Abstract
Chronic inflammation is suggested to contribute to the Philadelphia-chromosome−negative myeloproliferative neoplasm (MPN) disease initiation and progression, as well as the development of premature atherosclerosis and may drive the development of other cancers in MPNs, both nonhematologic and hematologic. The MPN population has a substantial comorbidity burden, including cerebral, cardiovascular, pulmonary, abdominal, renal, metabolic, skeletal, autoimmune, and chronic inflammatory diseases. This review describes the comorbidities associated with MPNs and the potential impact of early intervention with anti-inflammatory and/or immunomodulatory agents such as JAK-inhibitors, statins, and IFN-α to inhibit cancer progression and reduce MPN-associated comorbidity impact. Early intervention may yield a subset of patients who achieve minimal residual disease, thereby likely reducing the comorbidity burden and improving the cost-effective socioeconomic profile.
The myeloproliferative neoplasms
The classic, chronic Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) are acquired stem cell disorders characterized by clonal myeloproliferation and include essential thrombocythemia (ET), polycythemia vera (PV) and primary myelofibrosis (PMF) Citation[1]. Among the MPNs, varying degrees of bone marrow fibrosis and splenomegaly are common features that are usually most pronounced in PMF. MPN disease progression is associated with exacerbation of disease-specific constitutional symptoms, worsening of patient quality of life (QoL), leukemic transformation and decreased survival Citation[2]. Furthermore, a number of disease-related comorbidities affect patient role functioning and survival, and, until recently, an unmet medical need has existed for patients with MPNs.
Since the discovery of the Janus kinase 2 (JAK2) V617F mutation in 2005, our understanding of the biology of these neoplasms has increased substantially Citation[3]. The JAK2 V617F mutation is found in virtually all patients with PV (98%) and about 50% of patients with ET or PMF. In JAK2 V617F-negative patients with myeloproliferative cancer, other mutations have been detected within the JAK/STAT signal transduction pathway including JAK2 exon 12 mutations (<2%) Citation[4], thrombopoietin receptor (MPL) mutations (5–10%) Citation[5] or lymphocyte-specific adapter protein (LNK) mutations Citation[6]. Several other mutations have been associated with MPNs, including TET2, ASXL1, CBL, IDH1, IDH2 and IKZF1, but their significance with respect to disease initiation, clonal evolution and terminal blast crisis remains to be established Citation[7].
ET, PV and PMF have traditionally been considered to be closely related but distinct diseases with transitional forms Citation[8]. Recently, it has been suggested that the three classic MPNs could be considered to be different phenotypic presentations in a biological continuum from ET to PV to PMF Citation[1,9]. Likewise, prefibrotic myelofibrosis has recently been shown to be aligned along a clinical and biological continuum that includes PMF Citation[10]. Factors such as the accumulation of JAK2 V617F allele burden and increasing genetic instability contribute significantly to disease progression from chronic phase (ET and PV) to accelerated phase (PV with increasing splenomegaly and bone marrow fibrosis and PMF) as well as leukemic transformation Citation[1,11,12].
In this article, the potential impact of early intervention therapy with IFN-α2 and JAK inhibitors on the disease and comorbidity burden in patients with ET, PV and MF is discussed. It is emphasized that an early intervention strategy may be of great importance to inhibit cancer development Citation[9,13–17], in sharp contrast to a ‘wait-and-watch strategy’, whereby intervention occurs when the cancer has given rise to potentially life-threatening cell counts or debilitating complications such as major thrombosis and/or hemorrhage or when age qualifies for cytoreductive therapy (>60 years) Citation[18]. Furthermore, the socioeconomic consequences of an early intervention strategy in patients with MPNs are discussed.
Traditional therapies for MPNs
Patients with ET and PV have a significantly increased risk of arterial and venous thrombosis. In patients with PV, elevated hematocrit plays an independent role in increasing the risk of thrombosis Citation[19]. A recent study confirmed the importance of hematocrit levels in thrombotic risk; in patients with PV, it was shown that those with a hematocrit level of <45% had a significantly lower rate of major thrombotic events and cardiovascular death compared to patients with a hematocrit level of 45–50% Citation[20]. Age >60 years and a history of thrombotic events and other cardiovascular risk factors also increase the risk of thrombosis Citation[21]. There is little evidence that thrombocytosis correlates with thrombosis, whereas both leukocytosis and the presence of the JAK2 V617F mutation seem to be independent risk factors Citation[22]. Thus, traditional risk stratification is based on age >60 years and a history of previous thrombosis.
Low-risk patients in whom there is no contraindication are generally treated with aspirin (100 mg/day) Citation[23]. Abnormal bleeding or hypermetabolic symptoms, itching or mechanical discomfort as a result of splenomegaly are indications for cytoreductive therapy to reduce elevated leukocyte and/or platelet counts as well as the frequency of thrombosis. Conventionally, hydroxyurea (HU) or busulfan has been used as a cytoreductive therapy. Still, the most widely used cytoreductive agent, HU, has been shown to be well tolerated by most patients. In patients with ET and a high risk of thrombosis, HU has been shown to protect against thrombosis Citation[24], and the risk of arterial thrombosis appears to be lower compared with that in patients treated with anagrelide Citation[25]. The evidence basis for HU as a cytoreductive agent that prevents thrombosis in PV is lacking. HU therapy may be associated with potentially serious adverse events, including leg ulcers, which prevent continued therapy. Furthermore, it has been suggested that HU therapy may contribute to the development of skin cancer Citation[26]. There is also increasing concern about the possibility that HU is leukemogenic in patients with MPNs when used as a long-term therapy (>10 years). These serious adverse events have increasingly been reported in a subset of patients who did not achieve a satisfactory hematological response with an HU dose of 2–2.5 g daily Citation[27].
Although no randomized clinical trial has sufficiently demonstrated an increased leukemogenic potential for HU Citation[28], only prospective trials with a long median follow-up (>10 years) can accurately estimate the true leukemic risk because of the extended time period for transformation and development of myelodysplastic syndrome (MDS) or acute myelogenous leukemia (AML) Citation[27].
The only randomized clinical trial with a follow-up of this duration investigated the use of HU compared with pipobroman in patients with PV Citation[27]. After 15 and 20 years of follow-up, the cumulative incidences of leukemic events in the HU arm were 16.5 and 24.2%, respectively. While these rates in the HU treatment arm were roughly one-half of those in the pipobroman treatment arm, they were higher than had previously been reported for HU, further underscoring its potential leukemogenic effect. Moreover, a recent study showed that HU induced de novo copy number variants in normal human cultured fibroblasts, further bringing into question the wisdom of long-term therapeutic use of HU in MPNs and its role in leukemic transformation Citation[29].
Nondrug therapies have also been used to address symptoms of patients with MPNs. For example, in MF, to address the debilitating effects of splenomegaly, splenectomy is occasionally performed in patients who are refractory to other treatments. Although improvements in constitutional symptoms and portal vein hypertension have been observed with splenectomy, and the procedure has been improved, no association with increased survival has been seen Citation[30]. Alternatively, splenic irradiation may relieve symptoms for a short period of time (up to 6 months) but has been associated with life-threatening cytopenias Citation[31,32].
