Abstract
Immunoglobulin (IgG) replacement therapy has been the cornerstone of treatment for primary immunodeficiency disease for nearly 60 years. During this time, research has continually refined the target IgG trough level and IgG replacement dosages to allow patients with primary immunodeficiency disease to achieve effective protection from infection. Manufacturers have also improved IgG formulations to allow patients to receive clinically beneficial dosages of IgG replacement with improved safety and tolerability. This review will introduce Hizentra®, a highly concentrated (20%) IgG solution for subcutaneous (sc.) infusion, discuss its manufacturing process and pharmacokinetic profile and review its tolerability and efficacy data as evaluated in clinical trials. New highly concentrated sc. IgG products may improve patient quality of life and adherence to therapy because of the flexible dosing options, fewer infusion sites and less infusion time, compared with less concentrated sc. IgG products, resulting in favorable patient outcomes consistent with higher steady-state IgG levels.
Background
Over the past 35 years, there has been a substantial evolution in the treatment of primary immunodeficiency disease (PIDD) with IgG replacement therapy. This article will review the steps in this progression to the development of Hizentra®, a 20% subcutaneous (sc.) IgG (SCIG) that has been shown to be an effective, safe and well-tolerated long-term treatment for patients with PIDD.
History
Since the 1952 case report Citation[1] by Bruton first linked recurrent sinopulmonary infections with low serum Ig levels, more than 200 genetic mutations associated with PIDDs have been defined Citation[2]. Although PIDDs can be grouped into different categories based on genetic, immunologic and clinical features, deficient antibody production is the most common abnormality Citation[2–4]. The overall prevalence of PIDD is estimated to be between 1 in 1200 and 1 in 2000 individuals in the USA Citation[3,5]. Using the US estimated prevalence of 1 in 1200, there may be up to 6 million people with PIDD worldwide Citation[6].
Although PIDD may have once been considered a rare disease seen mainly in children, it has become increasingly evident that PIDD affects more people (and more adults) than previously believed. More than 50% of new PIDD cases are diagnosed in adults Citation[6]. The number of cases is expected to rise with the increasing awareness of the clinical signs and symptoms, the identification of new genetic phenotypes and the availability of new diagnostic algorithms Citation[2,6].
Some PIDDs, such as severe combined immunodeficiency, are associated with a life-threatening absence of functional T and B lymphocytes, rendering patients highly susceptible to both opportunistic and common infections Citation[7]. Typically diagnosed during the first year of life, state-mandated neonatal severe combined immunodeficiency screening is now increasingly common in the USA Citation[8]. Other PIDDs, such as X-linked agammaglobulinemia (XLA) or common variable immunodeficiency (CVID), are usually diagnosed after patients experience multiple recurrent, severe and/or unusual bacterial infections Citation[7]. The increased frequency and severity of infections is the typical clinical presentation of PIDD in patients, who often report symptoms for a number of years before a formal diagnosis is made Citation[3,9]. Antibody deficiencies are the most common manifestation of PIDD, occurring in about 50% of patients Citation[3]. While the use of prophylactic antibiotics and diligent personal hygiene practices are important elements in the care of PIDD patients, the cornerstone of therapy for PIDD associated with antibody deficiency is lifelong antibody replacement therapy Citation[10].
Immunoglobulin replacement therapy
By the mid-1950s, intramuscular Ig (IMIG) replacement was the most commonly used method of administration in patients Citation[11–13]. However, the therapeutic usefulness of this route was limited because the amount of Ig delivered (25–50 mg/kg/week or 100 mg/kg/month; 0.16–0.31 ml/kg/week or 0.7 ml/kg/month) Citation[14] could not achieve therapeutic IgG levels and patient adherence to the treatment was poor because of the pain associated with injection Citation[12,15,16]. Additionally, accidental injection of the IMIG product into a vein, resulting in an anaphylactoid reaction, was always a risk.
Manufacturers worked to develop purification procedures and Ig replacement formulations that could be delivered intravenously (iv.); an early challenge was that purified IgG in concentrated solutions aggregates and denatures. Denatured and/or aggregated IgG, when administered iv., is associated with severe adverse reactions, including fever, rigors, edema, hypotension and shock Citation[16]. Some improvements were made when small molecule excipients, carbohydrates and sodium chloride were employed to stabilize the solution. These additions and other modifications (such as sulfonation or enzymatic degradation of IgG) prevented aggregation and denaturation, enabling the introduction of intravenous Ig (IVIG) products by the early 1980s Citation[16]. IVIG replacement therapy has been the standard of care in the USA and most of Europe for the past 30 years for patients with PIDD Citation[17]. The dose of the first IVIG product was 100 mg/kg/month, which was based on the previously used intramuscular regimens. Subsequently, however, recommended and prescribed doses have increased steadily, as dose-related improvements in outcomes have been documented Citation[18–20]. The most recent American Academy of Allergy, Asthma and Immunology-associated guidelines recommend initially administering an IVIG dose of at least 400 mg/kg every 3–4 weeks Citation[10]. Subsequent dosing (up to a maximum of 600–800 mg/kg every 3–4 weeks Citation[3]) for each patient should be adjusted according to their individual clinical response. Based on evidence of a decreased risk of pulmonary infection Citation[18], 500 mg/dl has been considered the minimum trough level appropriate for treatment. There is evidence, however, that the risk of long-term lung damage may require maintenance trough levels >800 mg/dl Citation[21]. For patients with CVID, a minimum increase in the IgG level of 300 mg/dl above the pretreatment trough is used as a target by many clinicians Citation[21]. Bonagura et al. Citation[22] have introduced the concept of an individualized ‘biologic trough IgG level’ that is the minimum IgG level a particular patient requires to remain adequately protected from infection. Ultimately, IgG replacement dosing requirements for the individual patient should be determined by clinical outcomes.
Although IVIG therapy generally provides patients sufficient antibody replacement to effectively protect from infection, some aspects of the treatment regimen, such as systemic adverse events (AEs), the need for iv. access and low trough plasma IgG levels, may be problematic for some patients. Additionally, most patients will require a healthcare professional to initiate the infusion. A survey of patients registered in the Immune Deficiency Foundation database found that more than three-quarters of patients self-reported a systemic side effect resulting from IVIG at some time in the previous 12 months. These side effects included headaches (77%), muscle aches (45%) and fever/chills (40%) Citation[23]; muscle aches were the most frequently occurring reports (12.9 times on average) Citation[23]. Many patients also experience a ‘wear-off’ effect with IVIG in the days before their next scheduled infusion; 68% of respondents to an Immune Deficiency Foundation survey of Ig treatment experiences reported feeling a ‘wear-off’ of their IVIG treatment ‘usually’ or ‘sometimes’ Citation[24]. Patients tend to report more symptoms such as fatigue and increased susceptibility to infections in the days before their infusion is due, strongly suggesting a link between the clinically observed IVIG ‘wear-off’ effects and declining IgG levels. In addition, IVIG has been associated with an increased risk of rare but serious systemic reactions, including renal failure, thromboembolic events, aseptic meningitis, hemolysis and transfusion-related acute lung injury Citation[25].
Although continual improvements in product formulations have decreased the frequency and severity of AEs associated with IVIG, some patients still have difficulty tolerating IVIG treatment. In 1980, Berger et al. Citation[26] reported on slow sc. infusion of an IMIG product in three patients with CVID for whom other therapies were insufficiently efficacious or caused intolerable AEs. The report showed that sc. infusions could deliver higher Ig concentrations and improved infection control in patients without serious AEs Citation[26]. The slow infusion rates (1–2 ml/h), however, limited the widespread adoption of this route of administration.
