Abstract
Immunomodulator drugs (IMiDs) have contributed to the significant improvement observed in the survival of myeloma patients since the introduction of novel targeted therapies. The introduction of IMiDs in the myeloma pipeline has also significantly increased the incidence rate of thromboembolic events, either venous and/or arterial. The observation of an increasing number of deep venous thrombosis makes it compulsory to discuss prophylactic options in myeloma, particularly in those treated with IMiDs as immunomodulatory drugs. Although an attempt to propose guidelines was performed, it is clear that several questions remain unanswered, starting with the choice of the thromboprophylaxis and the definition of risk factors. We have reviewed evidence that might help to decide the optimal thromboprophylaxis, and also highlighted the unresolved area where further study is warranted.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/experthematology; (4) view/print certificate.
Release date: 7 December 2012; Expiration date: 7 December 2013
Learning objectives
Upon completion of this activity, participants will be able to:
• Analyze the risk for thromboembolic events associated with multiple myeloma (MM).
• Assess how the use of immunomodulator drugs (IMiDs) affects the risk for thromboembolic events among patients treated for MM.
• Distinguish the treatment most likely to be effective in preventing thromboembolic events among patients treated with IMiDs for MM.
• Evaluate the strengths and weaknesses of different treatments to prevent thromboembolic events among patients treated with IMiDs for MM.
Financial & competing interests disclosure
Editor
Elisa Manzotti
Publisher, Future Science Group, London, UK
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME Author
Charles P Vega, MD
Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA
Disclosure: Charles P Vega, MD, has disclosed no relevant financial relationships.
Authors and Credentials
Eileen M Boyle, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Eileen M Boyle, MD, has disclosed no relevant financial relationships.
Guillemette Fouquet, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Guillemette Fouquet, MD, has disclosed no relevant financial relationships.
Salomon Manier, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Salomon Manier, MD, has disclosed no relevant financial relationships.
Jordan Gauthier, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Jordan Gauthier, MD, has disclosed no relevant financial relationships.
Marie Pierre Noel, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Marie Pierre Noel, MD, has disclosed no relevant financial relationships.
Claire Borie, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Claire Borie, MD, has disclosed no relevant financial relationships.
Thierry Facon, MD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Thierry Facon, MD, has disclosed no relevant financial relationships.
Ismail Elalamy, MD, PhD
Service d'Hématologie Biologique Hôpital Tenon, ER2 UPMC, Paris, France
Disclosure: Ismail Elalamy, MD, PhD, has disclosed the following relevant financial relationships: received lecture fees and grant research from LeoPharma.
Xavier Leleu, MD, PhD
Service des Maladies du Sang, Hôpital Huriez, CHRU, Lille, France
Disclosure: Xavier Leleu, MD, PhD, has disclosed the following relevant financial relationships: received lecture fees and grant research from Celgene,
Janssen, Onyx, Novartis, Amgen and LeoPharma.
LMWH: Low-molecular-weight heparin.
Patients with cancer have a four to sixfold increased risk of thrombosis, mainly venous thrombosis Citation[1]. In some hematological malignancies, that risk is increased by 20-fold Citation[2]. Multiple myeloma (MM) is the second most common hematological malignancy. Patients with MM have a higher risk of thrombosis than the general population, but the incidence of spontaneous (without treatment) thromboembolic disease remains below 5%. Since the introduction of thalidomide therapy for MM, clinical trials have reported a higher incidence of thrombotic events of up to 30% in some studies. Myeloma has subsequently become the leading hematological malignancy in terms of venous thrombosis risk Citation[3]. The increased incidence of venous thromboembolisms (VTE), deep venous thrombosis with or without pulmonary embolism (PE), necessitates discussion of prophylactic strategies in patients treated with immunomodulator drugs (IMiDs). Several issues remain unanswered, including the optimal thromboprophylaxis for IMiDs used as monotherapy, the definition of VTE high-risk factors which are controversial, and the role of aspirin as a VTE prophylaxis in MM treated with an IMiD-based combination. This review summarizes the current knowledge on thromboembolic disease in IMiDs based treated myeloma and highlights zones where more work is needed before we can provide evidence-based recommendations.
Pathophysiology of thrombosis in MM patients treated with IMiDs
Hypercoagulable states have been described in plasma cell dyscrasias without being specifically related to MM. Indeed, the occurence of thromboembolic disease is estimated around 7% in monoclonal gammapathy of undetermined significance Citation[4,5], whereas it is roughly 10% in MM. Several mechanisms involving complex pathways are implicated, including either the paraprotein or the related tumor cells and all plasmatic and cellular hemostasis actors. First, it was shown that the M-protein interacts with platelets, enhancing platelet adhesion Citation[6] and aggregation. Further, data suggested that the M-protein increased the amount of fibrin protofibrils, therefore interacting with the process of fibrin assembling. In MM, the interaction between the tumor cells and their microenvironment, including the endothelial wall, may explain the increased expression of factor VIII (FVIII) and von Willebrand Factor, related to the neovascularization of the tumor-infiltrated bone marrow. Other mechanisms have been reported such as a procoagulant autoantibody activity or an increase incidence of protein C resistance Citation[7].
