Abstract
Tacrolimus is a cornerstone of the immunosuppression regimen for prevention of allograft rejection in kidney and liver transplantations, with efficacy proven in many clinical trials. The currently available and extensively used tacrolimus formulations are flawed by large inter- and intra-individual variability, low bioavailability, wide peak-to-trough fluctuations and a narrow therapeutic index. Drug delivery technology can significantly impact the pharmacologic action of a drug, influencing its pharmacokinetic and subsequent therapeutic profile. LCP-Tacro is a novel, prolonged-release, MeltDose® formulation of tacrolimus designed for once-daily administration. A hallmark differentiation between this formulation and other once- and twice-daily tacrolimus products is the proprietary MeltDose drug delivery technology which is designed to improve the bioavailability of drugs with low water solubility. Considering the studies conducted to date, once-daily LCP-Tacro has shown improved pharmacokinetic properties, rapid achievement of therapeutic trough levels, consistent exposure, non-inferior efficacy and similar safety, with lower tacrolimus dose than other tacrolimus formulations.
World-wide there were an estimated 77,800 kidney transplants performed in 2012 (69% of all transplants) and 23,986 liver transplants (21% of all transplants) Citation[1]. Data from the US show that, in 2013 more than half of the 28,953 transplants performed were kidney transplants (58.3%, n = 16,894) Citation[2]; liver transplants were the next most frequent, comprising 22.2% (n = 6455) of all US transplants Citation[2]. The number of individuals in need of organ transplantation outweighs the availability of organs – as of June 2014; data from the Organ Procurement and Transplantation Network showed that 100,967 individuals were on the waitlist for a kidney transplant and 15,758 individuals were on the waitlist for a liver transplant Citation[3]. For individuals who receive a transplant, lifelong administration of immunosuppressive medications are required to prevent organ rejection. The arsenal of immunosuppressive drugs has evolved greatly since the beginnings of successful organ transplantation. A combination of azathioprine and corticosteroids was the original mainstay regimen in the early period of organ transplantation. A major advance in immunosuppression for prevention of allograft rejection came in the late 1970’s with the advent of the calcineurin inhibitor (CNI) cyclosporine A Citation[4–6]. The ‘cyclosporine era’ of the 1980’s saw greatly improved short- and mid-term graft survival following transplantation. Immunosuppressive drugs have continued to evolve and the availability of improved drugs and regimens has contributed largely to the current high short- and long-term survival rates (survival following living donor transplant, 1-year kidney: 98%; 5-year kidney: 90%; 1-year liver: 90%; 5-year liver: 77%) Citation[2]. In 1994, the EMA approved another CNI, tacrolimus, formulated as twice-daily capsules (Prograf®, Astellas Pharma US, Inc.), for the prophylaxis of organ rejection in kidney, liver and heart transplant patients, while the US FDA approved Prograf in the same year with only the liver indication. Subsequently, the indication in the US was expanded to include prophylaxis of organ rejection in kidney transplant patients and heart transplant patients Citation[7]. Tacrolimus, marketed as Prograf, is now commercially available in more than 70 countries. A prolonged-release once-daily tacrolimus capsule has received similar approvals from European regulatory authorities for use in kidney and liver transplantation Citation[8], and recently in the US with similar indications Citation[9]. Tacrolimus is highly effective in preventing acute rejection after organ transplantation Citation[7,10]. As such, tacrolimus is used as part of the immunosuppression regimen for the majority of kidney and liver transplant recipients (in ∼91% of kidney and liver transplants Citation[11]), both early post-transplantation and as part of long-term maintenance regimens; Citation[11]; and is recommended in the 2009 Kidney Disease: Improving Global Outcomes Clinical Practice Guidelines for the care of kidney transplant recipients Citation[12]. Since gaining approval, use of tacrolimus has continued to grow and surpass that of cyclosporine A Citation[3].
Overview of the market
Tacrolimus is considered a narrow therapeutic index drug that requires individual dose titration to achieve a satisfactory balance between maximizing efficacy and minimizing dose-related toxicity Citation[13]. In clinical practice, drug monitoring is necessary to facilitate tacrolimus dose titration. The prescribing information for tacrolimus capsules includes use of therapeutic drug monitoring and recommended dosing and target whole blood tacrolimus trough levels Citation[7]. The pharmacokinetic (PK) profile of tacrolimus is characterized by a high degree of inter- and intra-individual variability Citation[14]. Although rapidly absorbed in the GI tract, the bioavailability of tacrolimus from the traditional twice-daily capsule formulation is low and variable, ranging from 17 to 23% Citation[7]. The relatively low bioavailability of tacrolimus is believed to be due to a number of factors including poor water solubility, extensive first pass metabolism, p-glycoprotein-mediated efflux and the ingestion of food Citation[15]. Tacrolimus twice-daily capsules are associated with a characteristic high peak (Cmax) following dosing, which may be associated with increased toxicity Citation[16–18].
Transplant recipients are often burdened with drug regimens that require taking numerous pills a day. A recent study found that 1 month after kidney transplantation, the median pill burden was 25 pills a day, and by 1 year after transplantation, 16 pills a day Citation[19]. Increasing pill burden is associated with decreased adherence Citation[20,21], and lack of adherence is associated with acute rejection Citation[22,23] and graft loss Citation[21,24–26]. While there are currently available prolonged-release tacrolimus hard capsule formulations designed for once-daily dosing, data from these formulations do not suggest that they offer improved bioavailability over tacrolimus twice-daily capsules Citation[27–29]. In a clinical trial in de novo kidney transplant recipients, another once-daily formulation already available on the market (Advagraf® in Europe; Astagraf XL® in the US) failed to demonstrate a reduction in peak concentration in comparison with the twice-daily formulation Citation[30].