In Scandinavia, there had been only limited use of IVIG, and in 1991 Gardulf et al. Citation[27] reported a faster method (17–20 ml/h) of SCIG replacement therapy using an IMIG product in 25 patients who were able to self-administer the weekly treatments at home after instruction. After 6 months of SCIG treatment, most patients showed improvement in trough IgG concentrations from those levels obtained with their previous IMIG regimen. Hospital admission rates also decreased. Patients experienced few systemic AEs and localized AEs were generally mild and resolved within a day Citation[27].
Over the next 15 years, additional reports were published describing the use of sc. administered antibody replacement, detailing its advantages over the iv. route, including better tolerability and elimination of the need for venous access Citation[28]. In parts of Europe, sc. rather than iv. administration was adopted early on and was frequently used by patients in that region Citation[29]. IgG products licensed for IM or iv. administration were commonly used because no IgG product was specifically licensed for sc. use. In 2006, a 16% human IgG product (Vivaglobin®; CSL Behring, Marburg, Germany) specifically formulated for sc. infusion was introduced in the USA.
Overview of the market
In the USA, the market for Ig continues to expand with a total estimated volume of 48,800 kg in 2012, which represented a 7.4% increase from 42,300 kg in 2010 Citation[30]. At the same time, SCIG use grew more rapidly (18%) and, in 2012, it comprised approximately 9.7% of the total Ig market (>4700 kg). Because SCIG products are only currently approved for PIDD and secondary immunodeficiencies, over 85% of SCIG is used for PIDD indications Citation[30].
Currently, 12 Ig replacement products are approved for the treatment of PIDD in the USA; of these, 9 products are licensed for iv. administration only Citation[31]. Two 10% IgG products, which were initially marketed as iv. formulations, were subsequently approved for sc. administration as well. Hizentra is the only Ig specifically approved for sc. administration, either weekly or biweekly (every 2 weeks). More recently, its use in alternative dosing regimens, such as rapid sc. push administration that allows for shorter, more frequent infusions (5–20 min, every 2–4 days), has been reported Citation[32].
Other products currently in late-stage development include sc. administration of recombinant human hyaluronidase followed by an IgG infusion, which allows for Ig administration using a single site every 3–4 weeks Citation[33]. Although recombinant human hyaluronidase is generally well tolerated with occasional use, a full understanding of the potential for adverse effects with chronic administration is unknown Citation[34]. Human hyaluronidase followed by an IgG infusion has been approved in Europe for use in adults, but has not yet been approved by regulatory authorities in the USA.
Ultimately, individual patient preference will guide the method of Ig administration and choice of Ig formulation. Some patients with PIDD may continue to choose IVIG because of needle phobia, a desire to minimize needle sticks and an unwillingness to self-administer Ig or to perform more frequent treatment. Other patients may prefer SCIG to have more control over their treatment by customizing the dosing interval and method of administration that provides the optimal clinical benefit with minimal disruption to their work and family lives.
Hizentra
As part of a program to improve the properties of the sc. infusion, clinical trials of a 20% formulation of proline-stabilized IgG began in 2006, and in March 2010 Hizentra [Immune Globulin Subcutaneous (Human)] was approved in the USA (it is also available in other areas including the EU, Australia, Canada and Japan) for antibody replacement in the treatment of PIDD in adults and pediatric patients 2 years of age and older. It is the only commercially available product to have a highly concentrated 20% IgG solution. It was initially approved in the USA and EU for recommended weekly sc. administration, but the US and EU approval was extended to include the option of biweekly (every 2 weeks) dosing in 2013.
Hizentra is ≥98% IgG, with a pH range of 4.6–5.2 . Its formulation also includes approximately 250 mmol/l (range, 210–290 mmol/l) of the non-essential amino acid l-proline as a stabilizer to reduce denaturation, dimerization and aggregation. There is also a very small amount of polysorbate 80 (range, 8–30 mg/l). Hizentra is stable at room temperature for 30 months. The IgA concentration is ≤50 μg/ml. Hizentra contains only trace amounts of sodium chloride and does not contain any carbohydrate stabilizers (e.g., sucrose, maltose) or preservatives.
Table 1. Composition of Hizentra®.
One of the reasons that l-proline was selected for use in the formulation of Hizentra is to decrease the viscosity of the finished product, allowing for efficient delivery. The mean viscosity of 20% Hizentra has been shown to be comparable to a 16% SCIG product in glycine (14.7 vs 14.4 mPa•s, respectively) Citation[35]. Physiologically, l-proline is necessary for collagen synthesis and neurologic function Citation[36]. Normal human serum proline levels, resulting from dietary protein intake and endogenous biosynthesis, vary by age and generally range from 58 to 336 μmol/l (in those aged ≥3 years) Citation[37]. Pharmacokinetic (PK) data from clinical trials of Hizentra show that although there is a transient increase in proline levels following administration, it is similar to the increase seen after the consumption of a protein-containing meal Citation[38]. Additionally, proline is rapidly metabolized and cleared, with no accumulation observed. No AEs related to the proline contained in Hizentra or its related IVIG formulation, Privigen® (10%; CSL Behring LLC, Kankakee, IL, USA), have been identified Citation[38]. IgG products that contain proline are contraindicated in patients who have extremely rare genetic conditions that result in reduced proline metabolism (hyperprolinemia, type I or II) Citation[39,40]. The safety of proline-containing IgG products has not been established specifically in patients who have chromosome 2q11.2 deletion syndrome (DiGeorge syndrome); however, as most of these patients do not have hyperprolinemia, they should not be specifically at risk Citation[38].
Manufacture
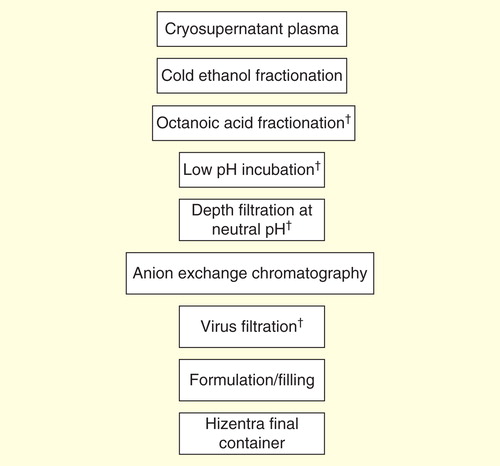
Hizentra is a ready-to-use, sterile 20% (0.2 g/ml) protein liquid preparation of polyvalent human IgG for sc. administration. It is manufactured from pooled source and recovered human plasma obtained following Quality Standards of Excellence, Assurance and Leadership criteria of the Plasma Protein Therapeutics Association. The IgG is purified using a combination of cold ethanol fractionation, octanoic acid fractionation and anion exchange chromatography Citation[40].
Plasma used in the manufacture of Hizentra is tested using licensed/approved serological and nucleic acid testing assays for hepatitis B virus, hepatitis B surface antigen, hepatitis C virus, HIV-1 and antibodies to HIV-1/2. All plasma units have been found to be non-reactive (negative) in these tests. In addition, the plasma is tested for parvovirus B19 (B19V) DNA by nucleic acid testing. Only plasma that passes virus screening is used for production, and the limit for B19V in the fractionation pool is set to be below 104 IU of B19V DNA per ml Citation[40]. Additionally, if required by local regulatory authorities, plasma pools also undergo specific viral testing.
