Abstract
Contrast-enhanced computed tomography and MRI are frequently used for the noninvasive diagnosis of hepatocellular carcinoma (HCC). Despite their important role in diagnosis and management of HCC, until recently, there has been no standardized system for their interpretation, reporting and data collection. In 2008, the American College of Radiology convened a committee to develop such a standardized system. This article reviews the role of computed tomography and MRI in the diagnosis and management of HCC; the need for a standardized imaging interpretation system; current HCC imaging criteria included in management guidelines endorsed by the European Association for the Study of Liver, American Association for Study of Liver Diseases, United Network for Organ Sharing and Asian Pacific Association for the Study of the Liver; and the limitations of these criteria. The article then provides an overview of the Liver Imaging Reporting and Data System and discusses future directions.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertgastrohep; (4) view/print certificate.
Release date: 28 February 2013; Expiration date: 28 February 2014
Learning objectives
Upon completion of this activity, participants will be able to:
• Report the role of CT and MRI in the diagnosis and management of HCC
• Provide the limitations of current HCC imaging guidelines addressed by the LI-RADs
• List the LI-RADS category codes
• Review the specificity of LR5 criteria
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Hien Nghiem, MD
Freelance writer and reviewer, Medscape, LLC
Disclosure: Hien Nghiem, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
An Tang, MD, Liver Imaging Group, Department of Radiology, University of California, San Diego; Department of Radiology, University of Montreal, Hôpital Saint Luc, Montreal, Quebec, Canada
Disclosure: An Tang, MD, has disclosed being supported by the Fulbright Program and the Canadian Institutes of Health Research (CIHR Fellowship Award 242199). An Tang, MD is a member of the ACR-supported LI-RADS Committee.
Irene Cruite, MD
Liver Imaging Group, Department of Radiology, University of California, San Diego; Department of Radiology, University of Washington Medical Center, Seattle, Washington
Disclosure: Irene Cruite, MD, has disclosed being a member of the ACR-supported LI-RADS Committee.
Claude B. Sirlin, MD
Liver Imaging Group, Department of Radiology, University of California, San Diego
Disclosure: Claude B. Sirlin, MD, has disclosed being a member of the ACR-supported LI-RADS Committee, the Chair of the American College of Radiology Liver Imaging Reporting And Data System (LI-RADS) Committee. He receives research grant support from General Electric Healthcare. He consults for Bayer Healthcare and is on a Bayer Healthcare speakers bureau.
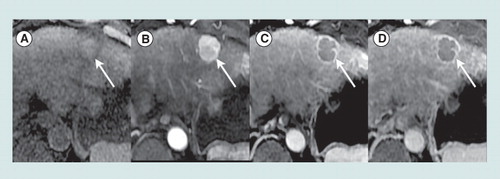
Axial T1-weighted fat-saturated magnetic resonance images obtained precontrast (A) and after injection of an extracellular gadolinium-based contrast agent, in the hepatic arterial (B), portal venous (C) and 5-min delayed (D) phases show a 2.5 cm mass in segment 2 (arrow) of the liver in a 63-year-old woman with cirrhosis due to hepatitis C viral infection. Relative to liver, the mass is mildly hyperintense precontrast, hyperenhances in the arterial phase, and hypoenhances in the portal venous and delayed phases (washout appearances). A peripheral rim of enhancement is evident in the portal venous and delayed phases (capsule appearance). In a patient with cirrhosis or other risk factors for HCC, a mass with these imaging features is categorized LI-RADS 5 (100% certainty observation is HCC).
HCC: Hepatocellular carcinoma; LI-RADS: Liver Imaging Reporting and Data System.
Contrast-enhanced computed tomography (CT) and MRI are frequently used for the noninvasive diagnosis of hepatocellular carcinoma (HCC) Citation[1–4]. Despite the important role of these modalities in the diagnosis and management of HCC, until recently, there has been no standardized system for their interpretation, reporting and data collection. The American Association for the Study of Liver Diseases (AASLD), the United Network for Organ Sharing (UNOS) Citation[101] and other international societies have released HCC management guidelines that include CT and MRI criteria for HCC diagnosis Citation[5–7]. While these guidelines represent important advances in HCC diagnosis and management, the diagnostic criteria included in the guidelines have limitations. Most importantly, the diagnostic criteria do not address the full spectrum of lesions and pseudolesions encountered in patients with cirrhosis or other risk factors for HCC and hence do not provide comprehensive guidance to radiologists in the interpretation of CT and MRI examinations performed in such patients. In addition, the diagnostic criteria do not provide a lexicon of precisely defined imaging terms or an atlas of illustrative examples and so may be prone to inconsistent application by radiologists in clinical practice and by investigators in research studies.