Tacrolimus has consistently demonstrated efficacy in preventing allograft rejection, however present formulations may not translate into best patient care. The less than optimal PK characteristics (poor bioavailability, high Cmax, fluctuations in peak-to-trough) of currently available tacrolimus formulations contribute to the risk of over-immunosuppression and under-immunosuppression while trying to maintain levels in the optimal therapeutic range (5–15 ng/ml) Citation[31]. The results of a large randomized multicenter Phase IV trial showed that low levels of tacrolimus (target level was 3–7 ng/ml; mean of ∼7 ng/ml) allow comparable outcomes with an improved safety and tolerability profile Citation[32]. In addition, the twice-daily formulation contributes to a dosing burden experienced by most transplant recipients.
Introduction to once-daily, LCP-Tacro, MeltDose® formulation tacrolimus tablets
LCP-Tacro, MeltDose® formulation tacrolimus (Envarsus®, Veloxis Pharmaceuticals, Hørsholm, Denmark) is a prolonged-release tablet formulation of tacrolimus designed for once-daily administration. A hallmark differentiation between LCP-Tacro and other forms of once- and twice-daily tacrolimus products is the proprietary MeltDose drug delivery technology (Veloxis Pharmaceuticals, Hørsholm, Denmark) Citation[33]. MeltDose is designed to improve the bioavailability of drugs with low water solubility (i.e., Biopharmaceutics Classification System Class II compounds) Citation[13]. Drug particle size is a crucial aspect affecting drug dissolution and absorption. The smaller the particle size, the greater the surface area of the drug and the faster the drug will be dissolved resulting in better absorption. MeltDose is a clinically validated drug delivery technology that is able to decrease a drug’s particle size to a molecular level; the particles are broken down into the smallest possible units as single molecules or what is referred to as a ‘solid solution’ Citation[33]. The technology advantages were clearly shown in Phase I and Phase II trials, which confirmed that LCP-Tacro enables broader absorption in the GI tract and sustains consistent tacrolimus concentrations Citation[34], even in patients that may be poor absorbers or rapid metabolizers [Veloxis, Pharmaceuticals, Data on File]. It therefore enables a more controlled, steady drug dissolution via a tablet matrix that helps establish a ‘flatter’ PK profile as compared to Prograf Citation[35]. Currently, there is another product on the market utilizing MeltDose technology – the first MeltDose-based product, Fenofibrate Immediate Release Tablets (Fenoglide™), is approved by the FDA and marketed in the USA Citation[36,37].
Chemistry
Tacrolimus, a macrolide produced by fermentation of Streptomyces tsukubaensis, is a potent immunosuppressive agent for the prophylaxis of organ rejection in allogenic organ transplantation. Tacrolimus blocks T-cell activation and proliferation by inhibiting the activity of the calcium-activated serine threonine phosphatase, calcineurin Citation[7,9,38,39]. Tacrolimus reduces the expression of several cytokine genes that are normally induced on T-cell activation. These include interleukin-2, whose synthesis by T-lymphocytes is an important growth signal for T cells Citation[39]. The suppression of T cell activation by tacrolimus inhibits the subsequent generation of cytotoxic lymphocytes and thereby downregulates the processes leading to acute graft rejection.
Pharmacodynamics
Tacrolimus is a macrolide antibiotic. It acts by reducing peptidyl-prolyl isomerase activity by binding to the immunophilin FKBP-12 (FK506 binding protein) creating a new complex. This inhibits both T-lymphocyte signal transduction and IL-2 transcription Citation[40].
PKs & metabolism
Tacrolimus is metabolized extensively by the liver, primarily by CYP3A isoenzymes Citation[14]. The major metabolite identified in incubations with human liver microsomes is 13-demethyl tacrolimus. In in vitro studies, a 31-demethyl metabolite has been reported to have the same activity as tacrolimus Citation[40].
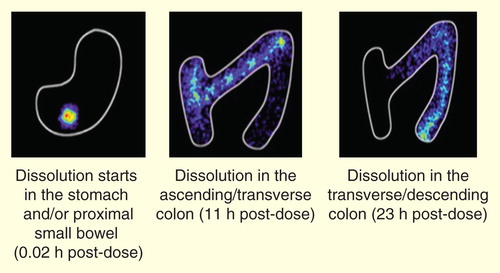
A key differentiator between LCP-Tacro tablets and the twice-daily capsule formulation of tacrolimus is the improved PK. Initial disintegration of LCP-Tacro tablets has been shown to occur in the stomach and/or proximal small bowel but complete disintegration (and presumably optimal absorption) Citation[41] occurs in the distal small bowel or the colon Citation[34]. There appears to be less gut CYP3A4 activity in the distal portions of the gastrointestinal system Citation[42] and thus less pre-systemic metabolism of tacrolimus, resulting in lower clearance and further increased bioavailability.
Figure 2. Depiction of the absorption of MeltDose® formulation of tacrolimus throughout the gastrointestinal tract across 24 h.
