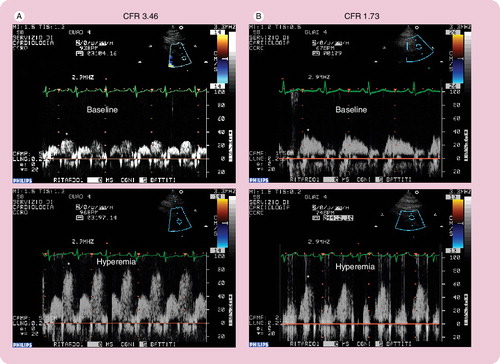
Figure 1. Normal (A) and pathological (B) coronary flow velocity reserve signal.
*Indicates the diastolic velocity in Doppler signal.
Systemic autoimmune diseases comprise a family of conditions that share common pathogenetic mechanisms as well as multiorgan involvement including the heart.
Autoimmune diseases occur as a result of the loss of tolerance to self-antigens, which is maintained under physiological conditions. Circulating antibodies do not always play a pathogenetic role but they represent specific markers of ongoing tissue damage.
In systemic autoimmune diseases, autoantibodies are against ubiquitous antigens (i.e., nuclear antigens in systemic lupus erythematosus [SLE]) and tissue damage is generalized. A large group of patients is affected by this group of diseases including rheumatoid arthritis (RA), SLE, primary antiphospholipid syndrome, systemic sclerosis and systemic vasculitis.
One of the leading causes of morbidity and mortality in these patients is represented by cardiovascular disease, which is associated with development of accelerated atherosclerosis Citation[1,2]. For example, in RA, cardiovascular mortality accounts for 40–50% of all deaths Citation[3]. Moreover, cardiovascular disease seems to occur in those of younger age compared with the general population. It is often asymptomatic, at least in early stages, and involves specific risk factors in addition to traditional risk factors shared with the general population. In particular, the excess of cardiovascular mortality and morbidity could be explained by chronic inflammation, duration and activity of the autoimmune disease and immunosuppressive therapy (glucocorticoids and methotrexate).
All components of the heart can be affected through several pathogenetic mechanisms; valves, coronary arteries, conduction system, myocardium, endocardium and pericardium could all be involved.
Clinical manifestations of cardiac involvement include: pericarditis, myocarditis and myocardial fibrosis, rhythm and conduction disturbances, coronaritis with ischemic heart disease, valvular diseases, pulmonary hypertension, syncope, diastolic or systolic heart failure Citation[1].
Recently, chronic inflammation has been recognized to play an important role in the development of atherosclerotic plaques Citation[4] and endothelial dysfunction seems to be the prime mover in this process Citation[5]. Reduced production of nitric oxide (NO) by NO synthase represents the leading feature of endothelial dysfunction. Asymmetric dimethyl arginine (ADMA) is an endogenous inhibitor of NO synthase and recently it has emerged as a novel cardiovascular risk factor. It has already been demonstrated that ADMA plasma levels are elevated in RA patients Citation[6].
Since autoimmune diseases are characterized by high cardiovascular risk and that cardiac involvement is associated with adverse outcomes and poor prognosis, an early identification of patients at higher risk becomes mandatory to improve the outcome. The knowledge of the mechanisms responsible for this process represents an important step to choose appropriate drugs that block/slow the development of atherosclerosis.
Diagnostic tools
Early phases of cardiovascular involvement in patients suffering from autoimmune diseases could be clinically silent and only a disorder at microcirculation level may be present.