In 2008, the American College of Radiology (ACR) convened a committee of diagnostic and interventional radiologists, surgeons, hepatologists and pathologists to oversee the development of a comprehensive reporting and data system for CT and MRI of the liver in patients with cirrhosis or other risk factors for HCC. Version 1.0 of the resulting Liver Imaging Reporting and Data System (LI-RADS) was completed and released by the ACR in 2011 Citation[102], and an update was released in January 2013 Citation[103]. The current version of LI-RADS (version 2013 [v2013]) addresses the full spectrum of lesions and pseudolesions in the cirrhotic liver, provides a lexicon of controlled imaging terminology, includes an illustrative atlas in development, offers general guidance on optimal CT and MRI technique, examination reporting, and diagnostic workup and management and incorporates some material on non-HCC malignancies. Future versions will include a more comprehensive atlas, a more detailed guidance on imaging technique, examination reporting (including a structured report template), and diagnostic workup, management and more comprehensive material on non-HCC malignancy. Future versions also will address hepatobiliary magnetic resonance (MR) contrast agents and assessment of treatment response, and will include supplementary educational and training materials. Future versions may address contrast-enhanced ultrasound. Eventually, LI-RADS may be expanded to address liver imaging in patients not at risk for HCC.
This article reviews the role of CT and MRI in the diagnosis and management of HCC, the need for a standardized imaging interpretation system, current HCC imaging criteria included in management guidelines endorsed by the European Association for the Study of Liver (EASL), AASLD, Asian Pacific Association for the Study of the Liver (APASL) and UNOS, and the limitations of these criteria. The article then provides an overview of LI-RADS, describes the history of LI-RADS development, reviews LI-RADS category codes and criteria and discusses future directions.
Role of CT & MRI in diagnosis & management of HCC
Early detection of HCC is important because aggressive treatments such as transplantation, resection and ablative therapies of localized-stage tumors improve long-term survival Citation[8]. However, detection of early, potentially curable HCC is not possible by clinical or physical examination findings and conventional laboratory tests are unreliable; detection of early HCC requires imaging surveillance of at-risk patients Citation[9]. For this reason, international societies have developed imaging-based screening and diagnostic guidelines for at-risk patients. The majority of such guidelines endorse ultrasound (US) for screening and surveillance. Contrast-enhanced CT or contrast-enhanced MRI are recommended to more definitively evaluate US-detected lesions, detect HCC missed by US in at-risk patients Citation[1–3], establish the noninvasive diagnosis of HCC Citation[1–4], stage tumor extent Citation[4,10,11], assess the severity of underlying liver disease and portal hypertension Citation[12], inform treatment decisions for HCC Citation[11], assess therapeutic response Citation[9,10,13,14] and determine eligibility and priority for liver transplantation Citation[9,13,15–18]. In addition, in many North American centers, CT and MRI are used for screening and surveillance and not just for evaluation of patients with positive findings at US surveillance.
Need for standardized imaging interpretation
While CT and MRI play critical roles in diagnosis and management of HCC, until recently, there has been no standardization with regard to technical parameters for performing CT and MR examinations, terminology for describing and interpreting liver lesions, structure and content of diagnostic CT and MRI reports or collection of data and monitoring of outcomes.
The lack of standardization has unfavorable consequences. Suboptimal technique may be used for CT and MRI examinations in at-risk patients, rendering images inadequate for accurate diagnosis. Inconsistent and imprecise terminology may be used by radiologists to describe liver lesions identified at CT and MRI, potentially hindering communication with referring providers, introducing within-reader and between-reader variability and leading to interpretation errors. The content of radiology reports may be non-uniform, thereby allowing inadvertent omission from reports of information relevant to patient management. Variable terminology is used in the published liver CT and MRI literature, making meta-analysis difficult and potentially unreliable. Finally, lack of a formal data collection system for liver CT and MRI reports limits the ability to amass large image databases, share data among institutions, perform data mining and monitor outcomes.
Overview of current imaging guidelines for HCC diagnosis
In the past 10 years, 18 consensus clinical practice guidelines have been published worldwide by various international working groups in an attempt to standardize the surveillance, diagnosis and management of HCC Citation[6,7]. Included in these guidelines are CT and MRI criteria for the diagnosis of HCC.
This section focuses on guidelines that have been proposed by major international scientific societies and organizations and whose CT and MRI criteria for the diagnosis of HCC are published in the English literature.
In 2001, the EASL working group proposed imaging criteria for the diagnosis of HCC in its guidelines following the Barcelona EASL Conference in 2000. The EASL guidelines defined the typical appearance of HCC on imaging as arterial phase hypervascularity at dynamic contrast-enhanced imaging Citation[10], regardless of lesion size.
To improve the specificity of the 2001 EASL guidelines and recognizing the diagnostic value of venous or delayed-phase hypoenhancement (‘washout’) as well as the significance of lesion size, AASLD issued a set of consensus guidelines in 2005 Citation[9]. These guidelines defined the typical appearance of HCC on imaging as arterial phase hypervascularity with venous phase ‘washout’ on two coincident dynamic contrast-enhanced imaging modalities for lesions measuring 1–2 cm and on one dynamic contrast-enhanced modality for lesions measuring >2 cm. In an update of the guidelines in 2010 Citation[5], the AASLD definition of typical HCC appearance on imaging remained unchanged, but a second dynamic contrast-enhanced imaging modality was required only if the imaging findings were not diagnostic on the first modality for lesions larger than 1 cm.