Classically, the only therapy considered to be a potentially curative option for patients with MF is allogeneic stem cell transplantation (ASCT). The benefits of ASCT include regression of bone marrow fibrosis, potential elimination of the mutant clone and potential cure Citation[33]. However, ASCT is only available for a small subset of patients who fit the criteria and are physically able to undergo transplantation. This often limits ASCT to patients younger than 50 years, which is well below the median age at diagnosis (65 years). Furthermore, with such a procedure, there is a risk of transplant-related mortality Citation[18]. Given that the MPNs are associated with chronic inflammation, with the recent advances in the development of specific tyrosine-kinase inhibitors, it is anticipated that these new treatment modalities, alone or in combinations, will likely lead to improved results.
Chronic inflammation & comorbidity burden
Chronic inflammation & cancer
It is well documented that chronic inflammation is involved in the pathogenesis of a variety of cancer types Citation[34,35]. It is also well documented that autoimmune diseases may predispose patients to the development of lymphoproliferative neoplasms Citation[36]. The association between chronic inflammation and myeloid cancer development has not been thoroughly investigated, although it has been shown that MDS may be preceded or accompanied by autoimmune and chronic inflammatory diseases Citation[37]. Additionally, it has recently been suggested that chronic immune stimulation may be instrumental in the development of MDS and AML Citation[38].
As with patients with MDS, patients with ET, PV and PMF often have a history of autoimmune or chronic inflammatory diseases. Indeed, an increased incidence of autoimmunity and infections has been well documented in MF including PMF, post essential thrombocythemia MF and post polycythemia vera MF Citation[39]. Furthermore, a distinct clinicopathological syndrome, primary autoimmune myelofibrosis, has been described Citation[40]. Studies have also documented immunological changes in the bone marrow and peripheral blood Citation[41], which may indicate that immunological mechanisms, such as a chronic inflammatory/immune stimulation, may be of pathogenetic importance.
A modest but significant association between autoimmune or inflammatory diseases and the development of Philadelphia chromosome-negative MPNs has been documented in a large epidemiological study Citation[42], providing further evidence that autoimmune or inflammatory diseases might be of pathogenetic importance for the development of myeloid cancers Citation[38,43]. Most recently, it has been suggested that chronic inflammation may be both a trigger and a driver of clonal evolution, premature atherosclerosis and secondary cancer development in MPNs Citation[15].
Chronic inflammation as the driving force for premature atherosclerosis & development of secondary cancer in MPNs
Based upon epidemiological, clinical and molecular studies on the association between inflammation and development of atherosclerosis, it is reasonable to suggest that chronic inflammation may contribute to the development of MPN-associated atherosclerosis as well Citation[15]. In addition to its role in myeloproliferation, the JAK2 V617F mutation mediates activation of circulating leukocytes, monocytes and endothelial cells Citation[21,22,44], which contribute significantly to thrombotic events Citation[45,46] and may further predispose patients with this mutation to early development of atherosclerosis. Thus, it is the author's clinical experience that even younger patients with MPNs may have atherosclerosis stigmata, which – if confirmed in clinical studies – may be supportive of the hypothesis that these neoplasms may predispose patients to premature atherosclerosis, which has been observed in chronic inflammatory diseases, such as rheumatoid arthritis Citation[47] and lupus erythematosus disseminates Citation[48]. Importantly, an additional clinical implication of MPN-associated chronic inflammation may be the development of secondary cancer, both hematological and nonhematological Citation[49,50].
The clinical complexity of the disease-related symptom & comorbidity burden in MPNs
Several recent studies have investigated symptom burden and QoL in patients with MPNs. In one such study, 456 patients with MF participated in an Internet-based symptom survey, in which the majority reported symptoms that included fatigue, night sweats and pruritus Citation[51]. Importantly, a considerable symptom burden was also reported in ET and PV patients Citation[51]. In the Controlled Myelofibrosis Study With Oral JAK Inhibitor Treatment (COMFORT)-I and COMFORT-II Phase III trials, an analysis of symptom burden of patients with MF at baseline reported similar findings Citation[52–54]. In an analysis of the baseline characteristics of patients enrolled in COMFORT-II prior to treatment randomization, patients with MF had a symptom burden and reduced QoL similar to what has been reported in other cancers such as AML, chronic myeloid leukemia (CML) and breast cancer Citation[55].
It is clear that patients with MF and associated MPNs experience an extensive burden of disease, yet the burden of MPN-related comorbidities is less clear Citation[56–58]. A recent analysis that compared comorbidity rates in a total of 25,145 patients with MF, PV and ET showed that patients with these MPNs displayed higher rates of bacterial and viral infections, cardiovascular complications and anemia compared with matched control patients without cancer Citation[56]. In this study, anemia was recorded in about 30% of patients with ET and about 10% of patients with PV. These figures likely reflect patients with post essential thrombocythemia MF and post polycythemia vera MF being wrongly classified as having ET and PV, respectively, although a subset of patients with ET may actually have anemia. However, the large majority of ET patients with anemia, elevated leukocyte counts and elevated lactic dehydrogenase likely are in the early stage of MF. In a similar study investigating the comorbidity rates in elderly MPN patients, significant increases in cardiovascular events, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, thromboembolism, renal disease, liver disease and infection were observed in patients with all or some of the MPNs investigated (PV, ET, MF and unspecific MPN) compared with matched controls Citation[57]. Lastly, in an analysis of 349 patients with PMF, the most common comorbidities observed included hypertension (31%), diabetes mellitus (DM: 14%) and prior solid tumor (11%). Surprisingly, patient comorbidity score, as assessed by the Adult Comorbidity Evaluation-27 form, was not identified as an independent predictor of survival, whereas age, performance status, platelet count, transfusion requirement and International Prognostic Scoring System score were found to be predictors of survival Citation[58].
Table 1. Comorbidity rates in MF, PV and ET compared with matched controls in a retrospective observational analysis of patients with MPNs.
Several investigations into MPN symptom burden and QoL assessments in patients with MPNs are ongoing, which will further uncover the impact of comorbidities. Such studies have recently been launched in Denmark including Internet-based QoL assessment. This novel design for burden assessment yields a complete, integrated signature of individual patients, capturing symptoms related both to MPNs and MPN-associated comorbidities. Although comorbidity score has not been shown to be an independent predictor of survival in PMF Citation[58], it is reasonable to assume that, in general, morbidity and mortality in MPNs are influenced by cardiovascular, pulmonary and abdominal complications as well as metabolic diseases, such as type 2 DM and osteopenia, likely due to inflammation-induced insulin resistance and inflammation-mediated osteopenia, respectively .
Figure 1. The assortment of comorbidities observed in patients with MPNs. Patients with MPNs display a variety of comorbidities that severely impact patient quality of life and survival. The increased morbidity and mortality in the MPNs are significantly affected by cardiovascular, pulmonary and abdominal complications as well as more global features including peripheral vascular insufficiency and an increased risk of developing fractures. A number of the listed comorbidities are related to the chronic inflammatory state that is associated with the MPNs and progression of disease.