Thalidomide has well demonstrated anti-inflammatory and antiangiogenic activities. Lenalidomide has a more potent immunomodulatory effect than thalidomide. Both drugs also increase plasma levels of FVIII Citation[8] and von Willebrand Factor and induce an acquired resistance to protein C activity, particularly during the first 2 months of treatment Citation[7,9]. They also reduce soluble thrombomodulin plasma levels Citation[10]. Unlike anthracyclines, thalidomide does not have a direct effect on endothelial cells Citation[8–11], but it may increase the expression of membrane surface proteins on endothelial cells such as protease-activated receptors Citation[11]. A higher incidence of constitutional prothrombotic abnormalities was questioned in MM treated with IMiDs presenting thrombosis, but not confirmed Citation[8]. A recent study proposed platelet activation related to granular release of proteolytic enzymes from immature leucocytes progenitors after the differentiation blockade induced by lenalidomide Citation[12].
Incidence of thrombosis in MM patients treated with IMiDs
Single-agent IMiD therapy
Using IMiDs as a single therapy Citation[13–21], the risk of thrombosis is low . In one of the first series reporting thalidomide use in MM patients, none of the 84 patients presented thrombosis even if they were treated with doses ranging from 200 to 800 mg daily. The incidence rate of thrombosis, mainly venous but also arterial, remained below 5% in subsequent studies using IMiDs as a single therapy, regardless of the number of previous lines of treatment, the treatment response or refractoriness of the disease and the tumor mass . However, the incidence of thrombosis was 6% without thromboprophylaxis in one of the two multicentric Phase III trials of lenalidomide used as MM maintenance therapy (with doses of 10–15 mg daily Citation[22,23]). Overall, although low the incidence rate of VTE events persisted when using IMiDs as single agent, and therefore should warrant systematic VTE prophylaxis.
Combined IMiDs-based therapy
Several studies have shown that thrombosis occurred mainly when IMiDs, either thalidomide or lenalidomide or pomalidomide, were used in combination with high-dose dexamethasone, anthracyclines or any chemotherapy (Tables 2 & 3); (Box 1) Citation[24,25]. It was also reported with other treatment associations Citation[25–30] such as alkylating agents, melphalan and cyclophosphamide, but with a lower incidence ranging from 3 to 9% Citation[30,31]. The incidence of thrombosis ranged from 9 to 20% without prophylaxis in the different Phase III trials comparing a melphalan–prednisone–thalidomide regimen to the historical melphalan–prednisone in front line therapy in elderly patients and in patients not eligible for autologous stem-cell transplantation. The median delay of thrombosis occurrence was 3 months, with a range of 2 to 6 months Citation[27,28]. There was no evidence supporting a correlation between the tumor mass and the increased thrombotic risk. Finally, the incidence of thrombosis was reduced in the relapse setting but thalidomide doses used were also lower. Although most of the studies reported mainly VTE events, there is also a less important but clear increase of arterial thrombosis incidence in MM-treated patients Citation[28]. The majority of trials, combining thalidomide found that the incidence of thrombosis dropped to less than 10% as soon as thromboprophylaxis was established Citation[24,26,27,31].
In the two multicentric Phase III trials in relapsed MM that led to lenalidomide approval, the ≥grade 3 adverse events included 11 and 15% VTE in absence of thromboprophylaxis in the two studies, respectively Citation[32,33]. These studies used high-dose dexamethasone for the first four cycles followed by low-dose dexamethasone thereafter. Thrombosis incidence was independent to the prior line of treatment with thalidomide or the number of previous lines of therapy, and the length on lenalidomide association did not influence the occurrence of thrombotic events. These results were then confirmed in an observational, multicentric study conducted in the USA and Canada among 1500 MM patients with relapsed MM receiving this combination for a median duration of 15 weeks Citation[34]. Prophylaxis was recommended and the incidence of thrombosis dropped to 8%. Since then several combinations with lenalidomide have been evaluated, and the thrombotic risk remained consistently below 10% with thromboprophylaxis Citation[24,27,35]. Studies conducted with pomalidomide did not show any increase of thrombosis occurrence with low-dose dexamethasone and systematic antithrombotic prophylaxis, so far Citation[36–38].
Beyond the necessity of thromboprophylaxis for all patients treated with IMiD combination, it was shown that dose reduction of dexamethasone significantly decreased the thrombosis occurrence Citation[24,27,31].
No protective effect of bortezomib in IMiD-based combinations
A review of Phase III trials combining IMiDs to bortezomib Citation[39] suggested a lowering of the expected number of thrombosis in MM. These data should nevertheless be handled with care since several studies have reported an excess of thrombotic events in IMiD regimens in combination to bortezomib, despite systematic recommended thromboprophylaxis Citation[40,41].