Instrumental diagnostic tools able to detect cardiac morphological damage could be classified into:
| • | Noninvasive imaging techniques (transthoracic echocardiography, tissue Doppler imaging) | ||||
| • | Semi-invasive techniques (transthoracic stress echocardiography, transesophageal echocardiography) | ||||
| • | Computed tomography (CT) and coronary magnetic resonance angiography | ||||
| • | Invasive techniques (angiography) | ||||
Transthoracic echocardiography
Transthoracic echocardiography is a reliable, noninvasive technique that allows for an accurate evaluation of valvular abnormalities, pericardial diseases and ventricular wall motion defects. Doppler analysis is useful to study left ventricular diastolic filling, valvular fuctioning and pulmonary pressures. Rexhepaj et al. used Doppler echocardiography to assess the prevalence of left and right ventricular diastolic dysfunction in patients with RA without clinical evidence of cardiovascular disease. They found significant differences in early diastolic flow velocity (E), atrial flow velocity (A) and E:A ratio between patients and controls, suggesting that a reduction in left and right ventricular function is present in asymptomatic RA patients, while left ventricular thickness, dimensions, systolic function and myocardial performance index were normal Citation[7].
Transthoracic stress echocardiography with coronary flow reserve evaluation
Recently, the evaluation of coronary flow reserve (CFR) by transthoracic dipyridamole stress echocardiography has been proven to be a highly sensitive (>90%) diagnostic marker for coronary artery disease Citation[8,9] and, when associated with the evaluation of the regional wall motion analysis, it also becomes highly specific Citation[10]. In literature, CFR of less than two assessed in the distal left antherior descending (LAD) artery has been shown to accurately predict the presence of coronary stenosis Citation[9]. In the absence of epicardial coronary stenosis, an abnormal CFR may reflect an impaired coronary microcirculation in patients with reperfused myocardial infarct, arterial hypertension with or without left ventricular hypertrophy, diabetes mellitus, hypercholesterolemia, syndrome X, hypertrophic cardiomyopathy and other diseases Citation[11]. The assessment of CFR has also been used to assess the prognostic value of different cardiac conditions and it has proven that a reduced CFR correlates with a negative prognosis Citation[12].
To perform dipyridamole stress echocardiography test with CFR evaluation, patients have to abstain from xanthine-containing food and drinks for at lease 24 h before the study. In a stable 90° left lateral recumbent position with a modified two-chamber view to identify distal LAD, CFR was evaluated in the LAD coronary artery before and during dipyridamole infusion (0.56 mg/kg in 4 min + 0.28 mg/kg in the next 2 min) with an 8-MHz transducer, assessing systolic and diastolic components of the Doppler signal.
Coronary blood flow in the mid-distal portion of the LAD artery is measured under the guidance of color-Doppler flow mapping, synchronizing electrocardiogram. The CFR is calculated as the ratio between peak diastolic velocity during hyperemia to diastolic velocity at baseline . At the end of the analysis, 125–250 mg of aminophylline should be administered to counteract the effect of dipyridamole.
Hirata et al. performed stress echocardiography to evaluate CFR in premenopausal women with SLE and they found a significant reduction of CFR compared with age- and sex-matched controls. They concluded that microvascular impairment in SLE could be explained by functional alteration of endothelium, which is responsible for the decrease in vasodilation in response to pharmacological stress Citation[13].
Tissue Doppler imaging
Recently, this new imaging modality has been proven to allow measurement of myocardial velocities with Doppler. The pulse wave Doppler cursor is placed at the mitral annulus from the apical window. In this way, we can measure velocities that represent the longitudinal contraction (positive systolic wave, Sa) and relaxation (early negative diastolic wave, Ea, and late negative diastolic wave). The Ea has proven to be a good index of left ventricular relaxation. It correlates with the time constant of isovolumic relaxation and with age. In particular, low E:A velocities with an E:A of less than one is considered an index of impaired ventricular relaxation.
Birdane et al. compared left and right ventricular tissue Doppler imaging parameters between RA patients and healthy subjects. They observed a significant reduction of E:A ratio in RA patients compared with controls; moreover, in RA patients the E:A ratio was correlated with patient age and use of steroids Citation[14].