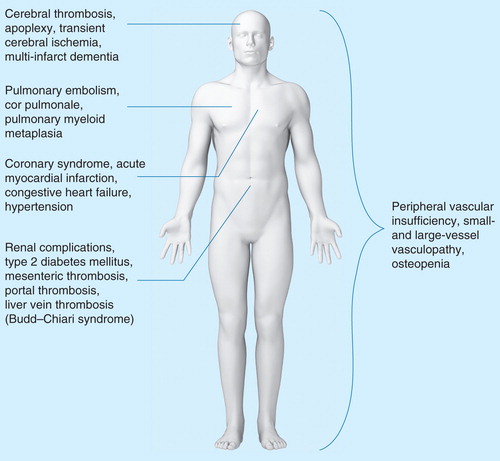
Thrombosis is a leading cause of morbidity and mortality in MPNs, with a reported cumulative rate of thrombotic events of ∼2–3% per patient per year in ET, 3–5% per patient per year in PV and ∼2% per patient per year in MF Citation[59]. The pathogenesis of thrombosis has been attributed to leukocyte, platelet and endothelial cell activation Citation[46,60–62]. Similarly, leukocyte, platelet and endothelial cell activation are characteristic of type 2 DM and has been shown to contribute to the increased risk of thrombosis in DM, apart from the vasculopathy, which is associated with increased blood glucose Citation[63]. Indeed, several common features exist between DM and the chronic MPNs in regard to vascular complications, suggesting that just as normalizing elevated blood glucose levels is an accepted goal in patients with DM, normalizing elevated cell counts in patients with MPNs should be a goal early in disease progression Citation[9]. In both diseases, type 2 DM and MPNs, a ‘wait-and-watch strategy’ would permit disease progression with an increased risk of thrombosis, cardiovascular dysfunction and ultimately multiorgan failure and death.
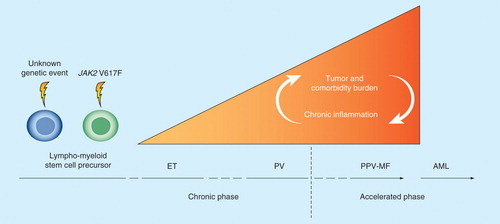
Given the strong association between JAK2 V617F allele burden and disease burden Citation[64], leukocytosis, and risk of developing cardiovascular complications Citation[65], and leukocyte, platelet and endothelial cell activation, and furthermore, the correlation between high tumor burden (ET < PV < MF) and MPN symptom burden Citation[51], it is strongly suggestive that the MPN symptom burden in patients with JAK2 V617F-positive MPNs is dependent on the JAK2 V617F allele burden at a given time, the burden of MPN-associated comorbidities and therapeutic regimen . In the JAK2 V617-negative ET and MF patients, the symptom burden is likewise considerable and reflecting tumor burden.
Figure 2. Tumor burden and comorbidity burden in patients with MPNs. Comorbidity burden increases from early disease stage (ET/PV) to the accelerated phase with myelofibrotic and leukemic transformation. In the JAK2 V617F-positive patient, the red area also depicts the steady accumulation of the JAK2 V617F mutation load, which promotes thrombosis and also induces increasing genomic instability, subclone formation and resistance to treatment. Long-term chronic inflammation induces increasing genomic instability and resistance to treatment and a heavy load of comorbidities, with ensuing impairment of quality of life. The ‘wait-and-watch strategy’ allows the cancer to progress and diminishes the possibility of a favorable outcome. The early intervention concept argues for treating the cancer at an earlier stage, preferably at the time of diagnosis, rather than at a later stage, when complications such as major thrombosis have occurred. The early-intervention concept implies early upfront combinatorial treatment with interferon-α2, a JAK inhibitor and statins.
Therapeutic strategies
The potential role of statins on comorbidity burden in MPNs
The pleiotropic effects of statins include potent anti-inflammatory and immunomodulatory effects Citation[66], and numerous studies have shown that statins reduce cardiovascular morbidity and mortality Citation[67]. Statin therapy has also been shown to inhibit the progression of chronic renal failure in patients with type 2 DM Citation[68]. This effect may be mediated by a reduced expression of TGF-β in mesangial cells and thus results in reduced fibrogenesis Citation[69].
Statin-related anti-inflammatory and immunomodulating effects have also been found in studies of statin treatment for pneumonia and sepsis; statin treatment has been associated with a significant reduction in both morbidity and mortality Citation[70]. The immunomodulatory effects of statins have also been shown in bone marrow transplant patients, in whom the risk of graft-versus-host disease was markedly reduced in patients receiving stem cells from donors treated with statins Citation[71]. Therefore, because of statins' anti-inflammatory, antithrombotic and cytoreductive potential, a rationale may exist for the use of statins, particularly in combination therapies, in the treatment of patients with MPNs and other cancers Citation[72,73]. Supporting this argument are the recent reports on the association of statin use and a reduction in cancer-related mortality in the general population Citation[74] as well as the ability of statins to inhibit MPN cell growth Citation[75]. Statins have also been shown to have a synergistic effect with JAK inhibitors, certainly a very exciting observation in light of the increasing use of combination therapy Citation[15,16,75].
Since hypocholesterolemia in MPNs has been associated with more advanced disease and large tumor burden, potentially having a negative impact on prognosis treatment with a cholesterol-lowering agent might theoretically give rise to some concern. However, a large number of MPN patients are being treated with statins due to cardiovascular events. Although not systematically studied, there have until now been no concern that statins might harm MPN patients. On the contrary, as outlined above, several studies in non-MPN patients have shown that statins are potent anti-inflammatory drugs that also impair oxidative stress, which has been shown to exist in MPN patients. In addition, statins downregulate activated leukocytes, trombocytes and endothelial cells which altogether might decrease the risk of thrombosis in MPNs since raised levels of activated leukocytes and platelets are highly thrombogenic per se Citation[60–62,75].
The impact of IFN-α2 on tumor burden & prospects for the role of IFN in MPN treatment
The beneficial effects of IFN-α, both in the traditional formulation and pegylated IFN, in patients with ET, PV or the hyperproliferative phase of PMF have been convincingly described in several studies Citation[13,17,76,77]. Importantly, it has been recently demonstrated that interferon-α2b (PegIntron) effectively induced deep molecular remissions and, in some patients, resulted in the normalization of abnormal bone marrow morphology Citation[78,79]. Patients who had undergone long-term treatment remained in molecular remission even after treatment discontinuation for up to approximately 4 years Citation[79]. Other patients had negligible or very protracted decline in JAK2 V617F burden Citation[17]. In this subset of patients, chronic inflammation associated with elevated levels of a number of cytokines Citation[16], including TNF-α, which facilitates clonal expansion Citation[80] may result in the inhibition of IFN-α. Furthermore, the protracted molecular effect of IFN-α may be the result of the development of blocking IFN antibodies Citation[81,82].
Most recently, IFN-α2 has been shown to stimulate previously dormant stem cells, resulting in their mobilization from the bone marrow Citation[83], inhibit JAK2 V617F cells at an early differentiation stage Citation[84] and exhaust stem cells with depletion of JAK2 V617F MPN-propagating stem cells Citation[85]; IFN-α2 may also enhance tumor killing and tumor immune surveillance in MPNs by mobilizing regulatory T cells from the bone marrow into circulation Citation[86,87] and upregulating HLA genes, which have been shown to be downregulated in MPNs Citation[88]. These multiple mechanisms suggest that IFN-α achieves molecular remission in patients with MPNs through its effects on MPN stem cells and immune cells, supporting the notion of combinatorial therapeutic approaches by concurrently depleting dormant JAK2 V617F MPN propagating stem cells with IFNα as well as targeting the proliferating downstream progeny with JAK-inhibitors.
In about 10–20% of patients, IFN-α therapy is poorly tolerated and is associated with the development of symptoms compatible with a systemic inflammatory response (SIR) including fatigue, weight loss and fever Citation[13,81,82]. Other patients may develop autoimmune manifestations and require treatment discontinuation. However, as discussed later, the IFN-induced SIR and the development of autoimmunity may actually reflect a positive effect of IFN-α, as has been observed in patients with CML and malignant melanoma Citation[81,82,89,90], which is not noticed and captured because of patient discontinuation.
