance. Dapagliflozin and sitagliptin combination caused a small increase from baseline in low-density cholesterol at week 24 (placebo-corrected mean change 95% CI: 3.6% [−1.3, 8.8]) Citation[43].
In the dapagliflozin and saxagliptin combination study, rates of overall AEs and hypoglycemia over 24 weeks with dapagliflozin 10 mg plus saxagliptin 5 mg were similar to the corresponding monotherapies Citation[44]. In the empagliflozin (10 or 25 mg)/linagliptin (5 mg) SPC study conducted among treatment-naïve patients, AE rates were comparable between all groups, with the lowest rates in the empagliflozin 25 mg groups with or without concomitant linagliptin therapy Citation[45]. Among patients taking metformin, higher proportions of patients in both of the empagliflozin monotherapy groups experienced AEs than the any other drug group Citation[46]. Confirmed hypoglycemic events (glucose ≤70 mg/dl) occurred at very low rates in all groups in both studies Citation[45,46].
Timing of adding SPC to the treatment regimen
Whether and when to start an SPC treatment depends on individual patient-specific factors, and the decision should be based on a risk–benefit analysis. Potential risks associated with an SPC treatment include increased AEs, whereas potential benefits include improved glycemic control and patient adherence Citation[55]. Although the percentage of T2DM patients meeting targets for HbA1c, blood pressure and lipids has risen over the past 10 years in the USA, between 33.4 and 48.7% patients still do not achieve their treatment goals Citation[56]. This is in part due to non-adherence to therapy. A large body of clinical evidence has shown that adherence rates are better with SPCs than with corresponding loose-pill combinations (LPCs) Citation[4,57,58]. Several factors account for this difference, including the reduced complexity of dosing, perception of medication, cost and patient–provider interaction Citation[57].
American Association of Clinical Endocrinologists’ (AACE) Comprehensive Diabetes Management Algorithm recommends dual therapy if HbA1c is ≥7.5% and advancement to triple therapy if the target HbA1c is not reached in 3 months Citation[59]. In such instances, initiating treatment with a combination of drugs is likely to provide more rapid glycemic control than sequential addition of drugs, thereby avoiding prolonged exposure to glucose Citation[60]. Specifically, combinations of drugs with complementary mechanisms of actions that could improve β-cell function and reduce obesity would be useful for early intensive therapy, given the multifactorial pathophysiology of T2DM Citation[60]. Metformin is the standard first component in a combination therapy, however, when it is insufficient for attainment of target HbA1c or is contraindicated or not tolerated, sequential addition of a DPP-4 inhibitor and SGLT2 inhibitor or simultaneous addition as a DPP-4/SGLT2-inhibitor combination depending on the desired level of reduction in HbA1c, could be a rational choice. Current T2DM treatment guidelines indicate use of DPP-4 and SGLT2 inhibitors at any stage of pharmacotherapy Citation[6,59]. For SGLT2 inhibitors, the AACE algorithm advises their use with caution and notes that clinical evidence is based on just three Phase III studies at the time of publication Citation[59]. Although SGLT2 inhibitors have only recently been incorporated into the treatment algorithms, they offer glucose-lowering efficacy with low risk of hypoglycemia, insulin-independent mode of action and positive effects on body weight Citation[61]. Thus, a DPP-4/SGLT2-inhibitor combination could support strategies that emphasize low risk of both hypoglycemia and weight gain.
The cost of SPC pills can be an important consideration for patients and payers because patients with T2DM are likely to use antidiabetic drugs for several years. LPCs of generic versions of individual components would be more affordable than the corresponding SPCs of branded drugs, but the use of SPCs instead of higher doses of monotherapy or corresponding LPCs has been shown to lower overall per-patient healthcare costs Citation[4,55,62–64].
Conclusion
Most patients with T2DM require more than one antidiabetic agent to attain the target HbA1c. From the patient’s perspective, the optimal drug combination depends on several factors besides efficacy, such as severity of side effects, dosing regimen and cost. Based on emerging data, DPP-4 inhibitors and SGLT2 inhibitors are two classes of oral antidiabetic drugs that are efficacious and well tolerated.