Impact of thrombosis on survival with IMiD-based therapy
The occurrence of thrombotic event does not seem to have an impact on the evolution or outcome of the MM disease. A clinical trial analyzed the impact of venous thrombosis on survival in a cohort of 668 patients treated with or without thalidomide Citation[2,42]. Most of the thrombotic events (95%) occurred within the first year after the treatment initiation. Overall survival and event-free survival were equivalent to those of patients who did not develop such a complication. It is worth noting that thalidomide was restarted with curative anticoagulant therapy after thrombosis in 75% of patients without further thrombosis.
Role of recombinant erythropoietin
There are evidence supporting the hypothesis that recombinant erythropoietin (EPO) might increase thrombosis due to its effect on increasing tissue factor expression Citation[43] and the induction of a hyperfibrinolytic state Citation[44]. The two registration trials of lenalidomide in relapsing MM patients are very informative in this matter. The incidence of thrombosis varied in both studies, 8.5% in the non-US trials and 15% in the US trial, while no difference was found in terms of lenalidomide association, however patients received more EPO in the US study, 23 versus 5% respectively Citation[32,33]. A recent study Citation[45] has showed that prophylactic EPO, thalidomide therapy and history of VTE, but not higher hemoglobin levels, were found to increase the risk of VTE among newly diagnosed myeloma receiving multiagent chemotherapy. It was also found a significant and independent marker in MELISSE and the study from Larocca and colleagues Citation[46,47]. Nevertheless, these data were not confirmed in other trials Citation[35,48] and the risk of thrombosis with EPO was mainly incriminated to high levels of hemoglobin above 13 g/dl. Facing these conflicting results, it is difficult to conclude whether EPO might increase the incidence rate of VTE in MM patients treated with IMiDs and low-dose dexamethasone when associated with an efficient VTE prophylaxis, but one might recommend systematic low-molecular-weight heparin (LMWH) prophylaxis. These studies have demonstrated that a systematic thromboprophylaxis is recommended in MM treated with IMiDs, especially in combination.
Thrombosis prophylaxis available in myeloma
The CLOT trial has significantly enhanced the understanding of thrombophylaxis in cancer patients Citation[49]. Nevertheless, only 10% of the included patients had hematological malignancies and the specificities previously described of thrombosis in myeloma, do not allow us to draw definite conclusions as far as thrombophylaxis for the myeloma treated with IMiDs patients. Current recommendations consider several drugs as suitable for thromboprophylaxis in myeloma treated with IMiDs and are both based on retrospective and prospective trials.
LMWH
A trial reported a decrease of thrombosis incidence from 20 to 3% in front-line elderly MM patients treated with the melphalan–prednisone–thalidomide regimen when LMWH therapy was introduced. The recommended dosage is within the prophylactic range Citation[31]. LMWH should be the prophylaxis of choice especially among cytopenic (mainly thrombocytopenic) patients. LMWHs have, however, some limits. Thrombopoiesis is reduced with LMWH secondary to an immunoallergic or an inflammatory phenomenon, increasing the risk of hemorrhages. Furthermore, most of the LMWHs are cleared via the renal route and LMWH therapy needs to be adjusted when the creatinine clearance is below 30 ml/min, to avoid the increase of bleeding risk by drug accumulation. Kidney function is often altered in MM patients, imposing a close appreciation of creatinine clearance in cases of LMWH therapy and therefore anti-Xa activity monitoring in MM patients with severe impaired renal function Citation[50,51]. One might recommend in MM patient to use preferably LMWH with an important proportion of long polysaccharide chains that has a greater reticulo–endothelial clearance rather than a kidney clearance.
Aspirin
Aspirin has been proven to reduce the risk of arterial complication but its role in venous thromboprophylaxis remains controversial Citation[52]. LMWH and warfarin-type drugs are regarded as more efficient options, indeed. Nevertheless, there are data demonstrating that aspirin reduces the risk of VTE in MM patients treated with IMiDs Citation[26,53,54]. One trial has reported a protective role of aspirin (180 mg, daily) in first-line therapy of MM patients treated with thalidomide and anthracyclines. Since then, other trials have shown a significant risk reduction in both arterial and venous thrombosis as soon as aspirin was introduced, although the risk of thrombosis remained present and higher than that reported in LMWH MM studies Citation[26,55,56]. The aspirin doses varied from 75 to 325 mg daily, but the optimal daily dose remains to be determined. Although rarely severe, the risk of bleeding is increased with the use of aspirin since many MM patients face thrombocytopenia.