Clinical data demonstrated that LCP-Tacro tablets are associated with a lower peak (Cmax) and reduced peak-to-trough fluctuations Citation[35]. LCP-Tacro showed a numerically higher correlation between Cmin and area under the curve versus twice-daily tacrolimus capsules Citation[35,43,44]. The PK profile of LCP-Tacro is characterized by flatter kinetics (i.e., less fluctuation and swing) compared to twice-daily tacrolimus , resulting in a steadier concentration-time profile that is more consistently within the therapeutic range/window over 24 h. Reduced fluctuations in drug plasma concentrations may result in a more continuous effect and the avoidance of high peak concentrations may reduce the incidence and/or intensity of drug toxicity-related adverse events. In addition to the increased tacrolimus bioavailability associated with LCP-Tacro, the reduced peak-to-trough found for LCP-Tacro compared to twice-daily tacrolimus capsules and the inter-day reproducibility in PK suggest a tighter and more consistent relationship between the dose given and the tacrolimus exposure level for LCP-Tacro compared to twice-daily tacrolimus capsules Citation[35]. In Phase II studies in which stable kidney or liver transplant recipients were converted from twice-daily tacrolimus capsules to once-daily LCP-Tacro tablets, an approximate 20% Citation[45] to 30% Citation[35,43] lower dose of LCP-Tacro resulted in similar AUC24 as twice-daily tacrolimus capsules. This was further confirmed by post-hoc analysis in a Phase III de novo clinical trial Citation[46]. A prolonged time to peak was also consistently demonstrated during Phase II trials, potentially decreasing the risk of overlapping of peak toxicities with other co-medications (e.g., mycophenolate mofetil Citation[47]/mycophenolic acid Citation[48]) In addition, LCP-Tacro showed similar PK regardless if administered in the morning or evening Citation[49].
Figure 3. Pharmacokinetic profiles of twice-daily and once-daily tacrolimus formulations. (A) Mean whole-blood tacrolimus concentration in stable kidney transplant patients on tacrolimus twice-daily (Day 7) converted to LCP-Tacro (Day 14) versus time. (B) Mean whole blood tacrolimus concentrations in stable liver transplant patients on tacrolimus twice daily (Day 7) converted to LCP-Tacro (days 14, 21 and in week 26) versus time.
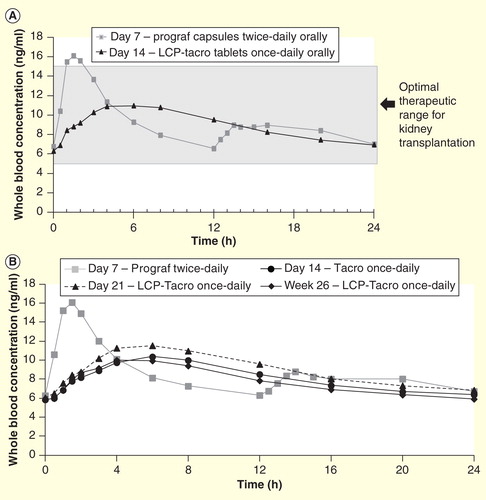
Table 1. Summary of PK results from Phase II trials.
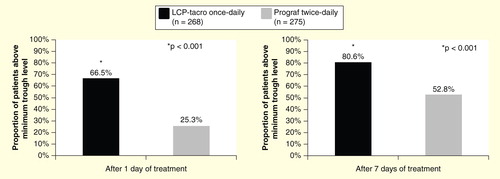
It is essential to achieve therapeutic exposure levels for immunosuppressive drugs to be effective. As stated in the Kidney Disease: Improving Global Outcomes clinical practice guideline for the care of kidney transplant recipients, the earlier that therapeutic blood levels of a CNI can be attained, the more effective the CNI will be in preventing acute rejection Citation[12]. Clinical data show that LCP-Tacro is associated with rapid and consistent exposure (AUC), with lower total daily tacrolimus dose in both kidney and liver transplant recipients who were converted from twice-daily tacrolimus capsules to once-daily prolonged release MeltDose tacrolimus tablets Citation[35,45], and in de novo kidney transplant recipients Citation[46]. As a result of the increased bioavailability afforded by LCP-Tacro, therapeutic tacrolimus levels are more rapidly achieved with LCP-Tacro compared to twice-daily tacrolimus capsules. In a study of de novo kidney transplantation, on Day 1, the majority of LCP-Tacro patients (66%) had trough levels above 6 ng/ml after the first day of dosing compared with 25% in the tacrolimus twice-daily group; after 7 days of treatment, 81% of patients in the LCP-Tacro group versus 53% of patients in the tacrolimus twice-daily group had levels greater than 6 ng/ml Citation[46]. Tacrolimus trough levels were notably higher in the LCP-Tacro group compared with the tacrolimus twice-daily group in the first 2 weeks after dosing; thereafter, trough levels in the two groups were similar through month 12 Citation[46].
The rapid achievement of therapeutic trough levels seen for LCP-Tacro is clearly different from that of the modified release version of tacrolimus currently approved by the EMA/FDA (Advagraf/Astagraf XL); published reports show lower initial tacrolimus exposure for once-daily, prolonged release tacrolimus formulations versus tacrolimus twice daily capsules Citation[27–29]. Data from studies in which patients were converted from twice-daily tacrolimus to once-daily tacrolimus (Advagraf) demonstrated the need for continuous dose increases over the first months to maintain consistent therapeutic trough levels Citation[50,51].
The majority of studies conducted to date for LCP-Tacro have been in comparison to twice-daily tacrolimus capsules, thus conclusions regarding direct PK comparisons between LCP-Tacro and other prolonged-release, once-daily, formulations of tacrolimus currently available are limited. A model using data from a Phase I study in healthy volunteers showed that LCP-Tacro had increased bioavailability compared to once-daily, prolonged release tacrolimus hard capsules (Advagraf) Citation[52]. It was estimated that the once-daily, prolonged release tacrolimus hard capsules would require an upward dose adjustment of a factor of approximately 1.5 to achieve similar exposure as once-daily LCP-Tacro tablets Citation[52].