DPP-4 inhibitors combined with SGLT2 inhibitors have shown no significant drug–drug interactions and can be administered together without dose adjustment. DPP-4/SGLT2-inhibitor SPCs could be expected to demonstrate similar pharmacokinetic/pharmacodynamic profiles as the corresponding components given alone or in LPCs. The two-drug combinations have shown superior antihyperglycemic efficacy than individual components with no significant increase in associated AEs. Low inherent risk of hypoglycemia associated with both drug classes, and added benefits of reduction in body weight and blood pressure with SGLT2 inhibitors make this combination an attractive option in overweight or obese patients and patients with increased risk of hypoglycemia. In clinical trials, SGLT2 inhibitors increased incidence of genital infections and to a lesser extent UTIs (both mostly in women), as well as frequency of urination. The infections were mild to moderate, treatable and occurred primarily during the initial months of treatment; however, patients on SGLT2 inhibitors should be monitored and educated about management of these possible AEs.
Expert commentary
Currently available SPCs for treatment of T2DM – metformin with another drug (a sulfonylurea, a thiazolidinedione or a DPP-4 inhibitor) contain at least one agent that can cause undesirable side effects including gastrointestinal reactions, weight gain, hypoglycemia, increased risk of fluid retention and related AEs (edema, heart failure or anemia), and fractures. DPP-4 inhibitors and SGLT2 inhibitors offer new treatment options that have shown to be inherently indisposed to these side effects. In addition, SGLT2 inhibitors alone and in combination with DPP-4 inhibitors have been associated with clinically meaningful reductions in body weight. Thus, a DPP-4/SGLT2-inhibitor SPC could be a desirable therapy for a wide spectrum of patients, including overweight or obese individuals or those at high risk of hypoglycemia. Because of the insulin-independent action of SGLT2 inhibitors and potential positive effects on insulin sensitivity, the combination could also be attractive in patients with long durations of diabetes and sub-optimal β-cell function and decreased insulin sensitivity. In patients requiring more than one oral antidiabetic drug, a DPP-4/SGLT2-inhibitor SPC with or without metformin could be a prudent choice. In patients taking the two drugs as loose pills, a SPC option could reduce pill burden. Potential for a DPP-4/SGLT2-inhibitor combination should be carefully considered in patients with RI. Linagliptin can be used without dose adjustment in patients with any degree of renal impairment Citation[65,66], whereas, sitagliptin and saxagliptin require dose adjustment in patients with moderate-to-severe RI Citation[67,68]. In patients with RI, renal function should be monitored during SGLT2 treatment, with manufacturer-recommended dosage adjustments or restrictions; loss of efficacy generally occurs with worsening degree of RI Citation[69–71]. Therapy should be discontinued if estimated glomerular filtration rate falls persistently below 60 ml/min/1.73 m2 for dapagliflozin or 45 ml/min/1.73 m2 for canagliflozin and empagliflozin Citation[69–71].
Five-year view
Given the record of the two latest drug classes and the advantages of SPC treatments, several DPP-4/SGLT2-inhibitor combinations are expected to be available over the next 5 years. Results of dedicated long-term cardiovascular outcome studies with drugs from both classes are also anticipated and will help establish a cardiovascular safety profile. Within the next 5 years, real-world data on both the drug classes should provide additional guidance with regard to their long-term safety and durability.
In patients with T2DM who need oral combination therapy, current treatments include metformin with another antidiabetic drug (a sulfonylurea, a thiazolidinedione or a DPP-4 inhibitor). These established drug combinations are generally efficacious, but contain at least one agent that can cause undesirable side effects.
DPP-4 inhibitors and SGLT2 inhibitors are newer glucose-lowering agents that demonstrated more favorable safety profiles than the traditional antidiabetic drugs because of the low inherent risks of hypoglycemia, weight gain, fluid retention and gastrointestinal reactions.
Pharmacokinetic studies of DPP-4 inhibitors combined with SGLT2 inhibitors have shown no significant drug–drug interactions, thus, they can be co-administered without dose adjustment. The two drug classes have complementary mechanisms of action. SGLT2 inhibitors block re-absorption of glucose, resulting in increased urinary glucose excretion. DPP-4 inhibitors slow inactivation of GLP-1, resulting in increased insulin secretion with reduced glucagon levels.