In 2008, the APASL developed consensus guidelines on HCC Citation[16]. The APASL criteria are similar to those endorsed by the Japanese Ministry of Health, Labor and Welfare, Japan Society of Hepatology and Asian Oncology Summit Citation[7].
The APASL definition of typical HCC appearance on imaging is comparable with that advocated by the 2010 updated AASLD guidelines, namely, arterial phase hypervascularity and venous phase washout on a single imaging modality, with the exception that the APASL criteria are applicable regardless of lesion size. However, the APASL guidelines address atypical lesions differently from the AASLD guidelines. For hypervascular lesions that do not have washout in the portal or delayed venous phases, the APASL guidelines advocate further evaluation with imaging tests (e.g., perfluorobutane microbubble-enhanced US or superparamagnetic iron oxide-enhanced MRI) that assess Kupffer cell density. The rationale is that HCCs generally have low Kupffer cell density and therefore can be distinguished on such tests from benign hypervascular lesions and pseudolesions, which tend to have normal or elevated Kupffer cell density.
In 2011, UNOS released its Organ Procurement and Transplantation Network (OPTN) imaging criteria (policy 3.6.4.4) for liver transplant candidates with HCC Citation[101]. These criteria were specifically designed to improve specificity for the diagnosis of HCC on imaging and determining eligibility for HCC exception points. Under the criteria, lesions must be classified according to the OPTN classification, with OPTN class 5 lesions corresponding to an imaging diagnosis of HCC. The imaging diagnosis of HCC is based on lesion hyperenhancement on late hepatic arterial images and, depending on lesion size, a combination of other features, including washout on venous/delayed-phase images, late capsule or pseudocapsule enhancement and growth.
In 2012, the EASL and the European Organization for Research and Treatment of Cancer (EORTC) updated the 2001 EASL guidelines Citation[6]. The new guidelines define the typical appearance of HCC on imaging as arterial-phase hypervascularity and portal venous or delayed-phase washout. Only one imaging modality is required for lesions measuring >2 cm. For 1–2 cm lesions, one modality is recommended when imaging is performed in centers of excellence with high-end technology and radiology expertise; two coincident modalities are recommended when imaging is performed outside such centers. The role of microbubble-enhanced US and angiography is considered controversial.
Limitations of current imaging guidelines for HCC diagnosis
The clinical practice guidelines developed by various international working groups are meant to serve as a guide for appropriate clinical decision-making by clinicians. The guidelines address several areas of importance for the management of patients with cirrhosis, including diagnosis, treatment, surveillance, epidemiology and prevention of HCC. While the CT and MRI criteria for HCC diagnosis included in these guidelines represent important advances, the criteria have limitations that potentially limit their utility in clinical practice and research.
The imaging criteria in most of these guidelines focus only on HCC and hence, in effect, categorize lesions in cirrhotic livers in a binary fashion as either diagnostic of HCC or not diagnostic of HCC Citation[5,9,10,15]. The criteria do not comprehensively address the full spectrum of lesions and pseudolesions, from benign to malignant, encountered in the cirrhotic liver at CT and MRI. While focusing on HCC is understandable in the context of the HCC management guidelines under which the criteria are proposed, it provides incomplete guidance to radiologists in the interpretation of CT and MRI findings in at-risk patients. Most at-risk patients have cirrhosis, which is characterized by extensive remodeling of the liver architecture and altered hepatic perfusion. The cirrhotic liver contains a spectrum of cirrhosis-associated nodules that includes cirrhotic nodules, also known as regenerative nodules, low-grade dysplastic nodules, high-grade dysplastic nodules and HCC. Pre-existing vascular lesions such as hemangiomas may be present, and in the setting of cirrhosis, these lesions often develop atypical imaging appearances that may make them difficult to distinguish at CT or MRI from HCC Citation[19]. Many benign pseudolesions also may be encountered in the cirrhotic liver, including arterioportal shunts, nodule-like or heterogeneous fat deposition, confluent fibrosis and hypertrophic pseudomasses; these benign pseudolesions may cause diagnostic confusion Citation[20,21]. Benign portal vein thrombosis is common in cirrhosis and may be mistaken for tumor thrombus caused by vasculo-invasive HCC Citation[22]. Thus, a plethora of non-HCC lesions and pseudolesions may be detected at CT or MRI in the cirrhotic liver. These imaging-detected abnormalities are common – in fact, collectively, they are far more common than HCC lesions. They are frequently diagnostically challenging and may lead to inappropriate management. Nevertheless, they are neglected or incompletely addressed by the HCC diagnostic criteria included in management guidelines. A comprehensive imaging interpretation system should address the full spectrum of imaging abnormalities.