Clinical efficacy
The efficacy of LCP-Tacro tablets in adult kidney transplant patients has been evaluated in seven completed clinical studies and more than 1,000 transplant recipients: two Phase III studies (Study 3001 in adult stable kidney transplant patients and Study 3002 in de novo kidney transplant patients) and five Phase II studies (Studies 2011 in adult stable kidney transplant patients; 2012 in stable liver transplant patients, with a 12 months extension period [2012E]; 2017 in de novo kidney transplant patients and 2018 in de novo liver transplant patients). The Phase II studies were primarily PK studies, with efficacy outcomes as secondary endpoints. In addition, a pooled analysis, including 861 stable and de novo kidney transplant recipients from studies 3001 and 3002, respectively, was conducted Citation[53]. An additional Phase IIIb study (STRATO Study) examined changes in tacrolimus-induced tremor among stable kidney transplant recipients converted from twice-daily tacrolimus to LCP-Tacro.
Phase II studies
Five Phase II trials have been completed to date, including two in kidney transplant recipients and three in liver transplant recipients . As previously stated, the Phase II studies did not include efficacy outcomes as primary objectives.
Table 2. Summary of Phase II trial efficacy results.
Kidney transplantation
Study 2011 was a Phase II, open-label, multicenter, prospective, conversion study in stable kidney transplant patients who were on a stable dose of twice-daily tacrolimus capsules, with trough levels of tacrolimus maintained at 7–12 ng/ml for at least 2 weeks before enrollment Citation[35]. Patients maintained their regimen of twice-daily tacrolimus capsules for the first 7 days after enrollment, on study Day 8, patients were switched to once-daily LCP-Tacro at a mean conversion dose ratio of 0.71. On Day 22, patients were then converted back to the pre-study twice-daily tacrolimus capsules dose Citation[35]. Study 2011 primarily evaluated the PK of LCP-Tacro tablets pre-conversion, and at days 7 and 14 post-conversion. Acute rejection episodes, graft loss and patient death were monitored as secondary safety endpoints. Of the 60 enrolled patients, 51 received at least one dose of study drug. There were no deaths, graft losses or allograft rejections Citation[35].
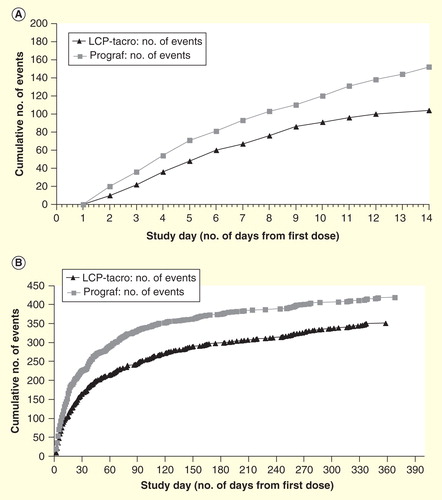
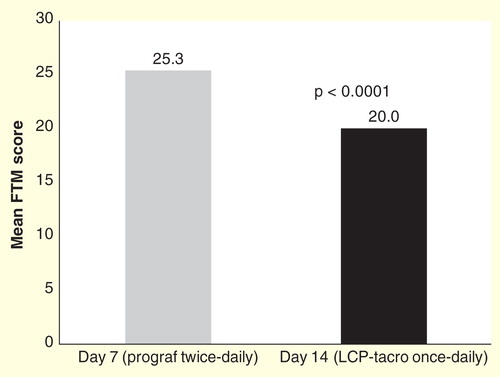
Study 2017 was an open-label, randomized study in which adult de novo kidney transplant patients were randomized 1:1 to receive either once-daily LCP-Tacro at a starting dose of 0.14 mg/kg/day (0.17 mg/kg/day for black patients) or twice-daily tacrolimus capsules at a starting dose of 0.2 mg/kg/day for 12 months Citation[54]. A total of 63 (once-daily LCP-Tacro, n = 32; twice-daily tacrolimus capsules, n = 31) patients received at least 1 study drug dose. Study 2017 primarily evaluated PK, all efficacy outcomes were secondary endpoints. Treatment failure (death, graft loss, rejection or lost to follow-up) was 6.3% (n = 2; 1 biopsy-proven acute rejection [BPAR] and 1 lost to follow-up) for once-daily LCP-Tacro and 9.7% (n = 3; 2 = BPAR and 1 = lost to follow-up) for twice-daily tacrolimus capsules (p = 0.67) at 12 months. No patients experienced graft failure during the study and there were no deaths Citation[54]. On Day 2 following first dose, the proportion of patients in the LCP-Tacro group with tacrolimus trough concentration that were within, above and below ideal target range of 6 to 11 ng/ml was 53, 11, and 37%, respectively. In addition, that study showed that dose adjustments were significantly (p = 0.0018) fewer with LCP-Tacro versus twice-daily tacrolimus in the early post-transplant period (i.e., through Day 14; ) (LCP-Tacro mean dose changes per patient: 3.25; tacrolimus twice-daily: 4.90), and over the whole 12-month study period (13.6 vs 15.7; p = 0.043; ). This suggests that patients on LCP-Tacro may achieve individualized targeted trough levels more consistently versus twice daily tacrolimus Citation[55].