In addition to selecting donors, testing donations and plasma pools for fractionation, the manufacturing process for Hizentra includes four manufacturing steps with virus reduction capacity using three different mechanisms (virus inactivation, virus removal by filtration and partitioning) to reduce the risk of virus transmission. These steps have been previously described for the related 10% proline-stabilized IVIG product (Privigen) Citation[41]. Two of these steps are dedicated virus clearance steps: incubation at pH 4 to inactivate enveloped viruses and virus filtration (also called nanofiltration) to remove both enveloped and non-enveloped viruses as small as approximately 20 nm. In addition, octanoic acid fractionation and a depth filtration step further contribute to the virus reduction capacity during the purification process Citation[41].
These steps have been independently validated in a series of in vitro experiments for their capacity to effectively and robustly inactivate and/or remove a diverse panel of both enveloped and non-enveloped viruses with a range of physicochemical characteristics. When only independently mechanistic steps are considered, the manufacturing process was shown to have a minimal overall reduction capacity of >11 log10 of enveloped and >7log10 of non-enveloped model viruses. These results showed that the manufacturing process steps provide a high margin of safety Citation[41]. The robustness of these steps is further illustrated by results that show that they inactivate/remove West-Nile virus and Chikungunya, two agents considered ‘emerging infections’ in the USA.
Infectious transmissible spongiform encephalopathy (TSE) agents (prions) are highly resistant to traditional virus inactivation methods (i.e., pasteurization and solvent/detergent treatment). Transmissible spongiform encephalopathy agents, however, can be efficiently cleared during the manufacturing process by virus filtration (also called nanofiltration) Citation[42], and by some manufacturing process steps that separate plasma protein impurities from IgG. For Hizentra, several production steps, including octanoic acid fractionation (≥6.4 log10), depth filtration (2.6 log10) and virus filtration (≥5.8 log10), have been shown to decrease infectivity of an experimental agent of transmissible spongiform encephalopathy, which provides reasonable assurance that if low levels of infective agents associated with variant or classical forms of Creutzfeldt–Jakob disease were present, they would be removed Citation[40].
Pharmacokinetics
Intravenous IgG is typically administered to patients every 3–4 weeks, resulting in fluctuations in serum IgG levels, as the levels immediately spike after the infusion bolus and then gradually decline as the IgG first redistributes from the vascular system into the interstitial fluid and is then catabolized at a first-order kinetic rate Citation[17,43]. The half-life of IVIG is approximately 28–32 days Citation[43]. High IgG peaks are thought to be responsible for AEs such as headache, myalgia and malaise, as well as increasing the risk for serious AEs. IgG levels are at their lowest (trough) in the days before the next infusion, leading to the ‘wear-off’ effects (such as fatigue) and an increased incidence of infections, as described previously Citation[44–47].
Because of the mode of administration and a more frequent dosing interval (every 1–2 weeks), SCIG administration results in relatively constant serum IgG concentrations that are more consistent with the levels seen in normal, healthy individuals Citation[43]. SCIG is introduced into the sc. tissue, from which it is slowly absorbed into the lymphatic system and then equilibrated with the intravascular space.
The bioavailability of SCIG, as measured by the area under the concentration–time curve (AUC), is lower than that of IVIG Citation[48,49], although the basis for this difference has not been fully established. Recent studies have shown that all SCIG products currently available in the USA have very similar bio-availabilities, ranging from 65.0 to 69.0% of that of IVIG Citation[49]. The rate of catabolism of IgG is similar whether it is of exogenous or endogenous origin Citation[50], and, because of serum concentration-dependent metabolism, the initially higher plasma IgG concentrations following IVIG infusion also may lead to more rapid elimination of IgG Citation[51,52].
The PK profile of Hizentra weekly dosing in patients with PIDD was evaluated as a part of the pivotal US Phase III clinical study (see section ‘Clinical efficacy studies’) Citation[17] to satisfy the US FDA requirement for the demonstration of bioequivalence to IVIG (similar AUC of serum IgG vs time). As expected, the study found some variability in Hizentra dosing requirements, with individual patients needing anywhere from 1.26- to 1.87-times their previous IVIG dose (overall mean dose adjustment = 1.53). After individual dosing adjustments were made, the standardized weekly steady-state AUCs with Hizentra were determined and compared with steady-state AUCs obtained while on IVIG treatment, resulting in a geometric mean ratio of 1.002 (range, 0.77–1.20). Comparing the dose adjustments and AUC ratios for 10 and 16% SCIG products studied in the USA to meet this FDA requirement, the results for all preparations are remarkably similar, suggesting that the apparent difference in bioavailability of SCIG versus IVIG is an intrinsic property of IgG itself, and is unrelated to the purification procedures, stabilizers or concentration of the product Citation[49]. The validity of using AUC rather than steady-state serum IgG levels (or trough levels for IVIG) as a major criterion for dosing has not been established, however, and it has been suggested that each patient’s regimen be individualized to keep that patient free from infection Citation[19,22].
The plasma concentration profile of biweekly dosing was characterized using PK modeling and simulation of plasma IgG concentration data from four separate Phase III clinical studies of Hizentra and the related 10% IVIG formulation, Privigen, to predict plasma concentrations and concentration–time profiles. The data included IgG levels measured in 3471 plasma samples from 151 patients who had participated in the Hizentra and Privigen pivotal trials Citation[53]. These studies allowed assessment of the effects of different dosing regimens . The model predicted near steady-state IgG levels for biweekly dosing of Hizentra, which compare favorably with the levels achieved with weekly administration, and simulated switching to Hizentra biweekly from either Hizentra weekly or IVIG monthly Citation[53]. The ratios of PK parameters such as AUCs, peak IgG concentration and trough IgG concentrations 7 and 14 days post-dosing ranged from 0.925 to 1.06 for weekly versus biweekly Hizentra administration. These ranges of the ratios, along with their associated 5th and 95th percentiles, were well within the accepted range of 0.8–1.25 for establishment of bioequivalence Citation[53,54]. The results of this study served as the basis for the FDA’s approval of the biweekly dosing option.
Figure 2. Pharmacokinetic simulation of steady-state concentration profiles for IgG following Hizentra weekly or biweekly dosing or Privigen monthly dosing. Total monthly sc. doses are 1.53-fold higher than iv. doses.
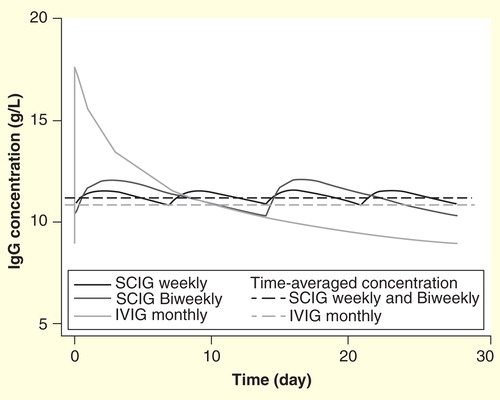
The effects on exposure when switching from monthly IVIG to biweekly Hizentra were also modeled. When a dose adjustment coefficient (DAC) of 1.53 for the switch from IVIG to Hizentra was used, biweekly Hizentra and monthly IVIG resulted in similar overall plasma exposure over 4 weeks, with a median AUC ratio of 1.03; however, biweekly Hizentra administration resulted in a 15% higher trough IgG concentration and a 25% lower peak IgG concentration Citation[53]. Recently, the results of another PK modeling and simulation study using a DAC of 1.37 for the switch from IVIG to Hizentra found an AUC ratio of 0.97 (95% CI: 0.90–1.04) with a 15% higher trough IgG concentration Citation[55].