Although the diagnostic criteria for HCC across the various guidelines have some imaging features in common – for example, arterial-phase hyperenhancement and venous or delayed-phase hypoenhancement (washout) Citation[5,9,10,15] – the criteria diverge in other features such as lesion size and growth as well as in the number of required imaging modalities. Furthermore, there are variations across the guidelines with regards to recommended imaging workup for lesions that do not meet diagnostic criteria for hypervascular HCC. For example, AASLD guidelines recommend pathological confirmation of lesions with vascular enhancement patterns atypical for HCC; APASL guidelines recommend use of Kupffer-specific superparamagnetic iron oxides at MRI or perfluorobutane microbubbles (where available) at contrast-enhanced ultrasound; while Japanese guidelines recommend use of gadoxetate-enhanced MRI for assessment of such lesions Citation[13]. Due to such variations and differences, the recommended imaging criteria included in these guidelines do not provide the expected standardization.
The imaging criteria advocated by most guidelines focus primarily on lesion vascularity and do not take into account all the imaging features that modulate the likelihood of HCC. Incorporating imaging features other than vascularity that increase or reduce the likelihood of HCC is important, as the features may improve diagnostic performance. Moreover, radiologists apply these features routinely in their clinical practice. Guiding radiologists in the appropriate application of these features therefore is relevant, but such guidance is not provided by the imaging criteria included in the management guidelines.
The imaging criteria in most current guidelines do not take into account hepatobiliary MR contrast agents, such as gadoxetate. Integration of hepatobiliary-enhanced MRI into diagnostic criteria for HCC is needed. These agents are used frequently in many parts of the world, especially in Asia and parts of Europe, and their use in North America is increasing. These agents may improve the diagnosis of HCC Citation[23–25] as they permit detection and characterization of lesions based on hepatocellular uptake/excretion in addition to vascularity Citation[26].
Current guidelines do not include imaging-based criteria for diagnosis of vascular invasion by HCC despite the importance of vascular invasion in tumor staging and treatment decision-making Citation[27,28]. The guidelines also lack criteria for infiltrative HCC Citation[29], in which imaging features are frequently atypical.
By design, the guidelines are intended mainly for tertiary liver centers where liver imaging is performed and interpreted by radiologists with expertise in liver imaging. The AASLD guidelines, for example, stated that CT and MRI examinations performed in patients in a surveillance program ‘should be read by radiologists with extensive expertise in liver radiology’ Citation[9]. The recent EASL-EORTC guidelines make a distinction between ‘centers of excellence with high-end radiological equipment’ and ‘centers where the technology at disposal or the local skills are not at the high-end level’, recommending one imaging technique for diagnosis of 1–2 cm nodules in the former case and two coincident imaging techniques in the latter Citation[6]. While the desire to have CT and MRI examinations in at-risk patients performed and interpreted in centers of excellence is understandable, many if not most at-risk patients with liver cirrhosis are not imaged at tertiary liver centers, and their liver images are not necessarily interpreted by radiologists with expertise in liver imaging. The development and promotion of a system appropriate for the non-expert as well as expert radiologists is a practical necessity.
Current guidelines lack both a lexicon of precisely defined imaging terms and a corresponding atlas of interpretive illustrations. For example, the AASLD diagnostic algorithm includes the terms ‘arterial hypervascularity’ and ‘venous or delayed-phase washout’, but these terms are not rigorously defined in the guidelines nor do they have standardized definitions in the radiology literature. In an important advance, the OPTN system provides provisional definitions for some essential imaging features of HCC, but the definitions are not accompanied by an illustrative atlas and not all relevant terms are defined. The lack of precise definitions and accompanying illustrative examples for all relevant terms may introduce ambiguity, inconsistency and discrepancy in the application of imaging criteria by clinical radiologists in daily practice and by radiology investigators in research studies and publications.
With the exception of the OPTN system, current diagnostic algorithms also do not provide detailed guidance on the optimal imaging technique or on the content and structure of radiology reports.
Overview of LI-RADS
The ACR-supported LI-RADS is a comprehensive system for standardized interpretation and reporting of CT and MR examinations performed in patients with cirrhosis or other risk factors for HCC Citation[102]. The system attempts to address the aforementioned limitations inherent to the current clinical practice guidelines.
The long-term aims of LI-RADS are to produce a lexicon of controlled imaging terminology and establish diagnostic criteria for image interpretation, standardize the content and structure of radiology reports, institute minimum acceptable technical parameters and create a formal data collection mechanism. It is expected that the successful completion of these aims will improve communication by radiologists with clinicians, reduce variability and error in image interpretation by radiologists, reduce omission of relevant information from radiology reports, reduce the frequency of technically inadequate examinations and facilitate outcome monitoring, performance auditing, quality assurance, research and meta-analysis of published manuscripts.