Warfarin-type drugs
Several trials have shown reduction in the incidence of venous thrombosis among MM patients treated with IMiDs and warfarin Citation[26,55,56]. Furthermore, patients may switch to vitamin K antagonists (VKA) from LMWH in the context of long-term exposure to IMiDs if the thromboprophylaxis is maintained. However, there are several limits to their use. First, warfarin-type drugs increase the incidence of hemorrhage when the international normalized ratio (INR) is above 3, especially in the elderly, corresponding to the majority of MM patients. This bleeding risk is further increased in patients who become thrombocytopenic particularly with a platelet count below 80 g/l. Furthermore, warfarin’s efficacy is related to patient-dependent fluctuations with a narrow therapeutic window and requires frequent INR controls.
Novel oral anti-Xa inhibitors
They were not studied in the context of cancer, however several studies should start soon to determine their potential and safety profile.
Comparison between available thromboprophylaxis in MM treated with IMiDs
There are few studies comparing the risk of thrombosis with aspirin versus LMWH, we summarized the three most recent below. A recent Phase III trial Citation[57] has compared aspirin (100 mg/day), low-dose warfarin (1.25 mg/day) and enoxaparin (40 mg/day) in 667 patients with previously untreated myeloma receiving a thalidomide-based regimen. The primary end point was a composite that included serious thromboembolic events, acute cardiovascular events or sudden deaths during the first 6 months of treatment. The most frequent complications were thromboembolic events that occurred in 5.9% in the aspirin group, 8.2% in the warfarin group and 3.2% in the LMWH group. Symptomatic PE episodes occurred only in the aspirin and warfarin groups. The absolute differences for serious thromboembolic events were +2.7% (p = 0.173) between aspirin and LMWH groups and +5.0% (p = 0.024) between warfarin and LMWH groups. These results did not reach a statistical significance in favor of LMWH over aspirin.
This group has also conducted a second Phase III trial Citation[46] to compare the efficacy and safety of thromboprophylaxis with low-dose aspirin or LMWH in newly-diagnosed MM patients, treated with lenalidomide and low-dose dexamethasone induction and melphalan–prednisone–lenalidomide consolidation. The authors included 342 MM patients randomized to receive aspirin 100 mg/day (n = 176) or LMWH enoxaparin 40 mg/day (n = 166). The incidence of VTE was not statistically different between aspirin and LMWH groups at 2.27 and 1.20%, respectively.
A common limitation might have hampered these two trials, in that physicians only included patients that could receive all available thrombophrophylaxis therapeutics, including aspirin. The patients with high-risk assessment of VTE were unlikely to be included in these studies as the patients may have been randomized in the aspirin prophylaxis arm, introducing a potential bias in the recruitment. The two trials populations might have studied, essentially, IMiDs-treated myeloma with a low risk of VTE.
A third study was conducted in France Citation[47], called MELISSE, that has prospectively evaluated the incidence and ought to identify the risk factors of VTE associated with IMiDs in MM patients in a large multicentric observational study. Although observational, this study is a picture of the real life. A total of 524 MM patients were followed during 1 year; among them, 84% patients started with a thromboprophylaxis: 58% with aspirin (75–160 mg/day), 17% with LMWH and 10% with vitamin K antagonists (target INR 2–3). The risk of VTE was assessed as high in only 14% of the population by the investigators. The investigators recorded 32 VTE events, including 12 PEs. The deep venous thrombosis/PE incidence was not different within the first 4 months as compared with the incidence within the 4–12-months time period, 3.5 and 2.5%, respectively. The incidence of VTE recorded was lower in LMWH group (3%) than in aspirin group (7%). Interestingly, none of the patients receiving VKA treatment (a curative strategy) developed any thrombotic event.