In Denmark, IFN-α has been used for 10–15 years in the treatment of patients with MPNs, and several reports on IFN-α2 have been published Citation[13,17,78,79,82], demonstrating for the first time that many years of treatment with IFN-α2 can induce minimal residual disease, or an operational cure, in some patients. Based on this experience, a national multicenter study (DALIAH) was launched in February 2012; patients with newly diagnosed ET, PV and hyperproliferative MF are initiating treatment with IFN-α2 (ClinicalTrials.gov identifier: NCT01387763). Furthermore, the observation that prolonged IFN-α2 treatment may induce deep molecular remissions will likely change the conventional risk-based treatment strategies in favor of early therapy intervention with IFN-α2 at the time of diagnosis, when the disease burden is the least and accordingly the chance of a favorable outcome with IFN-α2 treatment the greatest Citation[13,17,81,82,91]. Later in the course of the disease, with increasing disease burden, clonal evolution with genetic instability and development of subclones, a favorable outcome is less likely to be achieved.
The potential impact of JAK-inhibitor treatment on the comorbidity burden
The discovery of the JAK2 V617F mutation, as well as additional mutations that result in JAK/STAT signaling dysregulation in patients with MPNs, has led to the development of a number of JAK1/JAK2 inhibitors. Various preclinical and clinical studies have shown that treatment with JAK1/JAK2 inhibitors resulted in significant spleen reduction and improvement in hypermetabolic symptoms and, accordingly, has been associated with a significant improvement in QoL Citation[92–95].
Ruxolitinib, the first drug in this class, has recently been approved based on the results of the two large Phase III COMFORT trials Citation[94,95]. The impressive effect of ruxolitinib in the reduction of splenomegaly, normalization of proinflammatory cytokines and improvement in patient QoL may result not only in the amelioration of MPN-mediated disease activity, but most likely improvement in inflammation-mediated comorbidities as well Citation[96]. Preliminary data from the Danish MPN cohort that has been treated with ruxolitinib indicate that this treatment is occasionally associated with significant improvement in other inflammation-mediated diseases, such as psoriasis [Hasselbalch HC, Unpublished Data].
Results of studies of JAK1/JAK2 inhibitor therapy in patients with ET and PV have also shown significant reduction in spleen size, decreasing or alleviating the need for phlebotomies and ablation of pruritus and decreasing hypermetabolic symptoms in the vast majority of patients Citation[97,98]. The rapid reduction in spleen size and the decrease in leukocyte and platelet counts may primarily reflect the impact of the JAK1/JAK2 inhibition on the expanded myeloid compartment and the expanded hyperactive immune system. As previously noted, JAK2 V617F allele burden decreased in a subset of patients with PV and MF during treatment with ruxolitinib Citation[97,99]. It is possible that the JAK1/JAK2 inhibitor-mediated allele burden reduction is more easily achieved in the early stages in the biological continuum of the disease.
Preliminary studies of the efficacy and safety of JAK1/JAK2 inhibitors in the treatment of PMF and related diseases have shown very encouraging results. Thus, JAK inhibition may be an option not only for MF patients, but also for patients in early disease stage (ET and PV). These agents are associated with a marked reduction of proinflammatory cytokines and, accordingly, their effects are at least partially explained by their highly potent anti-inflammatory capacity. The reduction of proinflammatory cytokines may also be beneficial in the context of concurrent autoimmune/autoinflammatory diseases, particularly in the advanced MF stage in which the inflammatory cytokines and genes are often massively increased or dysregulated Citation[16]. Prospective studies that investigate these factors will be crucial for our understanding of the mechanisms of action of JAK1/JAK2 inhibitors.
Rationale for combination therapy with IFN & JAK1/JAK2 inhibitors & the potential of modification of MPN burden & associated comorbidity burden
Based on the previously mentioned considerations, a rational therapeutic intervention in patients with MPNs who are intolerant of IFN-α2 or do not respond satisfactorily to IFN-α2 treatment is a combinatorial treatment with a JAK1/JAK2 inhibitor. The reasons for such a combination therapy are several, as follows:
The potent anti-inflammatory effect of JAK1/JAK2 inhibitors may reduce or eliminate the (transient) SIR, likely mediated by the release of inflammatory cytokines in the context of the IFN-α2-mediated tumor killing.
The potent anti-inflammatory effect of JAK1/JAK2 inhibitors may induce significant downregulation of TNF-α, which will likely improve the tumor-reducing effect of IFN-α2 since TNF-α has been shown to facilitate clonal expansion.
Combination therapy may augment the effects of JAK1/JAK2 inhibition as IFN-α blocks the intramedullary release of cytokines from bone marrow stroma that has been shown to protect JAK2 V617F-bearing tumor cells from the JAK1/JAK2 inhibitor-induced tumor killing.
Combination therapy may result in enhancing immune system function via the mobilization of immune cells (immunosuppressive regulatory T cells from the bone marrow into the circulation) primarily mediated by IFN-α.
Combination therapy may prove to be more efficacious than single-agent therapy regarding the above anti-inflammatory actions, but also as IFN-α2 activates dormant stem cells and mobilizes them to be targets for potent JAK1/JAK2 inhibitors.
Combination therapy may be more efficacious by concurrently depleting dormant JAK2 V617F MPN propagating stem cells with IFN-α and targeting the proliferating downstream progeny with JAK2 inhibitors.
Both JAK inhibition and IFN-α2 may be associated with myelosuppression. However, in the context of treating MPN patients (ET, PV and hyperproliferativ MF) with elevated cell counts only (the pancytopenic myelofibrosis patient with severe myelofibrosis not a candidate for IFN-α2 and accordingly neither for combination therapy) myelosuppression is not likely to occur provided that low-dose IFN-α2 (e.g., Pegasys 45 μg × 1 sc/week) and low-dose ruxolitinib (e.g., Jakavi 10 mg × 2/day) is used. Otherwise, combination therapy with IFN-α2 + ruxolitinib is not expected to be associated with any particular risk or side effects. In fact, it is foreseen that the flu-like symptoms during the initial phase of IFN-α2 treatment may actually vanish when IFN-α2 is combined with a potent anti-inflammatory agent as ruxolitinib.
Improvement in comorbidity profile as a result of IFN-α & JAK1/2 inhibitor therapy
It is likely that combination therapy with IFN-α and a JAK1/JAK2 inhibitor will improve MF-related comorbidities, including cardiovascular, pulmonary, renal, metabolic/endocrine, skeletal and inflammatory diseases for a number of reasons. For example, atherosclerosis is an inflammation-mediated disease and the anti-inflammatory effects of ruxolitinib may reduce the impact of MPN-related atherosclerosis. Moreover, patients with impaired cardiac function and/or high blood pressure may benefit from combination treatment, which may improve cardiac output via the elimination or reduction in circulating inflammatory cytokines, with myocardial depressive effects, and a reduction in cardiovascular tone via reduced inflammation in the vessel wall Citation[100]. Similarly, abnormal blood glucose profiles (glycated hemoglobin) in patients with type 2 DM, a chronic inflammatory metabolic syndrome, may likely improve as a result of an improved glucose homeostasis consequent to a decrease in elevated levels of inflammatory cytokines and, accordingly, may lead to amelioration of inflammation-mediated insulin resistance. Combination therapy may likely improve osteopenia and, accordingly, decrease the risk of fractures, which has recently been documented in MPNs Citation[101]. Furthermore, in JAK2 V617-positive patients, combination therapy may be associated with a more rapid reduction in JAK2 V617F allele burden and therefore decrease the risk of thrombosis and disease progression, as the JAK2 V617F mutation is suggested to promote thrombosis and induce genetic instability with an inherent increased risk of subclone formation and development of resistance to therapy, respectively. Most recently, the JAK2 V617F mutation has also been shown to induce the accumulation of reactive oxygen species (ROS) in the hematopoietic stem cell compartment, overproduction of ROS being a mediator of JAK2 V617F-induced DNA damages that might promote disease progression Citation[102].