Recent short-term studies indicate that in patients with T2DM, SGLT2 inhibitors increased endogenous glucose production along with plasma glucagon concentration, possibly blunting the antihyperglycemic effects of the drugs. These observations suggest an intriguing hypothesis that a DPP-4 inhibitor co-administered with an SGLT2 inhibitor might reverse the stimulation of glucagon and endogenous glucose production, thereby enhancing the glycemic efficacy of the SGLT2 inhibitor.
Clinical trials with DPP-4/SGLT2-inhibitor combinations have shown superior glycemic efficacy relative to placebo or the respective monotherapies. The combination added-on to other glucose-lowering agents for up to 24 weeks achieved 0.5–0.75% reductions in mean HbA1c relative to placebo. Compared with the respective mono-treatments, the combination treatments for 24 weeks reduced mean HbA1c by 0.14 to 0.59% when used with or without additional antihyperglycemic agents.
DPP-4/SGLT2-inhibitor combination treatments up to 24 weeks elicited changes in mean body weight ranging from −1.9 to −2.78 kg relative to placebo. With both the empagliflozin (25 or 10 mg)/linagliptin 5 mg combinations, changes in mean body weight were clinically and statistically significant relative to linagliptin monotherapy (−1.2 to −2.3 kg), but not relative to empagliflozin monotherapies.
Rates of overall AEs were higher with DPP-4/SGLT2-inhibitor combination than with placebo for treatments lasting up to 48 weeks; however, the rates were similar to those with respective mono-treatments. Hypoglycemia incidence rates with the combinations were low and comparable with placebo or active mono-treatments, except when insulin, sulfonylurea or meglitinide were used as background therapies.
Dapagliflozin plus sitagliptin treatment for 48 weeks resulted in higher rates of signs, symptoms and events suggestive of genital infections than placebo (9.8 vs 0.4%), whereas rates of signs, symptoms and events suggestive of urinary tract infections were comparable between groups (6.7 vs 6.2%). The infections were not serious and responded to treatment.
Low rates of documented hypoglycemia in clinical trials with DPP-4/SGLT2-inhibitor combinations, with added benefits of reduction in body weight and blood pressure with SGLT2 inhibitors make this combination an attractive option in overweight or obese patients and patients with increased risk of hypoglycemia.
Over the next 5 years, results of the ongoing clinical trials with DPP-4/SGLT2-inhibitor combinations and of large cardiovascular outcome studies with both the drug classes along with real-world data should provide additional guidance with regard to their long-term safety and durability.
Financial & competing interests disclosure
The author D Singh-Franco has nothing to disclose. The author meets criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The author received no direct compensation related to the development of the manuscript. Medical writing was provided by R Narayan, of Envision Scientific Solutions, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Notes
References
- Boehringer Ingelheim. Aggrenox (aspirin/extended-release dipyridamole) label. Available from: www.aggrenox.com/
- Borghi C, Cicero AF. Rationale for the use of a fixed-dose combination in the management of hypertension: efficacy and tolerability of lercanidipine/enalapril. Clin Drug Investig 2010;30(12):843-54
- Gandhi M, Gandhi RT. Single-pill combination regimens for treatment of HIV-1 infection. N Engl J Med 2014;371(3):248-59
- Hutchins V, Zhang B, Fleurence RL, et al. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin 2011;27(6):1157-68
- Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ 2014;348:g3318
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-49
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368(9548):1696-705
- Hare KJ, Vilsboll T, Asmar M, et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010;59(7):1765-70
- Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003;88(6):2719-25
- Meier JJ, Kemmeries G, Holst JJ, et al. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes 2005;54(7):2212-18
- Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101(3):515-20
- Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995;44(9):1126-31
- Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 2011;13(1):7-18
- Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 2009;53(5):875-83