Liver transplantation
Study 2012 was a three-sequence, open-label, multicenter, prospective, study in stable liver transplant patients. Following study enrollment, each patient was monitored for 7 days on a fixed dose of tacrolimus capsules twice-daily to ensure stable tacrolimus trough concentrations between 5 and 12 ng/ml Citation[43]. On Day 8, each patient was converted to once-daily LCP-Tacro tablets using a dose conversion ratio targeting of 0.70, and ranging from 0.66 to 0.80; patients continued on a fixed dose of once-daily LCP-Tacro for days 8–21. On Day 22, patients were either converted back to their regular maintenance regimen of twice-daily tacrolimus capsules and followed for 30 days for safety assessment, or were enrolled in the extension phase of the study (study 2012E). In the open-label extension phase, subjects were maintained on once-daily LCP-Tacro for an additional 50 weeks at a dose determined by each center’s standard of care. The recommended whole blood trough level was 5–15 ng/ml Citation[43]. A total of 57 patients completed 2012 and 43 completed 2012E. That study, including the extension, was a PK study without planned efficacy endpoints. However, no patients experienced graft loss or death during the core study or extension phase Citation[43].
Study 2018 was an open-label, randomized study in adult de novo liver transplant recipients. After transplantation, patients were randomized to once-daily LCP-Tacro at 0.07–0.11 mg/kg once-daily (0.09–0.13 mg/kg for black patients) or tacrolimus capsules at 0.10–0.15 mg/kg/day (divided twice-daily). Subsequent doses of both drugs were adjusted to maintain tacrolimus trough levels of 5–20 ng/ml through Day 90 and 5–15 ng/ml thereafter Citation[45]. The primary objective of the study was to evaluate tacrolimus exposure and trough levels, all efficacy outcomes were secondary endpoints. Fifty-eight patients were randomized and 35 (once-daily LCP-Tacro, n = 17; twice-daily tacrolimus capsules, n = 18) completed 1-year follow-up. Incidence of BPAR at 12 months was once-daily LCP-Tacro, n = 6; twice-daily tacrolimus capsules, n = 4. Two patients in each group died during the study; neither death was suspected to be related to study drug; there were no cases of death-censored graft loss Citation[45].
Phase III studies
Two randomized Phase III trials and one Phase IIIb trial have been completed to date Citation[46,56]. Study 3001 (MELT trial) was an open-label, multicenter, prospective, randomized, two-arm parallel group study in stable kidney transplant patients. Following a 7-day run-in period during which patients continued on their current dose of twice-daily tacrolimus capsules, patients were randomized 1:1 to receive a reduced dose of once-daily LCP-Tacro or to continue on twice-daily tacrolimus capsules for 12 months. The initial dose of once-daily LCP-Tacro was based on a dose conversion ratio of 0.7 (0.85 for black patients). Subsequent study drug doses were based on clinical assessment and maintenance of target tacrolimus whole blood trough levels within the predefined range of 4–15 ng/ml Citation[56]. The primary efficacy endpoint was a composite efficacy failure outcome (death, graft failure, locally read BPAR or lost to follow-up) at 12 months after the first dose of study drug. There were 326 patients enrolled (162 in each treatment group). The primary efficacy failure rate for once-daily LCP-Tacro and twice-daily tacrolimus capsules was 2.5% (n = 4) for each of the treatments. The 95% CI for the treatment difference was ±4.2%, which was well within the predefined non inferiority margin of 9% Citation[56]. When centrally read BPARs were considered, the composite efficacy failure rate was numerically, though not statistically significantly (treatment difference: -1.85%, 95% CI: 6.51%, 2.30%; p = 0.502), lower for once-daily LCP-Tacro (n = 3, 1.9%) versus twice-daily tacrolimus capsules (n = 6, 3.7%) Citation[56].
Table 3. Summary table of Phase III trial results.
Study 3002 was a double-blind, double-dummy, multicenter, prospective, randomized, two-arm parallel group study in which adult de novo kidney transplant patients were randomized to receive either once-daily LCP-Tacro at a starting dose of 0.17 mg/kg/day or twice-daily tacrolimus capsules at a starting dose of 0.1 mg/kg/day for 24 months Citation[46]. After receiving the initial starting dose, the dose could then be adjusted to maintain tacrolimus whole blood trough levels within the predefined therapeutic range of 6–11 ng/ml for the first 30 days of the study, and 4–11 ng/ml for the remainder of the study Citation[46]. The primary efficacy endpoint was a composite efficacy failure outcome (death, graft failure, centrally read BPAR or lost to follow-up) at 12 months after the first dose of study drug. A total of 543 patients were randomized (once-daily LCP-Tacro, n = 268; twice-daily tacrolimus capsules, n = 275) Citation[46]. The primary efficacy failure rate was 18.3% for once-daily LCP-Tacro and 19.6% for twice-daily tacrolimus capsules, with a treatment difference (95% CI) of -1.35% (-7.94%, 5.27%), which was well below the predefined non inferiority margin of 10% Citation[46]. The incidence of BPAR at 12 months (once-daily LCP-Tacro: 13.1%; twice-daily tacrolimus capsules: 13.5%) is comparable to that reported in the literature among de novo kidney transplant recipients receiving tacrolimus formulations (e.g., range of 10–19% among de novo kidney transplant recipients receiving once- or twice-daily tacrolimus formulations Citation[28,57]). Within the first 3 months post-transplant, when kidney transplant recipients have the greatest rejection risk, treatment failure rates were once-daily LCP-Tacro: 10.4% and twice-daily tacrolimus capsules: 14.2%, p = 0.195 Citation[46]. A post-hoc analysis was done at the request of the FDA, in which several statistical models, examining tacrolimus trough levels as a time-dependent covariate, were evaluated to identify the most valid and parsimonious representation of the relationship between trough levels over time and risk of treatment failure. Baseline trough level (i.e., trough levels on initial dosing days 1, 2 and 3) and change in trough from baseline (i.e., every 1 ng/ml increase) were independently associated with significantly (p ≤ 0.02) decreased risk of treatment failure. In the absence of safety concerns, initial tacrolimus dosing should be started to target rapid attainment of therapeutic levels Citation[58].