Expert commentary
Current recommendations on thromboprophylaxis in myeloma patients treated with IMiDs-based regimens
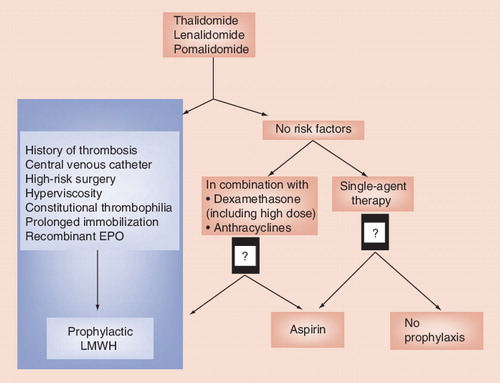
MM clearly comprises a state of hypercoagulability that occurs at diagnosis or at relapse periods in patients, although its mechanisms are multiple and complex. MM has become the leading hematological malignancy in terms of venous thrombosis since the introduction of IMiD therapy Citation[3,24]. The International Myeloma Working Group has therefore coordinated a guideline that specifically addressed VTEs in IMiD-based treated patients with myeloma, and that recommended a systematic thromboprophylaxis strategy in MM treated with IMiD-based therapy, especially in combination Citation[27]. This thromboprophylaxis should be based upon a risk-assessment model. Overall, the VTE risk factors, either classical or disease specific, can be classified into three categories; patient-related, disease-related or treatment-related. This guideline recommended that aspirin is used in patients with zero to one risk factors and LMWH is used in patients with two or more risk factors Citation[27]. However, the following unanswered issues remain:
• Several trials and observational studies have attempted to determine high-risk factors of thrombosis in MM patients treated with IMiD-based therapy Citation[46,47,57], however are quite heterogeneous regarding the context and the treatment strategy, and consequently the risk factors identified have varied as well. There was a tendency toward a higher risk of developing thromboembolic events in patients older than 60 years old presenting more than two comorbidities, with a Karnofsky performance status less than 70%, not receiving bortezomib; and receiving high doses of dexamethasone in one study Citation[46]. On the other hand, other variables independently predicted a higher risk to develop VTE in the MELISSE study: the time from diagnosis to occurrence of VTE and the association to EPO Citation[47];
• Some have stated that the presence of one of the risk factors increased the risk of thrombosis two- to 40-fold Citation[2,54,55,58,59], and other have proposed that the risk of thrombosis increased when at least two risk factors were present Citation[27,60];
• Furthermore, several studies conducted in IMiD-treated patients reported no increase in the incidence of VTE while patients were on aspirin and many of them presented with a high risk of VTE according to guidelines;
• Many patients that experienced a thrombotic event might have been misclassified with the current guideline;
• Finally, a patient that started on LMWH may switch at some point to either aspirin or VKA, but how to determine whether the initial high-risk factor has persisted and whether it is safer to use aspirin rather than VKA.
Overall, thromboprophylaxis has become compulsory since the introduction of IMiDs. Although recommendations are available on the optimal use of VTE prophylaxis, our knowledge concerning thrombosis in MM patients treated with IMiDs remains incomplete , and we believe there might be room for improvement to the guideline. Across all risk factors that are related to VTE, certain VTE risk factors, either classical or disease specific, are likely to be associated with an increased risk of thrombosis in IMiD-treated myeloma and have been repeatedly incriminated in studies Citation[27,60,61]. Therefore, certain risk factors listed in Box 1 are associated with high-risk occurrence of VTE indeed, and therefore might require – if present – systematic LMWH prophylaxis.
Five-year view
Over the next 5 years, the following areas must be addressed:
• Patients treated with IMiD-combined therapy, with or without dexamethasone, should be considered at risk of thrombosis and should be treated for thromboprophylaxis. Should patients treated solely with IMiDs as monotherapy and without other risk factors receive a thromboprophylaxis?
• Studies are designed to determine a scoring for thrombotic risk factors and allowing to adjust prophylaxis to the contextual risk: high-risk patients receiving LMWH, low-risk patients receiving aspirin. However, this definition is nevertheless difficult since the exact responsibility and the real burden of each risk factor such as diabetes, renal failure, heart failure and lung failure, for instance, are hard to determine. In the case of a risk factor being controlled, does it remain a risk factor?
• If the physician considers the patient at high risk of thrombosis, then LMWH is recommended, while aspirin might be kept for patients with low risk of thrombosis. Do we need more than one risk factor to consider a patient for high-risk thromboprophylaxis?
• Is the proposed list of risk factor exhaustive? What about genetic risk factors?
• The patients with high risk should be on LMWH, but for how long? At prophylactic doses? A switch to VKA might be recommended, but when to switch?
• It is also critical to identify the patients that will experience arterial rather than venous thrombotic events; more efforts are needed to understand the respective risk factors of either arterial or venous thrombotic events.
Table 1. Incidence of thrombotic events in multiple myeloma patients treated with immunomodulator drugs single agent.
Table 2. Incidence of venous thrombosis events in multiple myeloma patients treated with thalidomide-based regimens.
Table 3. Incidence of venous thrombosis events in multiple myeloma patients treated with lenalidomide- or pomalidomide-based regimens.
Box 1. Main risk factors of venous thrombosis in multiple myeloma patients treated with immunomodulator drugs-based therapy that might require systematic low-molecular-weight heparin prophylaxis.
Patient-related factors
• Past medical history of venous thrombosis
• Constitutional thrombophilia/blood-clotting disorders
• Immobilization/surgery
• Obesity (BMI over 30)
Disease-related factors
• Central venous catheter
• Hyperviscosity
Treatment-related factors
• High-dose dexamethasone
• Anthracyclines
• Multi-agent chemotherapy
• Erythropoietin
Key issues
• Thrombosis is not exceptional in multiple myeloma (MM) patients but thromboprophylaxis has become compulsory since the introduction of immunomodulator drugs (IMiDs). Indeed, IMiD-based combined chemotherapy increased significantly the risk of thrombosis.
• Although recommendations are available on the optimal use of venous thromboembolism (VTE) prophylaxis, our knowledge concerning thrombosis in MM patients treated with IMiDs remains incomplete. There are few randomized trials comparing different strategies, and many questions remain unanswered. Still, low-molecular-weight heparin should be proposed to myeloma treated with IMiDs that display a high risk of VTE.