Discussion, perspectives, expert commentary
MPNs are associated with chronic inflammation and cardiovascular complications. Chronic inflammation is likely of major importance in the development of a number of MPN-related comorbidities, including atherosclerosis, which seems to occur early in some patients, akin to the development of premature atherosclerosis in other chronic inflammatory diseases, such as rheumatoid arthritis, systemic lupus erythematosus, psoriasis and type 2 DM. Accordingly, mechanisms of thrombosis are shared among ET, PV and MF, in which early development of atherosclerosis might also be initiated by chronic inflammation mediated via the release of proinflammatory and prothrombogenic factors from hyperproliferating clonal leukocytes and platelets, which subsequently may also be a driver of clonal expansion Citation[15]. Indeed, the chronic MPNs may be considered as ‘A Human Inflammation Model’. In the context of the biological continuum from early cancer stage (ET/PV) to the advanced MF stage Citation[9] and the reported increased risk of secondary cancer Citation[49], these neoplasms may also be considered as ‘A Human Inflammation Model for Cancer Development’ Citation[14]. Therefore, the anti-inflammatory effects of JAK1/JAK2 inhibitors, alone or in combination with IFN-α and statins, will likely have a great impact on these related comorbidities, particularly when therapy is introduced at early stages of the disease.
JAK1/JAK2 inhibitor therapy has shown extremely promising results in patients with MF with respect to spleen size reduction and improvement in QoL. These improvements likely allow patients to lead more active lives and thus indirectly reduce their risk of related comorbidities. Furthermore, in addition to indirectly affecting MPN-related comorbidities, JAK1/JAK2 inhibitors also have a strong anti-inflammatory effect and therefore are suggested to directly improve the comorbidity profile of patients with MPNs. To this end, ruxolitinib treatment has been shown to have a survival advantage in patients with MF compared with historical controls Citation[103] as well as both placebo and best available therapy Citation[104,105]. In fact, improvement of comorbidity during treatment with a JAK inhibitor may contribute significantly to the prolonged survival.
The interferon battle against cancer
As outlined previously, IFN-α2 has been used in the treatment of patients with MPNs for many years in Denmark and other countries. In these countries, it has been shown that long-lasting treatment with IFN-α2, >5 years, is associated with induction of ‘minimal residual disease’ in a subset of patients with PV as evidenced by reversal of disease to a very early stage, in which patients have normal blood and bone marrow, and the disease is detectable in the blood only with highly sensitive molecular methods. This disease stage, when the patient feels healthy, can last several years even without treatment. These findings have allowed for launching the first randomized study in which all newly diagnosed Danish patients with ET, PV and hyperproliferative MF are offered treatment with either Pegasys or PegIntron (patients aged <60 years) or Pegasys, PegIntron or HU (patients aged >60 years).
However, IFN-α2 therapy may be associated with adverse reactions that necessitate discontinuation of treatment. From observations in the treatment of CML and malignant melanoma, adverse reactions to IFN-α may particularly affect patients who actually benefit from treatment Citation[81,82,89,90]. Adverse reactions, including fatigue, joint/muscle pain and fever, are believed to reflect ‘the interferon battle against cancer.' In this context, various inflammatory cytokines are thought to be responsible for the adverse events. In this time window, when the patient is burdened by inflammation symptoms, it may be rational to combine IFN-α2 treatment with a JAK1/2 inhibitor, for example ruxolitinib, which has a well-documented significant anti-inflammatory efficacy profile.
The ‘wait-&-watch strategy’ versus the ‘early intervention strategy’ in MPNs
Recently, there have been major advances in our understanding of the pathogenesis of MPNs. According to latest WHO classification, the diseases are classified as chronic myeloid neoplasias. These are clonal stem cell disorders, which evolve from a chronic phase with ET and PV to a more accelerated phase in the form of transitional PV and PMF. Therefore, these neoplasms are to be considered in a biological continuum with an inherent risk of development of MF and ultimately leukemic transformation.
The importance of chronic inflammation as one of the potential triggers in the development of MPNs as well as a driver of clonal evolution (from early ET over PV to PMF) has not previously been studied. With regard to the close link between chronic inflammation, atherosclerosis and thromboembolic disease and the fact that MPNs generate increasing chronic inflammation as the clone expands (ET to PV to MF), it is reasonable to assume that these patients have significant and progressive comorbidities, including cardiovascular, thromboembolic, renal, autoimmune and neoplastic diseases, derived from the chronic inflammation. Early diagnosis of these comorbidities will allow for early intervention therapy with anti-inflammatory agents, such as statins and JAK1/2 inhibitors, and cytoreductive and immune-enhancing therapy, such as IFN-α2. Early intervention with these agents is expected to result in improved patient QoL and prognosis Citation[13,15,16], since such a combinatorial approach may reduce patients' risk of developing cardiovascular and thromboembolic disease. In fact, recent studies have shown a very low incidence of thrombosis during treatment with IFN-α2 alone Citation[17,76,77]. Furthermore, by inhibiting clonal myeloproliferation, these early therapies may also prevent myelofibrotic and leukemic transformation Citation[13,15,16]. Importantly, conversion of the traditional ‘wait-and-watch’ strategy based on risk stratification of individual patients to an early intervention strategy is well founded, explicitly in the rationale that cancer is characterized by increasing genomic instability, subclone formation and ultimately metastasis and resistance to treatment. From the perspective that chronic inflammation may be a driver of these neoplasms, early treatment with IFN-α2 and anti-inflammatory agents may induce a state of minimal residual disease in a substantial number of patients. This may alter the natural history of the MPNs and the otherwise inevitable path toward thrombosis, irreversible MF and leukemic transformation Citation[13,15,16].
Figure 3. The MPN care pathway and the effect of early intervention. It is suggested that ET, PV and MF exist on a biological continuum and thus, early intervention with combination therapies including JAK1/2 inhibitors, IFN and/or statins is likely to result in the inhibition of disease evolution. It is important to note that in several centers, according to the EuLeuNet recommendations, younger ET patients without a history of thrombosis are not treated with cytoreductive agents unless the platelet count exceeds 1500 × 109/l or a thrombotic or hemorrhagic event has occurred. Similarly, PV patients may also be treated with aspirin and phlebotomies only rather than with cytoreductive agents.