Hizentra is approved for weekly or biweekly administration, increasing the flexibility for adapting long-term therapy to patients’ needs and preferences. Whether dosing is weekly or biweekly, the PK profile has interpatient variability; thus, monitoring of the clinical response is essential and periodic measurements of IgG levels may be a helpful adjunct.
Clinical efficacy studies
The efficacy and tolerability of Hizentra in patients with PIDD has been studied in three prospective, open-label, multicenter, single-arm Phase III clinical studies in the USA, the EU and Japan Citation[29,56,57]. The long-term efficacy and tolerability of Hizentra was evaluated in an extension study (up to 148 weeks) with patients who originally took part in the US and EU pivotal trials and in a follow-up study (up to 48 weeks) of patients who participated in the Japanese clinical trial Citation[58,59]. In all three pivotal studies, patients who were receiving regular IgG replacement therapy with a licensed IgG product by the iv. or sc. route and had one or more documented trough IgG levels of ≥500 mg/dl were switched to Hizentra. The primary end points of the studies were either rates of acute serious bacterial infections (aSBIs; US study) or comparison of serum IgG trough levels (EU and Japanese studies) during treatment with Hizentra as compared with previous IgG therapy. According to FDA criteria, aSBIs were defined as bacteremia/septicemia, bacterial meningitis, osteomyelitis/septic arthritis, bacterial pneumonia and visceral abscess Citation[60]. All studies also evaluated secondary end points, including the number of any infections, days missed from work/school/kindergarten/day care or unable to perform normal daily activities due to infections, days of hospitalization for infection and antibiotic use (either for prophylaxis or treatment) using data from patient diaries.
The safety assessments included the number of AEs and local tolerability, which were evaluated both by investigators and by individual patients and recorded in diary entries (although somewhat differently in each study; see section ‘Safety & tolerability’). Premedications to reduce the side effects of SCIG were not permitted in the EU and the US studies Citation[29,56].
US pivotal clinical study & extension study
In the US clinical study, patients with CVID or XLA who previously had received IVIG regularly for at least 3 months were eligible to participate Citation[56]. Patients received weekly Hizentra according to FDA requirements Citation[17,56]. The primary end point was the rate of aSBIs. A total of 38 patients (mean age, 36.3 years), including 6 children and 32 adults (age range, 5–72 years), entered the efficacy phase and 28 patients completed the study. The study met its primary end point; no patients experienced an aSBI during the trial, resulting in an annualized aSBI rate of 0 (upper 99% confidence limit: 0.132; ). During the efficacy period, the mean (SD) IgG trough concentration was 1253 (3.21) mg/dl, which is comparable to the IgG levels observed in the non-PIDD population. A total of 31 patients (81.6%) experienced 96 non-SBI infections, which corresponds to 2.76 infections/patient/year . The annualized rate of days missed from work or school was 2.06 days/patient/year based on a total of 71 days missed by 12 patients (31.6%). One patient (2.6%) was hospitalized for 7 days because of an infection, which corresponds to an annualized rate of 0.2 days/patient/year. The annual rate for antibiotic use was 48.5 days/patient/year calculated from 27 patients (71.1%) receiving antibiotics for a total of 1688 days.
Table 2. Published studies of Hizentra in patients with primary immunodeficiency disease: mean dose, IgG levels and acute serious bacterial infections.
Table 3. Secondary efficacy end points in Hizentra studies.
A total of 21 of the 28 patients from the US pivotal study chose to continue in the extension study, with 16 patients (76.2%) ultimately completing the study Citation[58]. Patients continued to receive the Hizentra dose that they received during the pivotal study, although dose adjustments were allowed if medically necessary or if the patient’s body weight changed. During the extension study, the median treatment period was 87 weeks (range, 11–104 weeks). A total of two aSBIs, both cases of bacterial pneumonia, were reported in two patients (9.5%), resulting in an annualized rate of 0.06 infections/patient/year (upper 99% confidence limit: 0.26; ).
The annualized rate of non-aSBI infection was 2.38 infections/patient/year (95% CI: 1.88–2.97), the rate of missed work/school days because of infection was calculated to be 4.28 days/patient/year and the annualized rate of antibiotic use was 72.13 days/patient/year . During the extension study, the IgG levels of the patients were measured every 24 weeks for 96 weeks (prior to the next scheduled infusion), and the mean (SD) of the individual median IgG values was 1198 (365) mg/dl Citation[58].
EU pivotal clinical study & extension study
In the EU pivotal clinical study, patients with CVID, XLA or autosomal recessive agammaglobulinemia who received IVIG (at 3- to 4-week intervals) or SCIG (weekly) for at least 6 months and had ≥3 serum IgG trough levels of ≥500 mg/dl were eligible for treatment with Hizentra during a 12-week wash-in/wash-out period followed by a 28-week efficacy period Citation[29]. In this study, there were two key differences from the US clinical study: no DAC was used (i.e., initial doses were equivalent to the calculated prior weekly IVIG or SCIG doses) and the primary end point was the comparison of IgG levels during treatment with 20% SCIG compared with the previous therapy regimen Citation[29]. Of the 51 patients enrolled in the study, 46 entered the efficacy phase and 43 completed the study. Mean (SD) IgG levels in patients treated with Hizentra were comparable to the IgG level on their previous Ig replacement therapy (810 [134] mg/dl vs 749 [157] mg/dl, respectively; ). There were no reports of aSBIs during the efficacy period. The 124 non-SBI infections experienced by 36 patients (78.3%) yielded an infection rate of 5.18 infections/patient/year . A total of 20 patients (43.5%) missed work/school for 198 days, resulting in an annualized rate of 8.00 days/patient/year, and 4 patients (8.7%) had infection-related hospitalizations for 86 days, resulting in an annual rate of 5.61 days/patient/year. Antibiotics were used by 32 patients (69.6%) for 1743 days, which corresponds to an annual rate of 72.75 days/patient/year Citation[29].
In the EU extension study, 40 of the 43 patients from the initial trial period were re-enrolled and 36 patients (90%) completed the study Citation[58]. The median treatment period was 148 weeks (range, 9–166 weeks).
A total of 5 aSBIs (all acute bacterial pneumonia) were reported by five patients (12.5%), corresponding to a rate of 0.05 infections/subject/year (upper 99% confidence limit: 0.13; ). The annualized rate of all infections was 3.33 infections/patient/year (95% CI: 2.99–3.70), the rate of missed work/school due to infections was calculated to be 6.77 days/patient/year, the annualized infection-related hospitalization rate was found to be 1.06 days/patient/year and the annualized rate of antibiotic use was 72.13 days/patient/year . During the extension study, the IgG levels of the patients were measured every 6 weeks for 42 months, and the mean (SD) of the individual median IgG values was 797 (117) mg/dl Citation[58]. The lower mean IgG levels seen in this study compared with the US trial are probably a function of the DAC (1.53-times the iv. dose) used in the US study as opposed to the 1:1 (iv.:Hizentra) dosing in the EU study. The significant difference in non-aSBI infections between the US and EU trials is most likely due to the higher mean IgG levels found in the US study during the treatment period (1198 vs 810 mg/dl), although other factors such as regional differences in the assessment of infections cannot be excluded.