• Further work should aim at further deciphering the risk factors of VTE in IMiD-treated MM patients in order to determine which patient should start on low-molecular-weight heparin to lower the risk of VTE.
• The future studies will have to focus on identifying risk factors of venous thromboembolism at the genetic level.
References
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case–control study. Arch. Intern. Med. 160(6), 809–815 (2000).
- Zangari M, Barlogie B, Thertulien R et al. Thalidomide and deep vein thrombosis in multiple myeloma: risk factors and effect on survival. Clin. Lymphoma 4(1), 32–35 (2003).
- Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N. Engl. J. Med. 344(25), 1951–1952 (2001).
- Sallah S, Husain A, Wan J, Vos P, Nguyen NP. The risk of venous thromboembolic disease in patients with monoclonal gammopathy of undetermined significance. Ann. Oncol. 15(10), 1490–1494 (2004).
- Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer 101(3), 558–566 (2004).
- Eby C, Blinder M. Hemostatic complications associated with paraproteinemias. Curr. Hematol. Rep. 2(5), 388–394 (2003).
- Elice F, Fink L, Tricot G, Barlogie B, Zangari M. Acquired resistance to activated protein C (aAPCR) in multiple myeloma is a transitory abnormality associated with an increased risk of venous thromboembolism. Br. J. Haematol. 134(4), 399–405 (2006).
- Cini M, Zamagni E, Valdré L et al. Thalidomide–dexamethasone as up-front therapy for patients with newly diagnosed multiple myeloma: thrombophilic alterations, thrombotic complications, and thromboprophylaxis with low-dose warfarin. Eur. J. Haematol. 84(6), 484–492 (2010).
- Zangari M, Siegel E, Barlogie B et al. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: implications for therapy. Blood 100(4), 1168–1171 (2002).
- Corso A, Lorenzi A, Terulla V et al. Modification of thrombomodulin plasma levels in refractory myeloma patients during treatment with thalidomide and dexamethasone. Ann. Hematol. 83(9), 588–591 (2004).
- Kaushal V, Kaushal GP, Melkaveri SN, Mehta P. Thalidomide protects endothelial cells from doxorubicin-induced apoptosis but alters cell morphology. J. Thromb. Haemost. 2(2), 327–334 (2004).
- Pal R, Monaghan SA, Hassett AC et al. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood 115(3), 605–614 (2010).
- Barlogie B, Desikan R, Eddlemon P et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a Phase 2 study of 169 patients. Blood 98(2), 492–494 (2001).
- Mileshkin L, Biagi JJ, Mitchell P et al. Multicenter Phase 2 trial of thalidomide in relapsed/refractory multiple myeloma: adverse prognostic impact of advanced age. Blood 102(1), 69–77 (2003).
- Neben K, Moehler T, Benner A et al. Dose-dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin. Cancer Res. 8(11), 3377–3382 (2002).
- Rajkumar SV, Gertz MA, Lacy MQ et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia 17(4), 775–779 (2003).
- Richardson PG, Schlossman RL, Weller E et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood 100(9), 3063–3067 (2002).
- Schey S, Ramasamy K. Pomalidomide therapy for myeloma. Expert Opin. Investig. Drugs 20(5), 691–700 (2011).
- Schey SA. Thalidomide in the management of multiple myeloma. Hematology 7(5), 291–299 (2002).
- Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA. Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br. J. Haematol. 141(1), 41–51 (2008).
- Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J. Clin. Oncol. 21(1), 16–19 (2003).
- Attal M, Lauwers-Cances V, Marit G et al.; IFM Investigators. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 366(19), 1782–1791 (2012).
- McCarthy PL, Owzar K, Hofmeister CC et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 366(19), 1770–1781 (2012).
- Musallam KM, Dahdaleh FS, Shamseddine AI, Taher AT. Incidence and prophylaxis of venous thromboembolic events in multiple myeloma patients receiving immunomodulatory therapy. Thromb. Res. 123(5), 679–686 (2009).
- Rus C, Bazzan M, Palumbo A, Bringhen S, Boccadoro M. Thalidomide in front line treatment in multiple myeloma: serious risk of venous thromboembolism and evidence for thromboprophylaxis. J. Thromb. Haemost. 2(11), 2063–2065 (2004).
- Baz R, Li L, Kottke-Marchant K et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin. Proc. 80(12), 1568–1574 (2005).
- Palumbo A, Rajkumar SV, Dimopoulos MA et al.; International Myeloma Working Group. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 22(2), 414–423 (2008).
- Rodeghiero F, Elice F. Thalidomide and thrombosis. Pathophysiol. Haemost. Thromb. 33(Suppl. 1), 15–18 (2003).
- Urbauer E, Kaufmann H, Nösslinger T, Raderer M, Drach J. Thromboembolic events during treatment with thalidomide. Blood 99(11), 4247–4248 (2002).