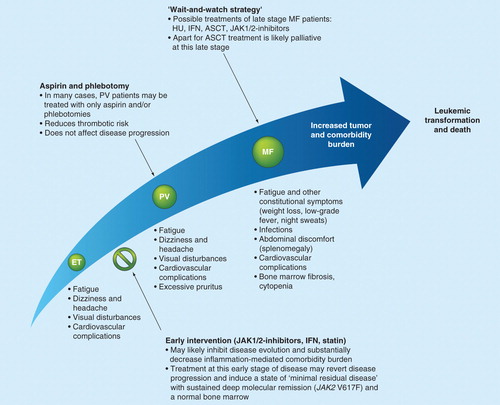
Furthermore, this strategy may reduce or eliminate the increased risk of secondary cancers, as most recently demonstrated in the Danish MPN cohort Citation[49]. Indeed, the most recent report that statins, one of the agents in the proposal for a combinatorial approach, reduce cancer-related mortality in the general population is certainly supportive of this notion Citation[74]. In addition, combinatorial treatment using JAK1/JAK2 inhibitors, statins and IFN-α may enhance the inhibition of clonal myeloproliferation, as most recently, statins have also been shown to inhibit MPN cell growth Citation[75] and exert a synergistic effect with a JAK inhibitor against JAK2 V617F-positive cells Citation[106]. As MPNs are chronic diseases characterized by severe cardiovascular complications, there is an unmet need for additional epidemiological and clinical studies that systematically identify and describe the comorbidity burden. These studies could be powered to monitor the impact and efficacy of early therapeutic intervention with potent anti-inflammatory agents including JAK1/JAK2 inhibitors, statin and IFN-α Citation[15,16]. Ruxolitinib has shown a survival benefit in MF, but its impact is not yet proven in ET and PV. Both agents are costly, and it remains to be shown by formal cost–effectiveness analysis that exposure to these agents for long periods of time is indeed cost effective.
Socioeconomic aspects of treating early-stage MPNs
Patients with ET and PV exhibit shorter survival than the general population due to various complications, such as thrombosis and hemorrhages Citation[11,12,19–22,44–46,59,64,65], and a heavy comorbidity burden Citation[56,57]. Recent studies have clearly shown that a large proportion of patients with ET and PV have a substantial impairment of QoL, which, for some patients, is equal to the QoL of patients with MF and metastatic cancer. Given the average age of patients diagnosed with MPNs and the pronounced impairment of QoL, MPNs greatly impact the working capacity of a large subpopulation. With a high prevalence, MPNs have a major impact upon the socioeconomic profile. Indeed, one study that investigated the medical costs, including inpatient, outpatient and emergency room costs, of patients with MF found that patients with MF incurred approximately five-times the healthcare expenditure compared with matched patients without MF Citation[107].
A population of cancer patients who are not actively treated, but are simply monitored for elevated blood cell counts according to a ‘wait-and-watch strategy’, would likely lead to serious socioeconomic consequences resulting not only from impairment of QoL but also from the increased risk of thrombosis and hemorrhage, both of which are documented even in the early stages of the disease Citation[108]. According to the conventional treatment strategy for MPNs, based on risk stratification, patients with low-risk disease are not offered cytoreductive treatment. Therefore, it is suggested that early intervention with JAK inhibitors, such as ruxolitinib, could profoundly impact the socioeconomic profile of the MPN population by not only improving QoL as a result of direct improvement in the MPN disease burden but also as a result of improvements in the MPN-related comorbidity burden. Accordingly, from a socioeconomic perspective, the capital expenditures with JAK1/JAK2 inhibitors and IFN likely contribute to significant future benefits for patients and society, including recovery of lost earning capacity, rehabilitation and, in the elderly population, diminished costs of social assistance measures costs for treatment of comorbidities may also decrease during JAK inhibitor treatment. Indeed, a personalized risk stratification should be a goal in the decision making when to treat MPNs. Not all patients may need to be treated early. The identification of novel biomarkers will likely help identifying those patients at higher or lower risk for thrombosis, MF transformation and AML. At least with regard to the latter, mutations such as ASXL1 may help identifying those MF patients with an inferior survival.
These issues will be addressed in prospective socioeconomic studies in Denmark on the impact of ruxolitinib on the ‘cost–benefit profile’ in individual patients. In an analysis to quantify the economic value of adding ruxolitinib to a formulary for the treatment of MF, it was shown that ruxolitinib was an economically acceptable treatment option for patients with MF Citation[109]. Since ruxolitinib has already been shown to have a highly impressive efficacy in the resolution of severe psoriasis, a chronic inflammatory disorder, ruxolitinib and similar JAK1/JAK2 inhibitors will likely have a beneficial effect on several complaints associated with concurrent inflammation-mediated comorbidities as well. Although combination therapy as described above is theoretically well founded, it needs to be proven systematically in prospective clinical trials, which should also focus upon potential toxicities using IFN-α2 + statin + ruxolitinib.
Five-year view
Based upon most recent clinical and molecular studies on the efficacy of IFN-α2 to induce deep molecular remissions in patients with ET and PV, novel information on the mechanisms of action of IFN, including exhaustion of MPN stem cells and its immune-enhancing properties, the avenue has been opened for combinatorial treatment with potent anti-inflammatory agents, including JAK1/2 inhibitors and statins. Since these three agents (IFN, JAK2 inhibitor and statins) may have strong synergistic effects as addressed above, it is foreseen that these agents may be one of several combination therapies to be used in the future treatment of MPNs. As in other diseases, statins may very rapidly reduce in vivo activation of leukocytes, monocytes, platelets and endothelial cells and, accordingly, the risk of thrombosis. Furthermore, statins have been shown to impair MPN cell growth and possess synergistic effects with JAK2 inhibitors. To this end, statins may reduce mortality related to second cancer in MPNs, a prospect in which the MPN population is highly relevant taking into account that statin use is associated with a reduced cancer-related mortality in the general population and that MPN patients have an increased risk of second cancer. The combinatorial approach is foreseen to be used, not only in the advanced metastatic cancer stage (MF), but in the early cancer stages (ET and PV) as well, thereby disrupting the vicious circle and prohibiting disease progression. This is in stark contrast to the steady increase in chronic inflammation being elicited by the evolving malignant clone itself if left untreated. Since inflammatory cytokines may inhibit the efficacy of IFN, combination therapy with JAK2 inhibitors and statins as described may also induce a faster reduction in the malignant clone and, accordingly, a much more rapid decline in the JAK2 V617F allele burden. This is critical as the JAK2 V617F mutation is both a promoter of thrombosis and a generator of ROS, the latter giving rise to DNA damage, genomic instability and ultimately mutations. In conclusion, in the 5-year view, a potential cure, at least an operational cure/minimal residual disease, is foreseen in a large proportion of patients with ET and PV, if combination therapy with IFN, JAK2 inhibitor and statins are initiated at the time of diagnosis. In patients with MF, the combination therapy may reverse disease progression with the resolution of bone marrow fibrosis after long-term treatment (3–5 years) and, in a subset of patients, likely result in the induction of minimal residual disease. This new ‘early intervention strategy’ is foreseen to substantially improve QoL by decreasing inflammation-mediated comorbidities and the risk of thrombohemorrhagic complications and furthermore, by improving prognosis by retarding or eliminating progression to MF and leukemic transformation.
Key issues
The Philadelphia-chromosome-negative myeloproliferative neoplasms (MPNs), essential thrombocythemia, polycythemia vera and myelofibrosis, depict a biological continuum with CD34+ cells (‘metastatic cancer cells’) egressing the bone marrow resulting in extramedullary hematopoiesis, primarily in the spleen.
As in numerous different cancers, chronic inflammation is suggested to be critical in the etiology and progression of the MPNs and may play a central role in the development of MPN-related comorbidities as well.
The MPN population displays a range of comorbidities including cerebral, cardiovascular, pulmonary, abdominal, renal, metabolic, skeletal, autoimmune and chronic inflammatory diseases.
Given the recent data of the therapeutic use of JAK inhibitors and Peg-IFN-α2 in patients with MPNs, it is highly suggestive that early intervention therapy will result in not only alleviating the severe disease burden, but may also alter the disease course resulting in increased patient survival.