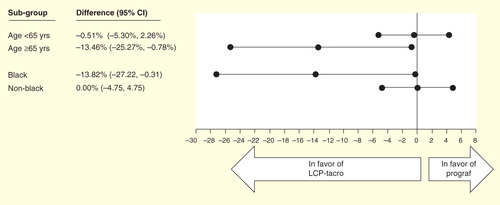
Figure 6. Efficacy at 1-year post-transplant in de novo kidney transplant recipients randomized to LCP-Tacro or tacrolimus twice-daily Citation[46].
![Figure 6. Efficacy at 1-year post-transplant in de novo kidney transplant recipients randomized to LCP-Tacro or tacrolimus twice-daily Citation[46].](/cms/asset/f6c90e81-aacb-41db-8fbc-159d0aa616b4/ierm_a_983903_f0006_b.jpg)
Data from the two Phase III studies were pooled to examine efficacy in specific patient subgroups. This analysis, which include 861 (LCP-Tacro n = 428; Prograf n = 433; 38% of patients were stable KTR [study 3001], and 62% were de novo KTR [study 3002]) patients found that treatment failure (death, graft failure, centrally read BPAR, or lost to follow-up) at 12 months was significantly lower with LCP-Tacro among black kidney transplant recipients (treatment difference and 95% CI: -13.82% [-27.22%, -0.31%]) and older kidney transplant recipients (-13.46% [-25.27%, -0.78%]) Citation[53].
Safety & tolerability
Increased immunosuppressive drug exposure is generally associated with increased toxicity risk; therefore, the goal is to balance adequate drug exposure to achieve the desired therapeutic effect, while mitigating unwanted toxic side effects. The safety profile of LCP-Tacro is similar to that of other tacrolimus formulations used for the prevention of rejection .
Table 4. Adverse events reported in Phase III trials of LCP-Tacro.
Immunosuppressed patients are at increased risk for opportunistic infections. In addition, tacrolimus has known adverse effects, including nephrotoxicity, new onset diabetes mellitus, hypertension and hyperkalemia. Neurotoxicity is a hallmark side effect of tacrolimus Citation[17,59], of which tremor represents the most common manifestation, occurring in 34–54% of kidney transplant recipients Citation[7]. The exact mechanism responsible for tacrolimus-related neurological adverse events is unknown. However, symptoms appear generally 2–3 h after administration, improve when the tacrolimus dose is reduced or when tacrolimus is withdrawn and it is a reasonable hypothesis that elevated tacrolimus blood levels may be associated with risk for toxicity Citation[16–18]. A two-sequence, open-label, multicenter, prospective Phase IIIb exploratory study was conducted in which stable kidney transplant recipients on Prograf or generic tacrolimus, experiencing tremor, were enrolled.
Following 7 days of their pre-enrollment twice-daily tacrolimus, patients were switched to LCP-Tacro. Tremor pre- and 7 days post-conversion was evaluated by two independent, blinded neurologists using the gold standard Fahn-Tolosa-Marin tremor rating scale and by an accelerometry device that measures frequency and amplitude of tremor (Tremorometer™). Patients completed the Patient Global Impression of Change scale and physicians completed the Clinical Global Impression of Improvement (CGI) scale; both are 7-point scales assessing tremor change ranging from very much improved (1) to very much worse (7) Citation[60]. Quality of life was assessed by the patient-completed Quality of Life in Essential Tremor scale, a subjective quality of life instrument consisting of 30 items divided into five dimensions (communication, work/finance, hobbies/leisure, physical and psychosocial). Data were available on 38 patients. There was a significant improvement in tremor as indicated by significant decrease (improvement) in the Fahn-Tolosa-Marin score and the Tremorometer score, and significant improvements in the patient global impression, clinical global impression and quality of life in essential tremor Citation[60].
Regulatory affairs
On July 18, 2014, the European Commission granted a marketing authorization valid throughout the European Union for Envarsus. Chiesi Farmaceutici S.p.A. (Parma, Italy) will be the Marketing Authorization Holder in EMA countries and applicant in other non-EMA countries. Indications approved were prevention of kidney and liver organ transplant rejection, and treatment of organ rejection resistant to other immunosuppressant drugs in adult patients.
In the US, the New Drug Application for Envarsus was accepted by the FDA for standard review for the prevention of organ rejection in adult kidney transplant patients. The FDA set a target review date under the Prescription Drug User Fee Act of October 30, 2014. The new drug application was submitted to the FDA on December 30, 2013 and is based on two Phase III studies, 3001 and 3002, in which Envarsus demonstrated non-inferiority compared to twice daily tacrolimus (Prograf) based on a composite endpoint of treatment failure at 1 year. The clinical program comprised 25 studies and enrolled over 1000 patients. In addition, Envarsus received Orphan Drug Designation by the FDA for prophylaxis of organ rejection in patients receiving allogeneic kidney transplants.
Conclusion
Tacrolimus is an effective immunosuppressive drug that is used immediately and long-term in the majority of kidney and liver transplant recipients. Currently available tacrolimus formulations are flawed by sub-optimal PK characteristics such as high inter- and intra-variability, high peak levels and low bioavailability. Each of these factors complicate maintaining tacrolimus exposure into the range necessary for effective and safe prevention of organ rejection – too low exposure increases the risk of rejection and too high increases the risk for toxicity. The novel MeltDose delivery system of LCP-Tacro results in a tacrolimus formulation that offers increased bioavailability and lower peak exposure, and at an approximately 30% lower dose and with once-a-day dosing. Randomized trials show that LCP-Tacro has a similarly good efficacy and safety profile as other tacrolimus formulations.