- Zangari M, Anaissie E, Barlogie B et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood 98(5), 1614–1615 (2001).
- Zangari M, Barlogie B, Anaissie E et al. Deep vein thrombosis in patients with multiple myeloma treated with thalidomide and chemotherapy: effects of prophylactic and therapeutic anticoagulation. Br. J. Haematol. 126(5), 715–721 (2004).
- Dimopoulos M, Spencer A, Attal M et al.; Multiple Myeloma (010) Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 357(21), 2123–2132 (2007).
- Weber DM, Chen C, Niesvizky R et al.; Multiple Myeloma (009) Study Investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N. Engl. J. Med. 357(21), 2133–2142 (2007).
- Chen C, Reece DE, Siegel D et al. Expanded safety experience with lenalidomide plus dexamethasone in relapsed or refractory multiple myeloma. Br. J. Haematol. 146(2), 164–170 (2009).
- Menon SP, Rajkumar SV, Lacy M, Falco P, Palumbo A. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer 112(7), 1522–1528 (2008).
- Lacy MQ, Allred JB, Gertz MA et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood 118(11), 2970–2975 (2011).
- Lacy MQ, Hayman SR, Gertz MA et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J. Clin. Oncol. 27(30), 5008–5014 (2009).
- Lacy MQ, Hayman SR, Gertz MA et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia 24(11), 1934–1939 (2010).
- Zangari M, Fink L, Zhan F, Tricot G. Low venous thromboembolic risk with bortezomib in multiple myeloma and potential protective effect with thalidomide/lenalidomide-based therapy: review of data from Phase 3 trials and studies of novel combination regimens. Clin. Lymphoma. Myeloma Leuk. 11(2), 228–236 (2011).
- Cavo M, Tacchetti P, Patriarca F et al.; GIMEMA Italian Myeloma Network. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised Phase 3 study. Lancet 376(9758), 2075–2085 (2010).
- Garderet L, Iacobelli S, Moreau P et al. Superiority of the Triple Combination of Bortezomib–Thalidomide–Dexamethasone Over the Dual Combination of Thalidomide–Dexamethasone in Patients With Multiple Myeloma Progressing or Relapsing After Autologous Transplantation: The MMVAR/IFM 2005-04 Randomized Phase III Trial From the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J. Clin. Oncol. 30(20), 2475–2482 (2012).
- Zangari M, Barlogie B, Cavallo F, Bolejack V, Fink L, Tricot G. Effect on survival of treatment-associated venous thromboembolism in newly diagnosed multiple myeloma patients. Blood Coagul. Fibrinolysis 18(7), 595–598 (2007).
- Fusté B, Serradell M, Escolar G et al. Erythropoietin triggers a signaling pathway in endothelial cells and increases the thrombogenicity of their extracellular matrices in vitro. Thromb. Haemost. 88(4), 678–685 (2002).
- Tobu M, Iqbal O, Fareed D et al. Erythropoietin-induced thrombosis as a result of increased inflammation and thrombin activatable fibrinolytic inhibitor. Clin. Appl. Thromb. Hemost. 10(3), 225–232 (2004).
- Anaissie EJ, Coleman EA, Goodwin JA et al. Prophylactic recombinant erythropoietin therapy and thalidomide are predictors of venous thromboembolism in patients with multiple myeloma: limited effectiveness of thromboprophylaxis. Cancer 118(2), 549–557 (2012).
- Larocca A, Cavallo F, Bringhen S et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 119(4), 933–939; quiz 1093 (2012).
- Leleu X, Daley L, Rodon P et al. MELISSE, a large multicentric observational study to determine criteria and risk factors of thromboembolism for patients with multiple myeloma treated with multiple myeloma treated with immunomodulator drugs. . Blood 21(116), 354 (2010).(abs.809)
- Galli M, Elice F, Crippa C, Comotti B, Rodeghiero F, Barbui T. Recombinant human erythropoietin and the risk of thrombosis in patients receiving thalidomide for multiple myeloma. Haematologica 89(9), 1141–1142 (2004).
- Lee AY, Levine MN, Baker RI et al.; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 349(2), 146–153 (2003).
- Ma JM, Jackevicius CA, Yeo E. Anti-Xa monitoring of enoxaparin for acute coronary syndromes in patients with renal disease. Ann. Pharmacother. 38(10), 1576–1581 (2004).
- Siguret V, Gouin-Thibault I, Pautas E, Leizorovicz A. No accumulation of the peak anti-factor Xa activity of tinzaparin in elderly patients with moderate-to-severe renal impairment: the IRIS substudy. J. Thromb. Haemost. 9(10), 1966–1972 (2011).
- Cohen AT, Skinner JA, Kakkar VV. Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ 309(6963), 1213–1215 (1994).
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 355(9212), 1295–302 (2000).