Proper combinations of JAK inhibitors, Peg-IFN-α2 and/or statins will also address the related comorbidities in patients with MPNs, and, with adequate safety profiles, may likely result in improved patient well-being and positively impact the socioeconomic profile of the MPNs.
Financial & competing interests disclosure
The author has participated in advisory board meetings for Novartis A/S and Sanofi. The author has received research grants from Novartis A/S. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Financial support for medical, editorial and graphic design assistance was provided by Novartis Oncology. The author thanks M Hoelzle (Articulate Science, USA) for his assistance with this manuscript.
Notes
References
- Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med 2006;355(23):2452-66
- Barosi G, Rosti V, Vannucchi AM. Therapeutic approaches in myelofibrosis. Expert Opin Pharmacother 2011;12(10):1597-611
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 2007;7(9):673-83
- Pietra D, Li S, Brisci A, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood 2008;111(3):1686-9
- Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108(10):3472-6
- Lasho TL, Pardanani A, Tefferi A. LNK mutations in JAK2 mutation-negative erythrocytosis. N Engl J Med 2010;363(12):1189-90
- Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 2010;24(6):1128-38
- Pettit JE, Lewis SM, Nicholas AW. Transitional myeloproliferative disorder. Br J Haematol 1979;43(2):167-84
- Hasselbalch HC. Myelofibrosis with myeloid metaplasia: the advanced phase of an untreated disseminated hematological cancer. Time to change our therapeutic attitude with early upfront treatment? Leuk Res 2009;33(1):11-18
- Barosi G, Rosti V, Bonetti E, et al. Evidence that prefibrotic myelofibrosis is aligned along a clinical and biological continuum featuring primary myelofibrosis. PLoS One 2012;7(4):e35631
- Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110(3):840-6
- Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 2010;24(9):1574-9
- Silver RT, Kiladjian JJ, Hasselbalch HC. Interferon and the treatment of essential thrombocythemia, polycythemia vera and myelofibrosis. Expert Rev Hematol 2013;6(1):49-58
- Hasselbalch HC. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis: a human inflammation model for cancer development? Leuk Res 2012;37(2):214-20
- Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012;119(14):3219-25
- Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev 2013;24:133-45
- Larsen TS, Iversen KF, Hansen E, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res 2013;37(9):1041-5
- Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29(6):761-70
- Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet 1978;2(8102):1219-22
- Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368(1):22-33
- Landolfi R, Cipriani MC, Novarese L. Thrombosis and bleeding in polycythemia vera and essential thrombocythemia: pathogenetic mechanisms and prevention. Best Pract Res Clin Haematol 2006;19(3):617-33
- Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost 2007;33(4):313-20
- Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350(2):114-24
- Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332(17):1132-6
- Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353(1):33-45
- Best PJ, Petitt RM. Multiple skin cancers associated with hydroxyurea therapy. Mayo Clin Proc 1998;73(10):961-3
- Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29(29):3907-13
- Spivak JL, Hasselbalch H. Hydroxycarbamide: a user's guide for chronic myeloproliferative disorders. Expert Rev Anticancer Ther 2011;11(3):403-14
- Arlt MF, Ozdemir AC, Birkeland SR, et al. Hydroxyurea induces de novo copy number variants in human cells. Proc Natl Acad Sci USA 2011;108(42):17360-5
- Mesa RA, Nagorney DS, Schwager S, et al. Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer 2006;107(2):361-70
- Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med 2009;60:233-45
- Mesa RA. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood 2009;113(22):5394-400
- Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood 2012;120(7):1367-79
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860-7
- Demaria S, Pikarsky E, Karin M, et al. Cancer and inflammation: promise for biologic therapy. J Immunother 2010;33(4):335-51
- Martin DN, Mikhail IS, Landgren O. Autoimmunity and hematologic malignancies: associations and mechanisms. Leuk Lymphoma 2009;50(4):541-50
- Al Ustwani O, Ford LA, Sait SJ, et al. Myelodysplastic syndromes and autoimmune diseases—case series and review of literature. Leuk Res 2013;37(8):894-9
- Kristinsson SY, Bjorkholm M, Hultcrantz M, et al. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol 2011;29(21):2897-903
- Hasselbalch H. Idiopathic myelofibrosis: a clinical study of 80 patients. Am J Hematol 1990;34(4):291-300
- Pullarkat V, Bass RD, Gong JZ, et al. Primary autoimmune myelofibrosis: definition of a distinct clinicopathologic syndrome. Am J Hematol 2003;72(1):8-12
- Rondeau E, Solal-Celigny P, Dhermy D, et al. Immune disorders in agnogenic myeloid metaplasia: relations to myelofibrosis. Br J Haematol 1983;53(3):467-75
- Kristinsson SY, Landgren O, Samuelsson J, et al. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica 2010;95(7):1216-20
- Anderson LA, Pfeiffer RM, Landgren O, et al. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer 2009;100(5):822-8
- Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109(6):2446-52
- Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol 2005;128(3):275-90
- Vannucchi AM, Guglielmelli P. JAK2 mutation-related disease and thrombosis. Semin Thromb Hemost 2013;39:496-506
- de Groot L, Posthumus MD, Kallenberg CG, Bijl M. Risk factors and early detection of atherosclerosis in rheumatoid arthritis. Eur J Clin Invest 2010;40(9):835-42
- Kahlenberg JM, Kaplan MJ. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus. Arthritis Res Ther 2011;13(1):203
- Frederiksen H, Farkas DK, Christiansen CF, et al. Chronic myeloproliferative neoplasms and subsequent cancer risk: a Danish population-based cohort study. Blood 2011;118(25):6515-20
- Vannucchi AM, Masala G, Antonioli E, et al. Increased risk of lymphoid neoplasms in patients with Philadelphia chromosome-negative myeloproliferative neoplasms. Cancer Epidemiol Biomarkers Prev 2009;18(7):2068-73
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109(1):68-76
- Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2013;31(10):1285-92
- Mesa RA, Shields A, Hare T, et al. Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res 2013;37:911-16
- Harrison CN, Mesa RA, Kiladjian JJ, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol 2013;162(2):229-39
- Kiladjian J, Gisslinger H, Passamonti F, et al. Health-related quality of life and symptom burden in patients with myelofibrosis in the COMFORT-II study. Haematologica (EHA Annual Meeting Abstracts) 2012;97(s1):0378
- Price GL, Pohl GM, Xie J, Walgren RA. A retrospective observational comparison of comorbidities between myeloproliferative neoplasm (MPN) patients and matched controls in a commercially insured US population. Blood (ASH Annual Meeting Abstracts) 2011;118(21):3140
- Davis KL, Price GL, Karve S, et al. Comorbidity burden in elderly persons with non-CML myeloproliferative neoplasms: real-world evidence from a United States Medicare population. Blood (ASH Annual Meeting Abstracts) 2012;120(21):1734
- Naqvi K, Tanaka M, Garcia-Manero G, et al. Patients' comorbidities and overall survival in primary myelofibrosis (PMF). Blood (ASH Annual Meeting Abstracts) 2011;118(21):5164
- Vannucchi AM. JAK2 mutation and thrombosis in the myeloproliferative neoplasms. Curr Hematol Malig Rep 2010;5(1):22-8