Expert commentary & five-year view
With the European approval of LCP-Tacro, and the imminent US approval, clinicians have an improved formulation of a tested, integral, immunosuppression drug available for use in prevention of organ rejection. Patients will have less pill burden with LCP-Tacro, and future data will show if the preliminary results showing a reduction in tremor among patients with this tacrolimus-induced side effect may translate into a reduction in other tacrolimus exposure-associated side effects.
Key issues
The overwhelming majority (∼91%) of kidney and liver transplant recipients are on a tacrolimus-based immunosuppression regimen.
Tacrolimus has a narrow therapeutic range/window, and it is essential that patients are within the range to avoid rejection (if too low) and toxicity (if too high).
Currently available tacrolimus formulations are highly effective but have less than desirable pharmacokinetic characteristics – for example, low bioavailability.
A novel, prolonged-release, once-daily, MeltDose®, tacrolimus tablet has been developed for prevention of rejection following kidney and liver transplantation.
The MeltDose drug delivery technology decrease’s tacrolimus’ particle size to a molecular level which in turn results in significantly improved bioavailability compared to other tacrolimus formulations and a smoother, flatter pharmacokinetic profile compared to other formulations of tacrolimus.
Rapid achievement of blood levels in de novo transplant patients immediately following Day 1 of dosing.
LCP-Tacro shows improved bioavailability, lower Cmax and less peak-to-trough fluctuation, and higher exposure at equivalent doses to the classical tacrolimus formulation, which results in a lower dosing benefit compared to twice-daily tacrolimus capsules.
LCP-Tacro is associated with a more rapid achievement of target trough levels, with more constant levels within the therapeutic window.
Fewer dose adjustments needed over the first 14 days and year post transplant.
LCP-Tacro is non-inferior in efficacy to twice-daily tacrolimus capsules with similar safety.
Additional potential benefits of prolonged-release, once-daily, MeltDose, tacrolimus tablet are less pill burden, and the possibility for less peak-related toxicity (e.g., tremor).
Financial & competing interests disclosure
Chiesi Farmaceutici (Parma, Italy) provided funding in support of this manuscript. S Petruzzelli is an employee of Chiesi Farmaceutici. K Kistler (Evidera) provided writing assistance, funded by Chiesi Farmaceutici. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Those 2012 data are based on the Global Observatory on Donation and Transplantation (GODT) data, produced by the WHO-ONT collaboration
- Available from: http://optn.transplant.hrsa.gov/latestData/rptData.asp [Accessed 3 June 2014]
- Available from: http://optn.transplant.hrsa.gov/latestData/rptData.asp Data as of June 27, 2014 [Accessed 2 July 2014]
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004;351:2715-29
- Colombo D, Ammirati E. Cyclosporine in transplantation - a history of converging timelines. J Biol Regul Homeost Agents 2011;25:493-504
- Calne R. Cyclosporine as a milestone in immunosuppression. Transplant Proc 2004;36:13S-5S
- Prograf, prescribing information. Astellas Pharma US, Inc; Northbrook, IL: 2013
- Advagraf 0.5 mg, 1 mg, 3 mg and 5 mg tacrolimus extended release capsules. Product Monograph. Astellas Pharma Canada, Inc. Markham, ON. 2010; Northbrook, IL
- ASTAGRAF XL - tacrolimus capsule, coated, extended release. Astellas Pharma US, Inc; Northbrook, IL: 2014)
- Webster AC, Taylor RRS, Chapman JR, et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005;19(4):CD003961
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2012 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration; Rockville, MD: 2014
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9(Suppl 3):S1-157
- Available from: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM181006.pdf
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004;43:623-53
- Provenzani A, Santeusanio A, Mathis E, et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol 2013;19:9156-73
- Abouljoud MS, Kumar MSA, Brayman KL, et al. Neoral® rescue therapy in transplant patients with intolerance to tacrolimus. Clin Transplant 2002;16:168-72
- Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 2000;13:313-26
- Eidelman BH, Abu-Elmagd K, Wilson J, et al. Neurologic complications of FK 506. Transplant Proc 1991;23:3175-8
- Hardinger KL, Hutcherson T, Preston D, et al. Influence of pill burden and drug cost on renal function after transplantation. Pharmacotherapy 2012;32:427-32
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296-310
- Tielen M, van Exel J, Laging M, et al. Attitudes to medication after kidney transplantation and their association with medication adherence and graft survival: a 2-year follow-up study. J Transplant 2014;2014:675301
- Morrissey PE, Reinert S, Yango A, et al. Factors contributing to acute rejection in renal transplantation: the role of noncompliance. Transplant Proc 2005;37:2044-7
- Vlaminck H, Maes B, Evers G, et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant 2004;4:1509-13
- Michelon T, Dominguez V, Losekan A, et al. Kidney graft failure due to noncompliance. Transplant Proc 1999;31:3031-2
- Jarzembowski T, John E, Panaro F, et al. Impact of non-compliance on outcome after pediatric kidney transplantation: an analysis in racial subgroups. Pediatr Transplant 2004;8:367-71
- Butler JA, Roderick P, Mullee M, et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 2004;77:769-76
- Wlodarczyk Z, Squifflet JP, Ostrowski M, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009;9:2505-13
- Krämer BK, Charpentier B, Bäckman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010;10:2632-43