- Hovens MM, Snoep JD, Tamsma JT, Huisman MV. Aspirin in the prevention and treatment of venous thromboembolism. J. Thromb. Haemost. 4(7), 1470–1475 (2006).
- Ikhlaque N, Seshadri V, Kathula S, Baumann MA. Efficacy of prophylactic warfarin for prevention of thalidomide-related deep venous thrombosis. Am. J. Hematol. 81(6), 420–422 (2006).
- Niesvizky R, Martínez-Baños D, Jalbrzikowski J et al. Prophylactic low-dose aspirin is effective antithrombotic therapy for combination treatments of thalidomide or lenalidomide in myeloma. Leuk. Lymphoma 48(12), 2330–2337 (2007).
- Palumbo A, Cavo M, Bringhen S et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a Phase III, open-label, randomized trial. J. Clin. Oncol. 29(8), 986–993 (2011).
- Ageno W, Squizzato A, Garcia D, Imberti D. Epidemiology and risk factors of venous thromboembolism. Semin. Thromb. Hemost. 32(7), 651–658 (2006).
- Bick RL. Cancer-associated thrombosis. N. Engl. J. Med. 349(2), 109–111 (2003).
- Palumbo A, Davies F, Kropff M et al. Consensus guidelines for the optimal management of adverse events in newly diagnosed, transplant-ineligible patients receiving melphalan and prednisone in combination with thalidomide (MPT) for the treatment of multiple myeloma. Ann. Hematol. 89(8), 803–811 (2010).
- Zonder JA. Thrombotic complications of myeloma therapy. Hematology Am. Soc. Hematol. Educ. Program, 348–355 (2006).
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR; Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J. Clin. Oncol. 24(3), 431–436 (2006).
- Cavo M, Zamagni E, Tosi P et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica 89(7), 826–831 (2004).
- Anagnostopoulos A, Weber D, Rankin K, Delasalle K, Alexanian R. Thalidomide and dexamethasone for resistant multiple myeloma. Br. J. Haematol. 121(5), 768–771 (2003).
- Schutt P, Ebeling P, Buttkereit U et al. Thalidomide in combination with vincristine, epirubicin and dexamethasone (VED) for previously untreated patients with multiple myeloma. Eur. J. Haematol. 74(1), 40–46 (2005).
- Zervas K, Dimopoulos MA, Hatzicharissi E et al.; Greek Myeloma Study Group. Primary treatment of multiple myeloma with thalidomide, vincristine, liposomal doxorubicin and dexamethasone (T-VAD doxil): a Phase II multicenter study. Ann. Oncol. 15(1), 134–138 (2004).
- Barlogie B, Tricot G, Rasmussen E et al. Total therapy 2 without thalidomide in comparison with total therapy 1: role of intensified induction and posttransplantation consolidation therapies. Blood 107(7), 2633–2638 (2006).
- Morgan GJ, Schey SA, Wu P et al. Lenalidomide (Revlimid), in combination with cyclophosphamide and dexamethasone (RCD), is an effective and tolerated regimen for myeloma patients. Br. J. Haematol. 137(3), 268–269 (2007).
- Richardson P. Management of the relapsed/refractory myeloma patient: strategies incorporating lenalidomide. Semin. Hematol. 42(4 Suppl. 4), S9–S15 (2005).
Immunomodulator drug-based therapy in myeloma and the occurrence of thrombosis
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/experthematology. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 70-year-old man with multiple myeloma (MM) and are considering the initiation of immunomodulator drugs (IMiDs). What should you consider regarding the general risk for thromboembolic disease and its consequences in cases of MM?
□ A The overall prevalence of thromboembolic events in untreated MM is approximately 25%
□ B MM is the leading hematologic malignancy associated with the risk for thromboembolic disease
□ C M-proteins reduce platelet aggregation
□ D Venous thromboembolism is associated with a higher risk for death among patients with MM
2. What should you consider regarding the risk for thromboembolic events associated with IMiD therapy in this case?
□ A Anticoagulant therapy is unnecessary when only 1 IMiD is used
□ B IMiDs can increase the risk for both venous and arterial thromboembolic events
□ C Use of bortezomib has been confirmed to normalize the risk for thromboembolic events associated with IMiDs
□ D The risk for thromboembolic events is similar regardless of whether 1 agent or combination therapy is used
3. You initiate combination IMiD therapy. What is the most effective treatment to prevent thromboembolism in this patient?
□ A Warfarin
□ B Unfractionated heparin
□ C Low-molecular-weight heparin (LMWH)
□ D Dabigatran
4. What else should you consider regarding thrombosis prophylaxis for this patient?
□ A LMWH should be avoided in cases of thrombocytopenia
□ B Aspirin is more proven in reducing the risk for arterial embolism than for venous thrombosis
□ C Patients receiving LMWH should generally not transition to therapy with warfarin
□ D Treatment with LMWH requires much higher doses than those generally used to prevent venous thromboembolism