Japanese clinical study
Recently, the efficacy and tolerability of Hizentra was reported in 24 Japanese adult (n = 13, 54.2%) and pediatric (n = 11, 45.9%) patients with CVID, XLA, autosomal recessive agammaglobulinemia or hyper IgM syndrome (mean age, 20.5 years) after a switch from a period of mandatory IVIG therapy (three infusions delivered every 3–4 weeks) Citation[57]. Similar to the European study, the initial weekly Hizentra dose was the same as the one-fourth of the previous monthly IVIG dose, and the primary end point compared serum IgG levels achieved on Hizentra during the 12-week efficacy period with trough levels while on IVIG using the geometric mean ratio. This study found that during Hizentra treatment, mean (SD) IgG levels were 715 (151) mg/dl, a 9.5% increase over the mean trough serum IgG concentrations of 653 (140) mg/dl during IVIG treatment. The calculated geometric mean ratio was close to 1 (mean, 1.09; 90% CI: 1.06–1.13; for the per protocol population), indicating that comparable IgG levels were achieved with the two treatments; thus, the primary efficacy end point of the study was achieved . No aSBIs were reported Citation[57].
In this study, 11 patients (52.4%) had 15 non-SBI infections, with an annualized rate of 2.98 infections/patient/year . Seven patients (33.3%) missed a total of 19 days from work/school because of infections, resulting in an annual of rate of 3.48 days/patient/year. One patient was hospitalized for infection for 3 days, resulting in an annualized hospitalization rate of 0.55 days/patient/year. Antibiotics, either for prophylaxis or treatment, were used by 16 patients (76.2%) for a total of 844 days, corresponding to an annualized rate of 167.42 days of antibiotics use/patient/year Citation[57]. A recent preliminary report from the Japanese follow-up study confirmed the continued efficacy of Hizentra up to 48 weeks Citation[59].
Safety & tolerability
During all the efficacy studies discussed, information on AEs was collected by investigators and recorded in patient diaries Citation[29,56–58]. A summary of the rates of AEs for each study and types of AEs are presented in . The vast majority (≥98.7%) of the AEs across all studies were of mild or moderate intensity. No serious AEs were causally related to Hizentra treatment, and discontinuation rates due to treatment-related AEs were low in the US (2/49 patients; 4.15%) and EU (3/51 patients; 5.9%) clinical trials. No patient discontinued Hizentra because of a treatment-related AE during the Japanese clinical study or during the US or EU extension studies. The reported rates of local reactions varied greatly among the studies, ranging from 15% (6/40) of patients in the EU extension study to 100% of patients (n = 49) in the US pivotal trial. The variability observed in the local reaction rates may be attributed to the notable differences in how data on local infusion site reactions were collected for each trial. Because the US pivotal study was the first Phase III trial of Hizentra, rigorous assessments of local site reactions were mandated with required evaluations by both investigators and patients within 15–45 min and 24 h post-infusion, respectively. Because the US study established that local site reactions were predominantly mild and quickly resolved without treatment, less stringent evaluations were used in the EU and Japanese primary studies, with patients required to note overall assessments of injection sites only at 24–72 h post-infusion. The EU extension study did not specify a time for evaluation of local reactions after each infusion; however, local reaction data were collected from diary records during study visits. Local reactions are commonly reported with sc. administration of IgG and are typically transient, with most reactions resolving within 24–72 h Citation[27,56,61]. The injection-site reactions reported by patients in these studies are not unexpected, as the infusion of fluid into the skin does have a physiologic effect. It is not possible to meaningfully assess differences in the rates of local site reactions among the studies because of the differences in the definitions of local site reactions, the timing of the assessment and the assessment scales used in the individual study protocols. For the same reasons, comparisons to other products are also not possible. Infusion-site reactions are not related to the IgG dose infused and, importantly, rates of infusion-site reactions decreased over time Citation[29,56,57].
Table 4. Summary of adverse events and adverse event rates in Hizentra efficacy studies.
Table 5. Summary of related adverse events, excluding local reactions, in >5% of patients in Hizentra primary efficacy studies.
Patient satisfaction
As part of the European clinical trial, the Treatment Satisfaction Questionnaire for Medication was used to assess patients’ satisfaction with Hizentra, with patients rating the effectiveness, side effects, convenience and overall satisfaction with treatment Citation[29]. At the end of the study, patients rated Hizentra therapy as more convenient than their previous IVIG treatment regimen with a median Treatment Satisfaction Questionnaire for Medication score of 83.3 compared with a baseline score of 55.6 (median change, 33.3; 95% CI: 22.2–38.9) Citation[29]. Data from participants in the US extension study indicate that patients continue to report high treatment satisfaction and quality-of-life scores Citation[62]. This finding is consistent with other surveys of patients with PIDD that found that the switch from IVIG therapy in the hospital/doctor’s office to home-based SCIG infusions was associated with increased quality of life and satisfaction measures Citation[63,64]. In these studies, patients reported that the flexibility of the self-infused SCIG treatment allowed for a greater sense of independence, more control over their treatment regimen and an improved perception of their overall health status. The authors of these studies suggest that the relatively stable plasma IgG concentrations between infusion intervals (thus avoiding the fluctuations in these levels associated with IVIG) and the low incidence of infections and AEs associated with SCIG therapy contribute to the improved patient satisfaction outcomes reported Citation[63,64].
Pediatric populations
A number of the attributes of SCIG, such as absence of the need for venous access, the smaller needles required for sc. infusions and the convenience of home administration by a parent or caregiver, may allow SCIG to be a more preferred route of administration in pediatric populations. An analysis of efficacy and safety data from the 18 children (2–11 years of age) and 5 adolescents (12–15 years of age) who participated in the EU pivotal study found Hizentra had similar efficacy in children and adolescents as adults Citation[65]. The percentage of AEs was similar across all patient groups, but children and adolescents experienced lower rates of related AEs and related, temporally associated AEs than adults. Data from this study also suggest that children and adolescents had better tolerability to local reactions (0.040 and 0.035 events per infusion, respectively) compared with adults (0.080 events per infusion). Discontinuation rates in children due to any AE (2/18, 11.1%) or a related AE (0/18) were lower than the rates found for the adult patient population (4/28 [14.3%] and 3/28 [10.7%], respectively). No adolescents discontinued the study. Additional case reports and retrospective studies have also been published that have demonstrated the successful use of SCIG in pediatric patients, including infants and toddlers Citation[66–70].
Conclusion
In summary, the results of all three pivotal clinical efficacy studies and the two extension studies demonstrate that treatment with Hizentra is comparably efficacious to IVIG in the prevention of aSBIs and the maintenance of IgG levels. Hizentra was also well tolerated by patients. Although patients received a mean Hizentra dose at 153% of their previous IVIG dose in the US study, Hizentra doses were equivalent to IVIG or previous SCIG doses in patients who participated in the European and Japanese studies. In all three pivotal studies, weekly Hizentra treatment resulted in higher IgG trough levels compared with previous IgG treatment. Hizentra provided effective protection from most occurrences of aSBIs, with very low annualized rates of aSBIs occurring during the efficacy periods of all three pivotal clinical studies. A small number of aSBIs were reported during both the US and EU extension studies; however, this finding is not unexpected, as some incidence of breakthrough infections can be expected in the PIDD patient population. The annualized rates of aSBIs during Hizentra treatment are notable, because they are substantially less than the efficacy criterion set by the FDA of 1.0/person/year Citation[60].