- Silva HT, Yang HC, Abouljoud M, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595-608
- Niioka T, Satoh S, Kagaya H, et al. Comparison of pharmacokinetics and pharmacogenetics of once- and twice-daily tacrolimus in the early stage after renal transplantation. Transplantation 2012;94:1013-19
- Rath T. Tacrolimus in transplant rejection. Expert Opin Pharmacother 2013;14:115-22
- Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357:2562-75
- MeltDose® Technology by the US Patent and Trademark Office. US7217431
- Nigro V, Glicklich A, Weinberg J. Improved bioavailability of MELTDOSE once-daily formulation of tacrolimus (LCP-Tacro) with controlled agglomeration allows for consistent absorption over 24 hrs: a scintigraphic and pharmacokinetic evaluation [abstract B1034]. American Transplant Congress, 2013
- Gaber AO, Alloway RR, Bodziak K, et al. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation 2013;96:191-7
- Holm P, Thomassen JQ, Rasmussen SR. MeltDose® a one step industrial process for the manufacturing of solid dispersion. 6th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology; 7th to 10th April 2008; CCIB, Barcelona, Spain
- Buch P, Holm P, Thomassen JQ, et al. IVIVC for fenofibrate immediate release tablets using solubility and permeability as in vitro predictors for pharmacokinetics. J Pharm Sci 2010;99(10):4427-36
- Advagraf. European public assessment report (EPAR). Available from: www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000712/human_med_000629.jsp&mid=WC0b01ac058001d124
- Vicari-Christensen M, Repper S, Basile S, et al. Tacrolimus: review of pharmacokinetics, pharmacodynamics, and pharmacogenetics to facilitate practitioners’ understanding and offer strategies for educating patients and promoting adherence. Prog Transplant 2009;19:277-84
- Available from: www.drugbank.ca/drugs/DB00864
- Gavhanea YN, Yadavb AV. Loss of orally administered drugs in GI tract. Saudi Pharm J 2012;20:331-44
- Thörn M, Finnström N, Lundgren S, et al. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. Br J Clin Pharmacol 2005;60:54-60
- Alloway RR, Eckhoff DE, Washburn WK, et al. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): phase II trial of stable liver transplant recipients. Liver Transpl 2014;20:564-75
- EMEA. Note for guidance on modified release oral and transdermal dosage forms: section II (pharmacokinetic and clinical evaluation). European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products; London: 1999
- Feng S, Chapman WC, DuBay D. A phase 2 randomized study of the pharmacokinetics, safety and efficacy of LCP-Tacro tablets once-a-day vs Prograf capsules twice-a-day in de novo liver transplants [abstract 709]. Presented at the 2012 American Transplant Congress; 2–6 June 2012; Boston, Massachusetts
- Budde K, Bunnapradist S, Grinyo JM, et al. Once daily LCP-Tacro MeltDose® tacrolimus vs. twice daily tacrolimus in de novo kidney transplants: one-year results of Phase 3, double-blind, randomized trial. Am J Transplant 2014; In press
- CellCept, (mycophenolate mofetil) summary of product characteristics (SPC, SmPC)
- Myfortic, (mycophenolate acid) summary of product characteristics (SPC, SmPC)
- Nigro V, Glicklich A, Weinberg J. Flexible dosing of once-daily LCP-Tacro tablets: morning vs. evening randomized crossover chronopharmacokinetic study. AST/ESOT Joint Meeting; 12–14 October 2012; Nice, France
- Barraclough K, Isbel N, Johnson D, et al. Once-versus twice-daily tacrolimus. Drugs 2011;71:1561-77
- Hougardy JM, Broeders N, Kianda M, et al. Conversion from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation 2011;91:566-9
- Gabardi S, Nigro V, Johnson J, et al. Evaluation of steady–state pharmacokinetic parameters of LCP-Tacro™and Advagraf® in healthy volunteers using a systems dynamic model [abstract 2308787]. Poster presented at the European Society of Transplantation. Vienna, 2013
- Bunnapradist S, Alloway R, West-Thielke P, et al. Lower treatment failures in blacks and older denovo and stable kidney transplant recipients treated with envarsus once-daily meltdose tablets vs. twice-daily prograf capsules: a pooled subgroup analysis of two phase 3 trials [abstract# 50]. World Transplant Congress (WTC); 26-31 July 2014; San Francisco, California
- Alloway R, Mulgaonkar S, Ueda K, et al. A phase 2 randomized study of the pharmacokinetics, safety and efficacy of LCP-tacro tablets once-a-day vs prograf capsules twice-a-day in de novo kidney transplants. Am J Transplant 2011;11:355
- Mulgaonkar S, Alloway RR. LCP-Tacro demonstrated significantly fewer dose adjustments: results of a phase 2 randomized study of lcp-tacro (meltdose once-daily prolonged release tacrolimus tablets) vs. prograf® capsules twice-a-day in de novo kidney transplant patients [abstract 51]. The European Society for Organ Transplantation (ESOT) and the American Society of Transplantation (AST); 17–19 October 2014; Madrid, Spain; 2014
- Bunnapradist S, Ciechanowski K, West-Thielke P, et al. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant 2013;13:760-9
- Albano L, Banas B, Klempnauer JL, et al. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013;96:897-903
- Grinyo JM, Rostaing L, Budde K, et al. Rapid attainment of tacrolimus trough levels early post-transplant reduces risk of treatment failure in de novo kidney transplant patients: a covariate analysis of a phase 3 double-blind study [abstract #1571]. World Transplant Congress; 26–31 July 2014; San Francisco, CA; 2014
- Paul LC. Overview of side effects of immunosuppressive therapy. Transplant Proc 2001;33:2089
- Gedaly R, Steinberg SM, Langone A, et al. LCP-Tacro–associated improvement in tacrolimus–induced tremors is also associated with improvement in quality of life: results of a switching study of kidney transplant patients with tremor (strato). European Society for Transplantation (ESOT); 2013