Transesophageal echocardiography
This examination is widely recognized to be more sensitive than the transthoracic approach for detecting valvular lesions Citation[15] and to identify intracardiac masses.
We followed-up 56 patients with primary antiphospholipid syndrome for 5 years. Transesophageal echocardiographic evaluation was performed in all patients. We observed a large prevalence of cardiac involvement. In 61% of patients, valvular thickening or vegetations and/or potential embolic sources were present Citation[16].
Computed tomography
Electron-beam CT is a highly sensitive technique for detecting also small amounts of calcium in the coronary arteries. Radiation doses received during a CT study are much lower than angiography Citation[17]. Recent studies using multislice CT associated with the administration of iodinate contrast medium to visualize the coronary artery lumen demonstrated a good accuracy for detection of coronary artery disease Citation[18].
Kiani et al. evaluated coronary calcium by means of helical CT in 200 asymptomatic SLE patients. They found that this population has increased coronary calcium that is significantly related to plasma ADMA levels Citation[6].
Coronary magnetic resonance angiography
This method makes it possible for the noninvasive visualization of the major epicardial coronary arteries in the majority of subjects. It has a high sensitivity, negative predictive value and overall accuracy for detecting coronary artery disease. It is not an exercise-dependent examination.
Compared with CT, coronary magnetic resonance angiography has the advantage of not requiring exposure to ionizing radiation or injection of contrast agents Citation[19].
Angiography
Angiography remains the ‘gold standard’ for diagnosing coronary stenosis allowing for detection of the presence, extent and position of atheromatous lesions. However, its use as a screening tool is limited by the invasiveness and potential high risk Citation[20].
In addition to instrumental diagnostic tools, there is increasing evidence for a strict association between plasma levels of ADMA and cardiovascular disease in autoimmune diseases. ADMA has proven to be a marker of endothelial dysfunction that represents an early stage of atherosclerosis. Increased ADMA plasma levels have been demonstrated in different pathological conditions characterized by high cardiovascular risk, such as hypercholesterolemia Citation[21], hypertrigliceridemia Citation[22], peripheral arterial disease Citation[23], hypertension Citation[24], Type 2 diabetes mellitus Citation[25], acute coronary syndrome Citation[26] and end-stage renal disease Citation[27]. Recently, Petri et al. described higher ADMA levels among SLE patients. In this group, ADMA levels appeared to be associated with coronary calcium and poor prognosis Citation[6].
Conclusion
Patients affected by systemic autoimmune diseases have an increased risk of developing cardiovascular diseases compared with the general population. It becomes mandatory to detect earlier endothelial dysfunction and impaired coronary microcirculation in asymptomatic subjects.
At this moment, coronary angiography remains the gold standard to diagnose coronary stenosis, however, new noninvasive and more reliable diagnostic tools have been introduced in clinical practice to detect subclinical microcirculation abnormalities. In particular, echocardiography with its numerous applications (CFR evaluation, tissue doppler imaging and transesophageal) seems to be the most suitable tool employed as a screening test. It is a noninvasive, reliable, sensitive and specific technique that allows for the identification of preclinical cardiac involvement in systemic autoimmune diseases. Finally, ADMA plasma levels could be used as a simple method to screen individuals at high cardiovascular risk.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Knonkaert DC. Cardiac involvement in systemic inflammatory diseases. Eur. Heart J.28, 1797–1804 (2007).
- Riboldi P, Gerosa M, Luzzana C et al. Cardiac involvement in systemic autoimmune diseases. Clin. Rev. Allergy Immunol.23, 247–261 (2002).
- Del Rincon I, Williams K, Stern MP et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum.44, 2737–2745 (2001).
- Sattar N, Mc Carey DW, Capell H et al. Explaining how ‘high-grade’ systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation108, 2957–2963 (2003).
- Arosio E, De Marchi S, Rigoni A et al. Forearm hemodynamics, arterial stiffness and microcirculatory reactivity in RA. J. Hypertens.25, 1273–1278 (2007).