A review of the data from the secondary study end points, rates of non-aSBI episodes, days missed from work or school due to infection and hospitalization for infections and antibiotic use, notes low overall annualized rates during treatment with Hizentra. There was, however, some variability in the secondary measures of efficacy among the studies. Haddad et al. compared the data from the US and UK Hizentra pivotal trials to investigate the effects of IgG dose on health outcomes Citation[71]. Significant differences in all the secondary health outcomes were found (p < 0.0001) that correlated with the differences in the mean weekly Hizentra doses (US study, 213 mg/kg vs EU study, 120 mg/kg; p < 0.0001) and in the resulting mean trough IgG levels (US study, 1254 mg/dl vs EU study, 810 mg/dl; p < 0.0001) Citation[71]. A post hoc analysis of the US and EU extension studies found that the rates of bronchopulmonary AEs were significantly higher in patients from the EU than from the US (ratio, 2.25; 95% CI: 1.21–4.18), which suggests that the higher mean IgG levels noted in patients from the USA versus the EU (1198 vs 797 mg/dl, respectively) appears to provide enhanced protection from infections Citation[58], but may also reflect the limitations of a comparison between two clinical trials.
Other studies have also observed a dose–response relationship with IgG replacement therapy. In a meta-analysis of 10 studies of IVIG therapy, it was found that there was an inverse relationship between the incidence of pneumonia and trough levels up to 1000 mg/dl Citation[20]. A meta-analysis of 10 prospective studies of SCIG therapy determined that higher IgG doses correlated with lower rates of all infections, with every 100 mg/dl increase in serum IgG level associated with a 0.38 events/patient/year decrease in the annualized rate of infections Citation[72]. In another analysis of seven studies of IgG replacement with SCIG, the incidence of non-SBI infections was inversely correlated with the mean steady-state serum IgG level Citation[73].
It should be noted that a recent analysis of data from 4580 patients found that the mean prescribed SCIG doses were less than IVIG doses (408.5 vs 568.3 mg/kg/month, respectively) suggesting that, despite the US prescribing information, use of SCIG without dose conversion (as has been widely used in Europe for many years) is practiced by some physicians in the USA Citation[74]. In clinical practice, the appropriate use of a DAC or 1:1 dosing in the switch to Hizentra may depend on the previous IVIG dose, previous IgG level and, ultimately, their individual protective IgG level (‘biologic trough’). Patients who previously received a high dose of IVIG or had a high IgG level may not require a DAC; however, those with an IVIG dose and level at the lower end of the suggested range may experience improved outcomes with the increased dose that results from using a DAC. Because IgG trough levels have been generally considered the biochemical surrogate test for the efficacy of IgG replacement products, appropriate monitoring of levels and dose adjustments may provide additional guidance for an individual patient Citation[75,76]. Each patient’s clinical response and ‘biologic trough IgG level’ including factors such as infection rate and quality of life, must be the primary consideration in establishing the appropriate dose of Hizentra. Bonagura et al. Citation[22] have suggested that the biologic trough IgG level may vary substantially between patients with PIDD and may differ over time in the individual patient, especially if there are other health status changes, such as becoming pregnant or developing kidney disease.
Expert commentary
Hizentra, a 20% IgG product for sc. administration, may offer multiple advantages to patients with PIDD in effective management of their disease. The high concentration of IgG allows the infusion of low volumes to achieve and maintain the higher steady-state IgG levels consistent with a decreased incidence of breakthrough infections. Additionally, the higher concentration of Hizentra usually allows fewer infusion sites and shorter infusion time than when using a lower concentration product, thereby increasing the flexibility of dosing. Although Hizentra and other SCIGs are typically delivered using programmable infusion pumps, a rapid push method using a syringe and a butterfly needle has been described Citation[32,69,77,78]. Instead of performing 1 weekly infusion, patients can choose to divide their dose into multiple short infusions (each of 5–20 min) per week, allowing patients another choice in customizing their treatment with regards to their individual needs. Individualization of IgG delivery may enhance patient adherence to the treatment regimen.
Rapid push administration was associated with lower Ig dosing but increased mean serum IgG levels to 1163.9 (278.8) mg/dl compared with IgG levels seen with 3 or 4 weekly IVIG treatments of 883.8 (317.8) mg/dl or levels of 1048.0 (298.5) mg/dl obtained using 1–3 weekly pumped infusions Citation[78]. In separate reports, including pediatric and obese patients, the relationship between higher mean IgG levels despite lower dosing with rapid push was also observed Citation[69,77]. Rapid push also increased the efficiency of SCIG administration by reducing the number of sites used and slightly increasing the volume infused per site, with adults and adolescents (ages 10–18 years) able to infuse an average of 15 ml or more per site Citation[69,77,78]. Administration of SCIG using rapid push was completed in 9 min or less in many patients compared with average pump infusion administration times between 45 min and 1 h, while achieving higher serum IgG levels than had been obtained with IVIG. Rapid push administration may be especially advantageous in infants and toddlers, as the required amounts of IgG can be delivered in a short time period without the added expense of an infusion pump and supplies. In these reports, adults also had a clear preference for rapid push, with the majority of those who chose rapid push continuing to use it or resuming its use after pump infusion administration Citation[69,77,78]. Expanding the use of rapid push administration of Hizentra represents another way that patients can individualize their PIDD therapy.
The product information change approved in the USA in 2013, allowing biweekly dosing, provides patients with another option to meet their treatment needs and may improve adherence to an IgG therapy regimen. Because Hizentra contains 20% (200 mg/ml) IgG, a total monthly dose of 1 g/kg is equal to sc. infusions of 1.25 ml/kg/week or 2.5 ml/kg/2 weeks, there is more flexibility in the treatment regimen. A recent retrospective analysis of 10 patients on a biweekly Hizentra regimen (median, 21.5 months) showed that it was effective in the prevention of aSBIs and the maintenance of therapeutic IgG levels Citation[79]. Another retrospective study comparing patients on weekly (n = 30) and biweekly (n = 22) Hizentra therapy found similar IgG levels and tolerability profiles in both patient groups during a median treatment duration of about 10 months Citation[80].
Patients with PIDD will benefit from individualized Ig therapy, which includes not only dosing based on their individual clinical response as reflected in their biological trough, but also a mode and schedule of delivery suitable to their needs and preferences, including IVIG every 2–4 weeks or Hizentra multiple times a week, once a week or biweekly (every 2 weeks).
Five-year view
The PIDD market is expected to continue to expand due to increased disease awareness and diagnosis. Analyses showing the association between higher trough IgG levels and improved clinical outcomes may lead to more individualization of dosing and targeting higher IgG trough levels. Additionally, it is expected that more patients will transition to SCIG because of the reduced incidence of serious and troublesome AEs, the elimination of the need for venous access and the increased flexibility associated with this infusion method. The clinical use of Hizentra may also be expanded to autoimmune neuropathies because its higher concentration may facilitate the higher doses generally indicated for these conditions. Preliminary studies have demonstrated the utility of SCIG in the treatment of chronic inflammatory demyelinating polyneuropathy Citation[81–85], multifocal motor neuropathy Citation[86,87], myasthenia gravis Citation[84,85] and dermatomyositis Citation[88,89].
Key issues
Long-term Hizentra administration is effective, safe and well-tolerated in patients with primary immunodeficiency disease of all ages.
Hizentra use may be associated with improvement in the clinical outcomes of patients with primary immunodeficiency disease resulting from adherence to treatment facilitated by the flexibility of the many dosing options available.