While the current version of LI-RADS (v2013) focuses on extracellular contrast agents, future versions will incorporate hepatobiliary contrast agents. LI-RADS covers the entire spectrum of benign, potentially malignant and definitely malignant lesions in patients with cirrhosis or other risk factors for HCC. It takes into account imaging features other than vascularity that modulate the likelihood of HCC. LI-RADS provides imaging criteria for vascular invasion by HCC. LI-RADS also provides a comprehensive lexicon of precisely defined imaging terms. To promote consistency and adoption by the radiology community, the LI-RADS terminology is being integrated with RadLex, a web-based term browser that serves as a unified source of radiology terms Citation[30]. LI-RADS includes an atlas of illustrative diagrams and examples; supplementary educational and training materials are being developed. Additionally, LI-RADS is designed for implementation by radiologists with or without expertise in liver imaging, for use both in and outside tertiary liver centers and for application both in clinical practice and research. LI-RADS provides general guidance for the CT and MRI techniques and a rationale for its recommendations. Diagnostic workup guidelines and a template for structured reporting are being developed.
History of LI-RADS development
To develop LI-RADS, in 2008, the ACR convened a committee of diagnostic radiologists with expertise in CT and MR imaging of HCC, interventional radiologists with expertise in locoablative therapy of HCC, transplant hepatologists, liver transplant surgeons, a hepatopathologist, informatics and lexicon experts, members in training and ACR staff members. The LI-RADS Committee began by considering functioning liver imaging systems from two of the committee members’ institutions, the University of California, (CA, USA) and the Thomas Jefferson University (PA, USA). Based on these systems, a provisional set of LI-RADS criteria that differed from both precursors was developed. The provisional LI-RADS criteria were subsequently expanded into a more comprehensive system for the imaging evaluation of all lesions encountered in patients with cirrhosis, spanning the spectrum from benign to malignant.
The LI-RADS criteria then underwent a 3-year iterative process of refinement that included weekly conference calls of a core group of radiologists from three different centers, monthly calls of LI-RADS Steering Committee, annual physical meetings of the entire LI-RADS Committee, active solicitation of feedback from radiologists at various levels of training and with various levels of expertise from multiple institutions around the world, one preliminary round of testing and two formal phases of testing. Through this iterative process of testing–refinement–retesting, the Committee modified the LI-RADS criteria to minimize variation and sources of ambiguity or errors identified by the testing. LI-RADS version 1.0 was completed and launched as a publicly available ACR-sponsored reporting system in March 2011 Citation[102].
In November 2011, UNOS released updated OPTN criteria (policy 3.6.4.4) for liver transplant candidates with HCC Citation[101]. These criteria defined OPTN Class 5 lesions. OPTN Class 1–4 lesions were deferred to LI-RADS. The OPTN 5 and version 1.0 LR5 criteria were similar but not identical. The main difference is that capsule appearance was considered a major feature of HCC in the OPTN system while it was considered an ancillary feature in version 1.0 of LI-RADS. The OPTN system and version 1.0 of LI-RADS also use slightly different thresholds for applying growth as a major feature of HCC. Due to the small but not insignificant discrepancies between version 1.0 LR5 and OPTN 5, the LI-RADS committee modified the LI-RADS criteria to make the LR5 and OPTN 5 categories concordant.
In addition to modified LR5 criteria, the updated version of LI-RADS features numerous enhancements, including an algorithmic display, an atlas in development and some material on non-HCC malignancies that may be encountered in patients with cirrhosis or other risk factors for HCC.
LI-RADS v2013 category codes & criteria
LI-RADS criteria are designed to provide an organized approach to image interpretation and reporting of observations in patients at risk for HCC. LI-RADS v2013 is intended for use only in patients with cirrhosis or other risk factors for HCC; the system should not be applied in patients without cirrhosis or other HCC risk factors as it may not be valid in the general population. Several concepts and principles guide the design of the system.
LI-RADS favors the term observation over the term lesion because some imaging abnormalities (e.g., arterioportal shunts, hypertrophic pseudomasses and artifacts) may represent pseudolesions rather than true lesions. LI-RADS assigns category codes summarizing suspicion levels for HCC to individual CT- or MRI-depicted observations in the liver. Category codes are assigned to individual observations rather than to the entire liver to reflect how HCC staging and treatment decisions are made. To match the diagnostic thought process, the LI-RADS category codes span a five-point ordinal scale ranging from LR1 (definitely benign) to LR5 (definitely HCC) . For each LI-RADS category, appropriate management may differ. In the absence of strong evidence to support rigid follow-up intervals, flexibility in follow-up intervals is permitted.
LI-RADS v2013 lists the types of observations that may appropriately be categorized LR1 (definitely benign) or LR2 (probably benign) and provides guidance for the assignment of the LR1 and LR2 categories, with a description of imaging features expected for each of these types of observations. Observations that are not definitely or probably benign are categorized LR3 (intermediate probability of HCC), LR4 (probably HCC) or LR5 (definitely HCC) based on major imaging features such as whether the observation is or is not a mass, presence or absence of arterial phase hyperenhancement, presence or absence of portal venous phase or delayed-phase washout appearance, presence or absence of capsule appearance and presence or absence of threshold growth. Observations associated with definite tumor in vein are categorized LR5V. LR5 observations that have undergone locoregional treatment are categorized as ‘LR5 treated’. Observations with features suggestive of non-HCC malignancy are categorized as ‘other malignancy’. At the radiologist’s discretion, ancillary-imaging features that favor HCC or that favor benignity may be applied to upgrade or downgrade the category, respectively. The current version of LI-RADS does not specify the exact manner in which radiologists should apply ancillary features to adjust the category code as the required supportive scientific evidence is lacking.