- Kiani AN, Mahoney JA, Petri M. Asymmetric dimethylarginine is a marker of poor prognosis and coronary calcium in systemic lupus erythematosus. J. Rheumatol.34, 1502–1505 (2007).
- Rexhepaj N, Bajraktari G, Berisha I et al. Left and right ventricular diastolic functions in patients with rheumatoid arthritis without clinically evident cardiovascular disease. Int. J. Clin. Pract.60, 683–688 (2006).
- Caiati C, Zedda N, Montaldo C et al. Contrast-enhanced transthoracic second harmonic echo Doppler with adenosine: a noninvasive, rapid and effective method for coronary flow reserve assessment. J. Am. Coll. Cardiol.34, 122–130 (1999).
- Hozumi T, Yoshida K, Ogata Y et al. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation97, 1557–1562 (1998).
- Rigo F, Richieri M, Pasanisi E et al. Usefulness of coronary flow reserve over regional wall motion when added to dual-imaging dipyridamole echocardiography. Am. J. Cardiol.91, 269–273 (2003).
- Dimitrow PP. Coronary Flow Reserve-Measurement and Application: Focus on Transthoracic Doppler Echocardiography Kluwer Academic Publishers, Boston, MA, USA (2002).
- Rigo F, Gherardi S, Galderisi M et al. The prognostic impact of coronary flow-reserve assessed by Doppler echocardiography in non-ischemic dilated cardiomyopathy. Eur. Heart J.27, 1319–1323 (2006).
- Hirata K, Kadirvelu A, Kinjo M et al. Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis Rheum.56, 1904–1909 (2007).
- Birdane A, Korkmaz C, Ata N et al. Tissue Doppler imaging in the evaluation of the left and right ventricular diastolic functions in rheumatoid arthritis. Echocardiography24, 485–493 (2007).
- Turiel M, Muzzupappa S, Gottardi B et al. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus9, 406–412 (2000).
- Turiel M, Sarzi-Puttini P, Peretti R et al. Five-year follow-up by transesophageal echocardiographic studies in primary antiphospholipid syndrome. Am. J. Cardiol.96, 574–579 (2005).
- Budoff MJ, Georgiou D, Brody A et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation93, 898–904 (1996).
- Achenbach S, Moshage W, Ropers D et al. Value of electron-beam computed tomography for the noninvasive detection of high-grade coronary-artery stenoses and occlusions. N. Engl. J. Med.339, 1964–1971 (1998).
- Kim WY, Danias PG, Stuber M et al. Coronary magnetic resonance angiography for the detection of coronary stenosis. N. Engl. J. Med.345, 1863–1869 (2001).
- Turiel M, Peretti R, Sarzi-Puttini P et al. Cardiac imaging techniques in systemic autoimmune disease. Lupus14, 727–731 (2005).
- Böger RH, Bode-Böger SM, Szuba A et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation98, 1842–1847 (1998).
- Lundman P, Eriksson MJ, Stuhlinger M et al. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J. Am. Coll. Cardiol.38, 111–116 (2001).
- Boger RH, Bode-Boger SM, Thiele W et al. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation95, 2068–2074 (1997).
- Surdacki A, Nowicki M, Sandmann J et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol.33, 652–658 (1999).
- Stuhlinger MC, Abbasi F, Chu JW et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA287, 1420–1426 (2002).
- Bae SW, Stuhlinger MC, Yoo HS et al. Plasma asymmetric dimethylarginine concentrations in newly diagnosed patients with acute myocardial infarction or unstable angina pectoris during two weeks of medical treatment. Am. J. Cardiol.95, 729–733 (2005).
- MacAllister RJ, Rambausek MH, Vallance P et al. Concentration of dimethyl-L-arginine in the plasma of patients with end-stage renal failure. Nephrol. Dial. Transplant.11, 2449–2452 (1996).