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14(1):5-14
- Singh SK, Gupta AK. SGLT2 inhibitors for treatment of type 2 diabetes mellitus: focus on canagliflozin. Muller J Med Sci Res 2014;4:166-73
- Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012;8(8):495-502
- Rosenwasser RF, Sultan S, Sutton D, et al. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab Syndr Obes 2013;6:453-67
- Karagiannis T, Paschos P, Paletas K, et al. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344:e1369
- Musso G, Gambino R, Cassader M, et al. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med 2012;44(4):375-93
- BI 10773 (Empagliflozin) cardiovascular outcome event trial in type 2 diabetes mellitus patients. Available from: http://clinicaltrials.gov/show/NCT01131676
- MARLINA - T2DM: efficacy, safety & modification of albuminuria in type 2 diabetes subjects with renal disease with Linagliptin. Available from: http://clinicaltrials.gov/ct2/show/NCT01792518?term=MARLINA&rank=1
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013;10(4):289-301
- Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124(2):499-508
- Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124(2):509-14
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122(1):4-12
- Edgerton DS, Johnson KM, Cherrington AD. Current strategies for the inhibition of hepatic glucose production in type 2 diabetes. Front Biosci (Landmark Ed) 2009;14:1169-81
- Muscelli E, Casolaro A, Gastaldelli A, et al. Mechanisms for the antihyperglycemic effect of sitagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab 2012;97(8):2818-26
- Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab 2007;92(4):1249-55
- Ahren B, Landin-Olsson M, Jansson PA, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004;89(5):2078-84
- Brand T, Macha S, Mattheus M, et al. Pharmacokinetics of empagliflozin, a sodium glucose cotransporter-2 (SGLT-2) inhibitor, coadministered with sitagliptin in healthy volunteers. Adv Ther 2012;29(10):889-99
- Friedrich C, Metzmann K, Rose P, et al. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther 2013;35(1):A33-42
- Rel. BA of Empagliflozin (BI 10773)/Linagliptin FDC Tbl, Comparison With Mono-components, With a Second FDC Tablet and Influence of Food. Available from: http://clinicaltrials.gov/show/NCT01189201
- Kasichayanula S, Liu X, Shyu WC, et al. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Diabetes Obes Metab 2011;13(1):47-54
- Drug interaction study of saxagliptin in combination with dapagliflozin in healthy participants. Available from: http://clinicaltrials.gov/ct2/show/results/NCT01662999?sect=Xmledba970156
- Chen L, Klein T, Leung PS. Effects of combining linagliptin treatment with BI-38335, a novel SGLT2 inhibitor, on pancreatic islet function and inflammation in db/db mice. Curr Mol Med 2012;12(8):995-1004
- Kern M, Kloting N, Glempler R, et al. A promising Combination for Future Treatment of Type 2 Diabetes: Coadministration of Empagliflozin (SGLT2 Inibitor) with Linagliptin (DPP-4 Inhibitor). Diabetes 2013;62(Suppl 1):A283
- Davidson JA. The placement of DPP-4 inhibitors in clinical practice recommendations for the treatment of type 2 diabetes. Endocr Pract 2013;19(6):1050-61
- Park H, Park C, Kim Y, et al. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother 2012;46(11):1453-69
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159(4):262-74
- Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)--a randomized placebo-controlled trial. Am Heart J 2013;166(2):217-23; e211
- Wysham CH, Woo VC, Mathieu C, et al. Canagliflozin (CANA) added on to DPP-4 inhibitors or GLP-1 agonists with or without other antihyperglycemic agents in Type 2 Diabetes Mellitus. Diabetes 2013;62(Suppl 1):A279
- Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care 2014;37(3):740-50
- Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in poorly controlled type 2 diabetes on metformin: randomized, double-blind trial of saxagliptin+dapagliflozin vs. saxagliptin and dapagliflozin alone. Diabetes 2014;63(Suppl 1A):LB32
- Lewin A, DeFronzo RA, Sanjay P, et al. Fixed dose combination of empagliflozin/linagliptin for 24 weeks in drug naïve patients with type 2 diabetes mellitus (T2DM). Diabetes 2014;63(Suppl 1A):LB33
- DeFronzo RA, Lewin A, Sanjay P, et al. Fixed dose combination of empagliflozin/linagliptin for 24 weeks as add-on to metformin in subjects with type 2 diabetes mellitus (T2DM). Diabetes 2014;63(Suppl 1A):LB33