A key concept is that a LR5 categorization is intended to convey near 100% positive predictive value for HCC . Thus, a LR5 categorization indicates that the radiologist is certain not only that the observation is HCC but also that, if resection or explanation were performed, HCC would be identified in the corresponding portion of the pathology specimen. The motivation for seeking near 100% positive predictive value is to prevent false-positive diagnosis of HCC, which can lead to inappropriate transplantation or unnecessary treatment. LR5 observations contribute to radiology T staging and can be treated empirically without biopsy.
To provide the required degree of certainty, LR5 criteria have high specificity for HCC but at the cost of imperfect sensitivity. For example, only observations with hepatic arterial-phase hyperenhancement can be categorized LR5 based on major imaging features. Observations without arterial-phase hyperenhancement cannot be categorized LR5, as the differential diagnosis includes not only hypovascular HCCs but also benign lesions and non-HCC malignancies such as cholangiocellular carcinomas. To maintain high specificity, more stringent criteria are applied to <20 mm than ≥20 mm observations, because <20 mm observations are more likely to result in false-positive interpretations Citation[31]. Thus, unlike the updated AASLD criteria Citation[5] as well as the criteria included in other guidelines Citation[10,17], LI-RADS draws a distinction between <20 mm versus ≥20 mm observations. In addition, <10 mm observations should not be categorized LR5 regardless of other imaging features because subcentimeter nodules may be missed at pathology and, even if detected, are difficult to colocalize on pathology specimens and preoperative images.
Due to the high specificity but imperfect sensitivity, strict application of LR5 criteria will cause some HCCs, especially those that are small and/or hypovascular, to be categorized LR3 or LR4. Radiologists, referring healthcare providers and patients should be aware that LR3 and LR4 categorization does not exclude HCC, and patients with LR3 or LR4 observations would require additional evaluation, which may include biopsy, or close follow up.
LI-RADS v2013 applies to multiphase CT and MRI examinations performed with conventional extracellular contrast materials. MRI with hepatobiliary agents and contrast-enhanced US are not addressed in v2013. The current version of LI-RADS focuses on HCC and on assessment of untreated observations. Provisional material on the evaluation of malignancies other than HCC, such as cholangiocellular carcinoma and metastases, is provided.
LI-RADS future directions
Between version updates, the LI-RADS Committee remains active and provides an ongoing mechanism for further refinement or modification of LI-RADS as knowledge accrues and in response to feedback from users. The Committee is composed of working groups, each charged with specific responsibilities. One working group, for example, is responsible for the continued development and refinement of the lexicon and the atlas, while another is responsible for developing the reporting requirements and producing the structured report template. New working groups recently have been convened to expand LI-RADS to address hepatobiliary MR agents and assessment of treatment response. Together, the various working groups are developing supplementary educational materials. To promote collaboration, cross-fertilization and coordination with RadLex and UNOS, several LI-RADS committee members are active or past members of RadLex subspecialty lexicon development committees or of the UNOS imaging working group.
Future versions of LI-RADS will incorporate the progress made by the working groups and will feature an expanded atlas; more detailed guidance on optimal imaging technique, diagnostic workup and management and examination reporting including a structured report template; more comprehensive discussion of non-HCC malignancy; and supplementary educational and training materials. Future versions will also include criteria applicable to MRI performed with hepatobiliary agents as well as criteria for assessing locoregional and systemic treatment response. LI-RADS currently focuses on CT and MRI in patients at risk for HCC. In the future, the scope may be expanded to include contrast-enhanced US and, it is hoped, to observations in noncirrhotic liver. In parallel with these refinements, it is anticipated that the LI-RADS lexicon and category codes eventually will be integrated into radiology information systems and picture archiving and communications systems. Such integration will provide a mechanism for the creation of prospective registries that will inform continued evidence-based refinements in radiology criteria and management guidelines.
Expert commentary
In this brief review, we cover the important role of CT and MRI in the diagnosis and management of HCC and emphasize the need for standardized imaging interpretation. In recent years, numerous international societies and clinical networks have developed clinical practice guidelines for HCC and these guidelines have included CT and MRI criteria for the noninvasive diagnosis of HCC. While these guidelines represent important advances in HCC diagnosis and management, the diagnostic criteria included in the guidelines do not address the full spectrum of lesions and pseudolesions encountered in patients with cirrhosis or other risk factors for HCC and hence provide only partial guidance to radiologists in the interpretation of CT and MRI examinations performed in at-risk patients. Moreover, as the criteria from the various guidelines have discrepancies and are not accompanied by a lexicon of precisely defined imaging terms or an atlas of illustrative examples, the criteria may be applied inconsistently by radiologists in clinical practice and by investigators in research studies. LI-RADS is an ACR-supported initiative to develop a comprehensive, standardized reporting and data collection system for CT and MRI of the liver in patients with or at risk for HCC. LI-RADS is the first system to address the full spectrum of observations, from benign to malignant, encountered in patients at risk for development of HCC and it thus fills a gap not previously addressed. LI-RADS was released in 2011 and updated with numerous enhancements in January 2013. The current version focuses on extracellular agents and future versions will be expanded to include hepatobiliary agents. An illustrative atlas has been developed and will continue to be improved and expanded.