Hizentra’s highly concentrated IgG formulation (20%) should enable patients to receive their prescribed IgG therapy doses more efficiently with lower infusion volumes and fewer infusion sites required.
Hizentra is the only subcutaneous immunoglobulin indicated for weekly or biweekly administration.
Acknowledgements
Medical editorial support was provided by LM Havran and RH Sayce at Complete Publication Solutions, LLC; this support was funded by CSL Behring, LLC.
Financial & competing interests disclosure
Apart from the abovementioned writing assistance, RL Wasserman has served as a consultant for ADMA Biologics, Baxter, CSL Behring, Biotest, BPL, the Korean Green Cross, Kedrion and Therapure; has served as an investigator for ADMA Biologics, Baxter, CSL Behring, BPL and Kedrion and has served as a speaker for Baxter and CSL Behring. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Bruton OC. Agammaglobulinemia. Pediatrics 1952;9(6):722-8
- Bousfiha AA, Jeddane L, Ailal F, et al. A phenotypic approach for IUIS PID classification and diagnosis: guidelines for clinicians at the bedside. J Clin Immunol 2013;33(6):1078-87
- Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol 2005;94(5 Suppl 1):S1-63
- Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2014;5:162
- Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol 2007;27(5):497-502
- Bousfiha AA, Jeddane L, Ailal F, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immunol 2013;33(1):1-7
- Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol 2010;125(2 Suppl 2):S182-94
- Gaspar HB, Hammarstrom L, Mahlaoui N, et al. The case for mandatory newborn screening for severe combined immunodeficiency (SCID). J Clin Immunol 2014;34(4):393-7
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999;92(1):34-48
- Yong PL, Boyle J, Ballow M, et al. Use of intravenous immunoglobulin and adjunctive therapies in the treatment of primary immunodeficiencies: a working group report of and study by the Primary Immunodeficiency Committee of the American Academy of Allergy Asthma and Immunology. Clin Immunol 2010;135(2):255-63
- Janeway CA, Rosen FS. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med 1966;275(15):826-31
- Long AA, Denburg JA, Dent PB. Hypogammaglobulinemia: therapeutic rationale. CMAJ 1987;137(9):793-7
- Hypogammaglobulinaemia in the United Kingdom. Summary report of a Medical Research Council working-party. Lancet 1969;1(7587):163-8
- Stiehm ER. Immunologic disorders in infants and children. 4th edition. W.B. Saunders; Philadelphia, PA, USA: 1996
- Eibl MM. History of immunoglobulin replacement. Immunol Allergy Clin North Am 2008;28(4):737-64, viii
- Skoda-Smith S, Torgerson TR, Ochs HD. Subcutaneous immunoglobulin replacement therapy in the treatment of patients with primary immunodeficiency disease. Ther Clin Risk Manag 2010;6:1-10
- Wasserman RL, Melamed I, Nelson RP, et al. Pharmacokinetics of subcutaneous IgPro20 in patients with primary immunodeficiency. Clin Pharmacokinet 2011;50(6):405-14
- Roifman CM, Levison H, Gelfand EW. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet 1987;1(8541):1075-7
- Lucas M, Lee M, Lortan J, et al. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol 2010;125(6):1354-60. e4
- Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol 2010;137(1):21-30
- Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2006;117(4 Suppl):S525-53
- Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol 2008;122(1):210-12
- Immune Deficiency Foundation. Treatment experiences and preferences among patients with primary immunodeficiency diseases. National Survey of Patients (2008). Available from: http://primaryimmune.org/idf-survey-research-center/idf-surveys
- Immune Deficiency Foundation. Treatment experiences and preferences of patients with primary immune deficiency diseases: first national survey. 2003. Available from: http://primaryimmune.org/publications#idf_surveys
- Ballow M. Safety of IGIV therapy and infusion-related adverse events. Immunol Res 2007;38(1-3):122-32
- Berger M, Cupps TR, Fauci AS. Immunoglobulin replacement therapy by slow subcutaneous infusion. Ann Intern Med 1980;93(1):55-6
- Gardulf A, Hammarstrom L, Smith CI. Home treatment of hypogammaglobulinaemia with subcutaneous gammaglobulin by rapid infusion. Lancet 1991;338(8760):162-6
- Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol 2004;112(1):1-7
- Jolles S, Bernatowska E, de Gracia J, et al. Efficacy and safety of Hizentra in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol 2011;141(1):90-120
- US IVIG-SCIG forecast 2012-2017: for cost of the intravenous and subcutaneous immunoglobulin (IVIG/SCIG) market in the United States between 2012 and 2017. The Marketing Research Bureau, Inc; Orange, CT, USA: 2011
- US FDA. Immune globulins. 2014. Available from: www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm127589.htm
- Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol 2010;30(2):301-7
- Wasserman RL, Melamed I, Stein MR, et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol 2012;130(4):951-7. e11
- Baxter Healthcare Corporation. HyQvia: immune globulin infusion 10% (human) with recombinant human hyaluronidase. 2014. Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM407013.pdf
- Maeder W, Lieby P, Sebald A, et al. Local tolerance and stability up to 24 months of a new 20% proline-stabilized polyclonal immunoglobulin for subcutaneous administration. Biologicals 2011;39(1):43-9
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 2009;37(1):1-17
- Wu A. Tietz clinical guide to laboratory tests. 4th edition. Saunders; Philadelphia, PA, USA: 2006
- Hagan JB, Wasserman RL, Baggish JS, et al. Safety of L-proline as a stabilizer for immunoglobulin products. Expert Rev Clin Immunol 2012;8(2):169-78
- Privigen. Immune globulin intravenous [human], 10% liquid Full prescribing information. CSL Behring, LLC, Kankakee, IL, USA. 2011
- Hizentra. In immune globulin subcutaneous (human), 20% liquid CSL Behring LLC Kankakee, IL, USA. 2013
- Stucki M, Boschetti N, Schafer W, et al. Investigations of prion and virus safety of a new liquid IVIG product. Biologicals 2008;36(4):239-47
- Cai K, Groner A, Dichtelmuller HO, et al. Prion removal capacity of plasma protein manufacturing processes: a data collection from PPTA member companies. Transfus (Paris) 2013;53(9):1894-905
- Bonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am 2008;28(4):803-19, ix
- Gardulf A. Immunoglobulin treatment for primary antibody deficiencies: advantages of the subcutaneous route. BioDrugs 2007;21(2):105-16
- Bonagura VR. Using intravenous immunoglobulin (IVIG) to treat patients with primary immune deficiency disease. J Clin Immunol 2013;33(Suppl 2):S90-4
- Haddad E, Barnes D, Kafal A. Home therapy with subcutaneous immunoglobulins for patients with primary immunodeficiency diseases. Transfus Apher Sci 2012;46(3):315-21
- Kobrynski L. Subcutaneous immunoglobulin therapy: a new option for patients with primary immunodeficiency diseases. Biologics 2012;6:277-87
- Berger M, Rojavin M, Kiessling P, Zenker O. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol 2011;139(2):133-41
- Berger M, Jolles S, Orange JS, Sleasman JW. Bioavailability of IgG administered by the subcutaneous route. J Clin Immunol 2013;33(5):984-90