- Falanga A, Marchetti M, Vignoli A, et al. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol 2005;33(5):523-30
- Jensen MK, de Nully Brown P, Lund BV, et al. Increased platelet activation and abnormal membrane glycoprotein content and redistribution in myeloproliferative disorders. Br J Haematol 2000;110(1):116-24
- Jensen MK, de Nully Brown P, Lund BV, et al. Increased circulating platelet-leukocyte aggregates in myeloproliferative disorders is correlated to previous thrombosis, platelet activation and platelet count. Eur J Haematol 2001;66(3):143-51
- Bartnik M, Norhammar A, Ryden L. Hyperglycaemia and cardiovascular disease. J Intern Med 2007;262(2):145-56
- Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia 2008;22(7):1299-307
- Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 2010;24(9):1574-9
- Kinlay S, Selwyn AP. Effects of statins on inflammation in patients with acute and chronic coronary syndromes. Am J Cardiol 2003;91(4A):9B-13B
- Lardizabal JA, Deedwania PC. The anti-ischemic and anti-anginal properties of statins. Curr Atheroscler Rep 2011;13(1):43-50
- Gonzalez GL, Manrique CM, Sowers JR. High cardiovascular risk in patients with diabetes and the cardiometabolic syndrome: mandate for statin therapy. J Cardiometab Syndr 2006;1(3):178-83
- Li YB, Yin JJ, Wang HJ, et al. Effect of simvastatin on expression of transforming growth factor-β and collagen type IV in rat mesangial cells. Pharmacology 2011;88(3–4):188-92
- Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther 2011;9(11):1013-33
- Rotta M, Storer BE, Storb RF, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood 2010;115(6):1288-95
- Hasselbalch HC, Riley CH. Statins in the treatment of polycythaemia vera and allied disorders: an antithrombotic and cytoreductive potential? Leuk Res 2006;30(10):1217-25
- Saggini A, Anogeianaki A, Maccauro G, et al. Cholesterol, cytokines and diseases. Int J Immunopathol Pharmacol 2011;24(3):567-81
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2013;368(6):576-7
- Griner LN, McGraw KL, Johnson JO, et al. JAK2-V617F-mediated signalling is dependent on lipid rafts and statins inhibit JAK2-V617F-dependent cell growth. Br J Haematol 2013;160(2):177-87
- Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-α. Cancer 2006;107(3):451-8
- Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112(8):3065-72
- Larsen TS, Bjerrum OW, Pallisgaard N, et al. Sustained major molecular response on interferon alpha-2b in two patients with polycythemia vera. Ann Hematol 2008;87(10):847-50
- Larsen TS, Moller MB, de Stricker K, et al. Minimal residual disease and normalization of the bone marrow after long-term treatment with alpha-interferon2b in polycythemia vera. A report on molecular response patterns in seven patients in sustained complete hematological remission. Hematology 2009;14(6):331-4
- Fleischman AG, Aichberger KJ, Luty SB, et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood 2011;118(24):6392-8
- Hasselbalch HC, Larsen TS, Riley CH, et al. Interferon alpha in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Status and perspectives. Curr Drug Targets 2011;12(3):392-419
- Hasselbalch HC. A new era for IFN-α in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev Hematol 2011;4(6):637-55
- Essers MA, Offner S, Blanco-Bose WE, et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 2009;458(7240):904-8
- Hasan S, Lacout C, Marty C, et al. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNα. Blood 2013;122(8):1464-77
- Mullally A, Bruedigam C, Poveromo L, et al. Depletion of Jak2V617F myeloproliferative neoplasm-propagating stem cells by interferon-α in a murine model of polycythemia vera. Blood 2013;121(18):3692-702
- Riley CH, Jensen MK, Brimnes MK, et al. Increase in circulating CD4+ CD25+ Foxp3+ T cells in patients with Philadelphia-negative chronic myeloproliferative neoplasms during treatment with IFN-α. Blood 2011;118(8):2170-3
- Swierczek S, Kelley TW, King KY, et al. Salutary effect of pegylated interferona in PV and ET as evaluated by quantitation of pre-JAK2V617F and JAK2V617F-bearing stem cells and granulocytes and correlation with circulating regulatory T cells and HSC cell cycle status. Blood (ASH Annual Meeting Abstracts) 2012;120(21):807
- Skov V, Riley CH, Thomassen M, et al. Whole blood transcriptional profiling reveals significant downregulation of HLA class I and II genes in essential thrombocythemia, polycythemia vera and myelofibrosis. Leuk Lymphoma 2013;54(10):2269-73
- Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 2006;354(7):709-18
- Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol 2006;24(19):3164-71
- Hasselbalch HC, Kiladjian JJ, Silver RT. Interferon alfa in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. J Clin Oncol 2011;29(18):e564-5
- Verstovsek S. Therapeutic potential of Janus-activated kinase-2 inhibitors for the management of myelofibrosis. Clin Cancer Res 2010;16(7):1988-96
- Tefferi A. Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N Engl J Med 2012;366(9):844-6
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366(9):799-807
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366(9):787-98
- Mesa RA. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs 2010;13(6):394-403
- Verstovsek S, Passamonti F, Rambaldi A, et al. Durable responses with the JAK1/JAK2 inhibitor, INCB018424, in patients with polycythemia vera (PV) and essential thrombocythemia (ET) refractory or intolerant to hydroxyurea (HU). Blood (ASH Annual Meeting Abstracts) 2010;116(21):313
- Verstovsek S, Passamonti F, Rambaldi A, et al. Long-Term efficacy and safety results from a phase II study of ruxolitinib in patients with polycythemia vera. Blood (ASH Annual Meeting Abstracts) 2012;120(21):804
- Vannucchi A, Kiladjian JJ, Gisslinger H, et al. Reductions in JAK2 V617F allele burden with ruxolitinib treatment in COMFORT-II, a phase 3 study comparing the safety and efficacy of ruxolitinib to best available therapy (BAT) [abstract 0373]. Poster presentation at: the European Hematology Association Annual Meeting; 14–17 June 2012; Amsterdam, Netherlands
- Oikonomou E, Tousoulis D, Siasos G, et al. The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol 2011;52(1):30-40
- Farmer S, Horváth-Puhó E, Vestergaard H, et al. Chronic myeloproliferative neoplasms and risk of osteoporotic fractures; a nationwide population-based cohort study. Br J Haematol 2013;165(5):603-10
- Marty C, Lacout C, Droin N, et al. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia 2013;27(11):2187-95
- Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood 2012;120(6):1202-9
- Verstovsek S, Mesa R, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib treatment in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologic 2013. [Epub ahead of print]
- Vannucchi A, Kiladjian J, Cervantes F, et al. Long-term outcomes from a phase III study comparing ruxolitinib with best available therapy for the treatment of myelofibrosis; a three-year update of COMFORT-II [abstract S1111]. Oral presentation at: the European Hematology Association Annual Meeting; 14 June 2013; Stockholm, Sweden
- Griner LN, McGraw KL, Johnson JO, et al. A mechanistic rationale for the use of statins to enhance JAK inhibitor therapy in MPNs. Blood (ASH Annual Meeting Abstracts) 2011;118(21):2816
- Wang H, Mehta J, Iqbal U, Mesa RA. Analysis of the impact and burden of illness of myelofibrosis in the US. Blood (ASH Annual Meeting Abstracts) 2012;120(21):972
- Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120(7):1409-11
- El Ouagari K, Knight CJ, Mendelson ET. Cost-effectiveness of ruxolitinib versus best-available therapy for medical treatment of myelofibrosis: Canadian Societal Perspective. Blood (ASH Annual Meeting Abstracts) 2012;120(21):4255