- Lewin A, Defronzo R, Patel S, et al. Fixed dose combinations of empagliflozin and linagliptin for 52 weeks in drug naive patients with type 2 diabetes. Diabetologia 2014;57(Suppl 1):S346[851]
- Patel S, DeFronzo R, Lewin A. Fixed dose combinations of empagliflozin/linagliptin for 24 weeks as add-on to metformin in subjects with type 2 diabetes. Diabetologia 2014;57(Suppl 1):S7
- Goossen K, Graber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab 2012;14(12):1061-72
- Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract 2014;103(3):373-81
- Leiter LA, Cefalu WT, de Bruin TW, et al. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc 2014;62(7):1252-62
- Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382(9896):941-50
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14):1317-26
- Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR - TIMI 53 randomized trial. Circulation 2014;130(18):1579-88
- Bell DS. Combine and conquer: advantages and disadvantages of fixed-dose combination therapy. Diabetes Obes Metab 2013;15(4):291-300
- American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 2014;37(Suppl 1):S14-80
- Garcia-Perez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013;4(2):175-94
- Vittorino Gaddi A, Benedetto D, Capello F, et al. Oral antidiabetic therapy in a large Italian sample: drug supply and compliance for different therapeutic regimens. Public Health 2014;128(1):70-6
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013;19(2):327-36
- Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol 2014;13:102
- Cefalu WT, Buse JB, Del Prato S, et al. Beyond metformin: safety considerations in the decision-making process for selecting a second medication for type 2 diabetes management: reflections from a diabetes care editors’ expert forum. Diabetes Care 2014;37(9):2647-59
- Colombo GL, Rossi E, De Rosa M, et al. Antidiabetic therapy in real practice: indicators for adherence and treatment cost. Patient Prefer Adherence 2012;6:653-61
- Cheong C, Barner JC, Lawson KA, et al. Patient adherence and reimbursement amount for antidiabetic fixed-dose combination products compared with dual therapy among Texas Medicaid recipients. Clin Ther 2008;30(10):1893-907
- Wertheimer AI. The economics of polypharmacology: fixed dose combinations and drug cocktails. Curr Med Chem 2013;20(13):1635-8
- Boehringer Ingelheim. Tradjenta (linagliptin) prescribing information. Available from: www.tradjenta.com/
- Boehringer Ingelheim. Trajenta (linagliptin) summary of product characteristics. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002110/WC500115745.pdf
- Bristol-Myers Squibb, AstraZeneca. Onglyza (saxagliptin) prescribing information. Available from: www.onglyza-hcp.com/hcp/hcp.aspx
- Merck & Co. Januvia (sitagliptin) prescribing information. Available from: www.januvia.com/sitagliptin/januvia/consumer/prescribing-information.jsp
- Boehringer Ingelheim. Jardiance® (empagliflozin) prescribing information. Available from: www.jardiance.com/
- Bristol-Myers Squibb, AstraZeneca. Farxiga (dapagliflozin) prescribing information. Available from: www.farxiga.com/
- Janssen Pharmaceuticals. Invokana (canagliflozin) prescribing information. Available from: www.invokanahcp.com/dosing-and-prescribing-information
- Safety and efficacy of the combination of empagliflozin and linagliptin compared to linagliptin alone over 24 weeks in patients with type 2 diabetes. Available from: https://clinicaltrials.gov/ct2/show/NCT01734785
- Linagliptin as add on therapy to empagliflozin 10 mg or 25 mg with background metformin in patient with type 2 diabetes. Available from: https://clinicaltrials.gov/ct2/show/NCT01778049
- Safety and efficacy of dapagliflozin in triple therapy to treat subjects with type 2 diabetes. Available from: https://clinicaltrials.gov/ct2/show/NCT01646320
- Safety and efficacy of saxagliptin in triple therapy to treat subjects with type 2 diabetes. Available from: https://clinicaltrials.gov/ct2/show/NCT01619059
- A study to evaluate the efficacy and safety of the addition of canagliflozin in participants with type 2 diabetes mellitus with inadequate glycemic control on metformin and sitagliptin. Available from: https://clinicaltrials.gov/ct2/show/NCT02025907
- Drucker DJ. The Biology of incretin hormones. Cell Metab 2006;3(3):153-65