- Waniewski J, Gardulf A, Hammarstrom L. Bioavailability of gamma-globulin after subcutaneous infusions in patients with common variable immunodeficiency. J Clin Immunol 1994;14(2):90-7
- Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med 1999;340(3):227-8
- Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – a prospective, multi-national study. J Clin Immunol 2006;26(2):177-85
- Landersdorfer CB, Bexon M, Edelman J, et al. Pharmacokinetic modeling and simulation of biweekly subcutaneous immunoglobulin dosing in primary immunodeficiency. Postgrad Med 2013;125(6):53-61
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry: statistical approaches to establishing bioequivalence. 2014. Available from: www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf
- Sidhu JS, Rojavin M, Berger M, et al. Pharmacokinetic modeling predicts different IgG exposures using different IVIG-SCIG dose conversion factors in patients with primary immune deficiency. J Allergy Clin Immunol 2014;133(2):AB181
- Hagan JB, Fasano MB, Spector S, et al. Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. J Clin Immunol 2010;30(5):734-45
- Kanegane H, Imai K, Yamada M, et al. Efficacy and safety of IgPro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency diseases. J Clin Immunol 2014;34(2):204-11
- Jolles S, Borte M, Nelson RP Jr, et al. Long-term efficacy, safety, and tolerability of Hizentra® for treatment of primary immunodeficiency disease. Clin Immunol 2014;150(2):161-9
- Imai K, Kanegane H, Yamada M, et al. Safety, tolerability, and efficacy of Hizentra® in Japanese patients with primary immunodeficiency over 48 weeks. J Allergy Clin Immunol 2014;133(2):AB182
- Food and Drug Administration and Center for Biologics Evaluation and Research. Guidance for industry: Safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency. U.S. Department of Health and Human Services, Food and Drug Administration; 2005
- Ochs HD, Gupta S, Kiessling P, et al. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol 2006;26(3):265-73
- Jones CA, Rojavin M, Baggish JS. Patients with primary immunodeficiency receiving subcutaneous immune globulin Hizentra maintain health-related quality of life and treatment satisfaction in a multicentre extension study of efficacy, tolerability and safety. J Pharm Health Serv Res 2012;3:41-7
- Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol 2006;26(1):65-72
- Berger M, Murphy E, Riley P, Bergman GE. Improved quality of life, immunoglobulin G levels, and infection rates in patients with primary immunodeficiency diseases during self-treatment with subcutaneous immunoglobulin G. South Med J 2010;103(9):856-63
- Borte M, Bernatowska E, Ochs HD, Roifman CM. Efficacy and safety of home-based subcutaneous immunoglobulin replacement therapy in paediatric patients with primary immunodeficiencies. Clin Exp Immunol 2011;164(3):357-64
- Meth MJ, Rosenthal DW, Bonagura VR. Subcutaneous immunoglobulin infusion to treat infants and toddlers with antibody deficiencies. Ann Allergy Asthma Immunol 2010;105(2):187-8
- Sundin M, Nordin K, Jostemyr Y, Winiarski J. Subcutaneous IgG replacement after pediatric SCT. Pediatr Transplant 2012;16(8):866-71
- Duff CM, Faulkner E, Schirm Z, et al. Effective use of subcutaneous immune globulin (SCIG) for an infant with X-linked agammaglobulinemia (X-LA). J Allergy Clin Immunol 2009;123(2):S64
- Shapiro RS. Subcutaneous immunoglobulin: rapid push vs. infusion pump in pediatrics. Pediatr Allergy Immunol 2013;24(1):49-53
- Church JA, Howard V, Sleasman JW, et al. Subcutaneous immunoglobulin replacement therapy in infants and children with primary immunodeficiencies. J Allergy Clin Immunol 2011;127(2):AB213
- Haddad E, Berger M, Wang EC, et al. Higher doses of subcutaneous IgG reduce resource utilization in patients with primary immunodeficiency. J Clin Immunol 2012;32(2):281-9
- Orange JS, Belohradsky BH, Berger M, et al. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin Exp Immunol 2012;169(2):172-81
- Berger M. Incidence of infection is inversely related to steady-state (trough) serum IgG level in studies of subcutaneous IgG in PIDD. J Clin Immunol 2011;31(5):924-6
- Huang F, Feuille E, Cunningham-Rundles C. Home care use of intravenous and subcutaneous immunoglobulin for primary immunodeficiency in the United States. J Clin Immunol 2013;33(1):49-54
- Shapiro RS, Wasserman RL, Bonagura V, Gupta S. Emerging paradigm of primary immunodeficiency disease: individualizing immunoglobulin dose and delivery to enhance outcomes. J Clin Immunol 2014. [Epub ahead of print]
- Fadeyi M, Tran T. Calculating the dose of subcutaneous immunoglobulin for primary immunodeficiency disease in patients switched from intravenous to subcutaneous immunoglobulin without the use of a dose-adjustment coefficient. P T 2013;38(12):768-70
- Shapiro R. Subcutaneous immunoglobulin (16 or 20%) therapy in obese patients with primary immunodeficiency: a retrospective analysis of administration by infusion pump or subcutaneous rapid push. Clin Exp Immunol 2013;173(2):365-71
- Shapiro RS. Subcutaneous immunoglobulin therapy given by subcutaneous rapid push vs infusion pump: a retrospective analysis. Ann Allergy Asthma Immunol 2013;111(1):51-5
- Wasserman RL, Fatteh S, Khan JM, Haddad E. Retrospective analysis of the clinical utility of biweekly dosing with high-concentration subcutaneous immunoglobulin in 10 patients with primary immunodeficiency. J Allergy Clin Immunol 2014;133(2):AB184
- Malcolmson C, Jones A. Safety and efficacy of biweekly Hizentra® administration in patients with primary immunodeficiency diseases: A retrospective single-center study. J Allergy Clin Immunol 2014;133(2):AB180
- Magy L, Ghorab K, Calvo J, Vallat JM. Subcutaneous immunoglobulin as maintenance therapy in intravenous immunoglobulin-responsive CIDP patients. Long-term response in 16 patients. J Peripher Nerv Syst 2009;14:94
- Cocito D, Serra G, Falcone Y, Paolasso I. The efficacy of subcutaneous immunoglobulin administration in chronic inflammatory demyelinating polyneuropathy responders to intravenous immunoglobulin. J Peripher Nerv Syst 2011;16(2):150-2
- Lee DH, Linker RA, Paulus W, et al. Subcutaneous immunoglobulin infusion: a new therapeutic option in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2008;37(3):406-9
- Zampelli A, Zacek L, Levasseur MC. Successful use of subcutaneous immunoglobulin in chronic neurology patients. Neurology 2012;78:1
- Kazamel M, Sultan S, Alsharabati M, et al. Subcutaneous immunoglobulin use in neuromuscular patients who could not use or tolerate intravenous immunoglobulin: case series. J Clin Neuromuscular Dis 2013;14(3):154
- Misbah S, Comi G, Fazio R, et al. Efficacy and safety of subcutaneous immunoglobulin, Vivaglobin, in patients with multifocal motor neuropathy. J Neurol 2010;257(Suppl 1):S105-6
- Dacci P, Riva N, Scarlato M, et al. Subcutaneous immunoglobulin therapy for the treatment of multifocal motor neuropathy: a case report. Neurol Sci 2010;31(6):829-31
- Schleinitz N, Jean E, Benarous L, et al. Subcutaneous immunoglobulin administration: an alternative to intravenous infusion as adjuvant treatment for dermatomyositis? Clin Rheumatol 2008;27(8):1067-8
- Danieli MG, Moretti R, Gambini S, et al. Open-label study on treatment with 20 % subcutaneous IgG administration in polymyositis and dermatomyositis. Clin Rheumatol 2014;33(4):531-6