The implementation and continued refinement of LI-RADS are important. LI-RADS are the most comprehensive image interpretation and reporting system developed to date for liver imaging and through the ongoing effort of the standing ACR-supported LI-RADS Committee, it will be further expanded, refined and improved in response to feedback from users and as knowledge advances. We encourage radiologists, hepatologists, surgeons, oncologists and pathologists to become familiar with LI-RADS, to apply the system in clinical practice and clinical research studies, and to provide feedback to the LI-RADS committee to guide further refinement. Eventually it may be possible to unify LI-RADS and the various imaging criteria endorsed by different societies and organizations into a single diagnostic system. We believe that having a unified diagnostic system, rather than separate competing systems, for the reporting of CT and MRI examinations for HCC surveillance will enhance the care of patients with or at risk for HCC, facilitate research and advance the field.
Five-year view
The authors offer the following predictions for the areas where they expect to see significant progress in the next 5 years toward standardized reporting and data collection for liver imaging in patients with or at risk for HCC:
• Unification of LI-RADS and imaging criteria endorsed by different international societies and clinical networks into a single diagnostic system that comprehensively addresses the full spectrum of lesions and pseudolesions encountered in patients with or at risk for cirrhosis, which is applicable to hepatobiliary as well as extracellular contrast agents, and which includes a lexicon of precisely defined terminology and an illustrative atlas;
• Development of standardized imaging criteria, terminology and illustrative atlas to assess HCC treatment response to locoregional ablative therapies such as radiofrequency ablation, microwave ablation, cryoablation and transarterial chemoembolization as well as to systemic therapy;
• Integration of the unified system into clinical practice guidelines endorsed by the AASLD, UNOS and other international societies and clinical networks;
• Integration of the unified system into radiology information systems and picture archiving and communications systems;
• Adoption of the unified system into clinical practice and research;
• Creation of national and international prospective registries with data collected using the unified system;
• Prospective scientific validation of the system;
• Continued refinement of the system based on user feedback and advances in knowledge made possible by the registries and by prospective studies.
Table 1. Summary of Liver Imaging and Reporting Data system (version 2013) category codes.
Key issues
• Contrast-enhanced computed tomography (CT) and MRI play important roles in the diagnosis, staging and management of hepatocellular carcinoma (HCC).
• Despite the important role of these modalities, until recently, there has been no standardized system for their interpretation, reporting and data collection.
• Numerous organizations have released HCC management guidelines that include CT and MRI criteria for HCC diagnosis. While these guidelines represent important advances in patient care, the diagnostic criteria included in the guidelines have limitations.
• One important limitation is that these criteria do not address the full spectrum of lesions and pseudolesions encountered in patients with cirrhosis or other risk factors for HCC, and hence do not provide comprehensive guidance to radiologists in the interpretation of CT and MRI examinations performed in such patients.
• Another limitation is that the diagnostic criteria do not provide a lexicon of precisely defined imaging terms or an atlas of illustrative examples and, therefore, may be prone to inconsistent application by radiologists in clinical practice and by investigators in research studies.
• Liver Imaging Reporting and Data System (LI-RADS) is an ACR-supported initiative to develop a comprehensive, standardized reporting and data collection system for CT and MRI of the liver in patients with or at risk for HCC.
• LI-RADS is the first system to address the full spectrum of observations, from benign to malignant, encountered in patients at risk for development of HCC. It also provides a lexicon of precisely defined imaging terms and an atlas in development. LI-RADS thus fills a gap not previously addressed.
• LI-RADS will continue to be expanded, refined and improved in response to feedback from users and as knowledge advances.
References
- Taouli B, Krinsky GA. Diagnostic imaging of hepatocellular carcinoma in patients with cirrhosis before liver transplantation. Liver Transpl. 12(11 Suppl. 2), S1–S7 (2006).
- Krinsky G. Imaging of dysplastic nodules and small hepatocellular carcinomas: experience with explanted livers. Intervirology 47(3–5), 191–198 (2004).
- Burrel M, Llovet JM, Ayuso C et al.; Barcelona Clínic Liver Cancer Group. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology 38(4), 1034–1042 (2003).
- Hanna RF, Kased N, Kwan SW et al. Double-contrast MRI for accurate staging of hepatocellular carcinoma in patients with cirrhosis. AJR. Am. J. Roentgenol. 190(1), 47–57 (2008).
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 53(3), 1020–1022 (2011).
- European Association for the Study of the Liver and European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 56(4), 908–943 (2012).
- Song P, Tobe RG, Inagaki Y et al. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 32(7), 1053–1063 (2012).
- Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population. Am. J. Surg. 195(6), 829–836 (2008).
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 42(5), 1208–1236 (2005).
- Bruix J, Sherman M, Llovet JM et al.; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 35(3), 421–430 (2001).
- Ito K, Mitchell DG, Siegelman ES. Cirrhosis: MR imaging features. Magn. Reson. Imaging Clin. N. Am. 10(1), 75–92, VI (2002).
- Brancatelli G, Federle MP, Ambrosini R et al. Cirrhosis: CT and MR imaging evaluation. Eur. J. Radiol. 61(1), 57–69 (2007).
- Kudo M, Izumi N, Kokudo N et al.; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig. Dis. 29(3), 339–364 (2011).
- Memon K, Kulik L, Lewandowski RJ et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 141(2), 526–535, 535.e1 (2011).
- Freeman RB, Mithoefer A, Ruthazer R et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 12(10), 1504–1511 (2006).
- Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 21(Suppl. 5), v59–v64 (2010).
- Omata M, Lesmana LA, Tateishi R et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 4(2), 439–474 (2010).
- Pomfret EA, Washburn K, Wald C et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 16(3), 262–278 (2010).
- Brancatelli G, Federle MP, Blachar A, Grazioli L. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology 219(1), 69–74 (2001).
- Jeong YY, Yim NY, Kang HK. Hepatocellular carcinoma in the cirrhotic liver with helical CT and MRI: imaging spectrum and pitfalls of cirrhosis-related nodules. AJR. Am. J. Roentgenol. 185(4), 1024–1032 (2005).
- Baron RL, Peterson MS. From the RSNA refresher courses: screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics 21, S117–S132 (2001).
- Catalano OA, Choy G, Zhu A, Hahn PF, Sahani DV. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology 254(1), 154–162 (2010).
- Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255(2), 459–466 (2010).
- Motosugi U, Ichikawa T, Sou H et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology 256(1), 151–158 (2010).
- Lee S, Kim SH, Park CK, Kim YS, Lee WJ, Lim HK. Comparison between areas with Gd-EOB-DTPA uptake and without in hepatocellular carcinomas on Gd-EOB-DTPA-enhanced hepatobiliary-phase MR imaging: pathological correlation. J. Magn. Reson. Imaging 32(3), 719–725 (2010).
- Schneider G, Reimer P, Mamann A, Kirchin MA, Morana G, Grazioli L. Contrast agents in abdominal imaging: current and future directions. Top. Magn. Reson. Imaging 16(1), 107–124 (2005).
- Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur. J. Surg. Oncol. 34(8), 900–905 (2008).
- Takizawa D, Kakizaki S, Sohara N et al. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig. Dis. Sci. 52(11), 3290–3295 (2007).
- Kanematsu M, Semelka RC, Leonardou P, Mastropasqua M, Lee JK. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J. Magn. Reson. Imaging 18(2), 189–195 (2003).
- Langlotz CP. RadLex: a new method for indexing online educational materials. Radiographics 26(6), 1595–1597 (2006).
- Forner A, Vilana R, Ayuso C et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 47(1), 97–104 (2008).
Websites
- OPTN/UNOS policy 3.6.4.4. http://optn.transplant.hrsa.gov/policiesAndBylaws/policies.asp
- American College of Radiology. Liver Imaging Reporting and Data System version 1.0. www.acr.org/Quality-Safety/Resources/LIRADS/Archive/
- American College of Radiology. Liver Imaging Reporting and Data System version 2013.1. www.acr.org/Quality-Safety/Resources/LIRADS/.
Toward a standardized system for hepatocellular carcinoma diagnosis
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertgastrohep. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. A 50-year-old man presents to your clinic for follow-up of liver ultrasound. On his last visit, the patient was found to have significantly elevated liver enzymes on a routine laboratory examination. He has a history of hepatitis C and an extensive history of alcohol abuse. His ultrasound findings reveal a lesion suspicious for hepatocellular carcinoma (HCC). You recommend he obtain a contrast-enhanced CT of the liver. The patient inquires what more information will be obtained with a CT. You inform him the results may provide the following:
□ A Predict the symptoms that he will develop
□ B Determine his prognosis
□ C Stage tumor extent
□ D Determine how long the lesion has been there
2. A few weeks later, you receive the CT results of your patient in question 1. The results were reported according to the LI-RADS criteria. In contrast to other international HCC imaging guidelines, the LI-RADS addresses:
□ A The full spectrum of lesions and pseudolesions in the cirrhotic liver
□ B Provides a lexicon of controlled imaging terminology
□ C General guidance on optimal CT and MRI technique
□ D All of the above
3. The CT results of your patient reveal that he has a lesion consistent with LR5. This indicates that the lesion is:
□ A Probably benign
□ B Intermediate probability of HCC
□ C Probably HCC
□ D Definitely HCC
4. The CT results of your patient reveal that he has a lesion consistent with LR5. This indicates that the lesion is:
□ A Probably benign
□ B Intermediate probability of HCC
□ C Probably HCC
□ D Definitely HCC
