Adapted from Citation[1].
![Figure 2. Composition of the fungal cell wall.Adapted from Citation[1].](/cms/asset/ceb87f9d-9145-459b-b28d-1f0718c6694b/ierz_a_11222338_f0002_b.jpg)
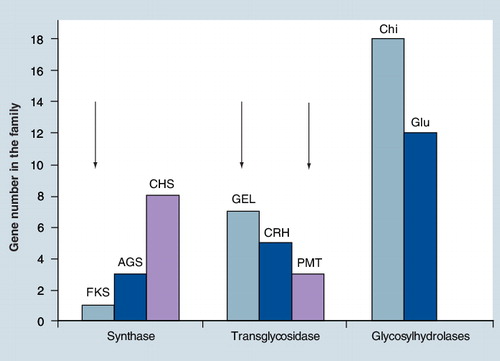
Arrows indicate that one gene has been shown to be essential in the family.
AGS: α-1,3-glucanosyltransferase; Chi: Endo-chitinases; CHS: Chitin synthase; CRH: Glucan-chitin transferase; FKS: β-1,3-glucan synthase; GEL: β-1,3-glucanosyltransferase; Glu: Endo-β-1,3-glucanase; PMT: O-mannosyltransferase.
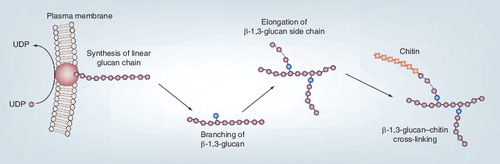
UDP: Uridine diphosphate.
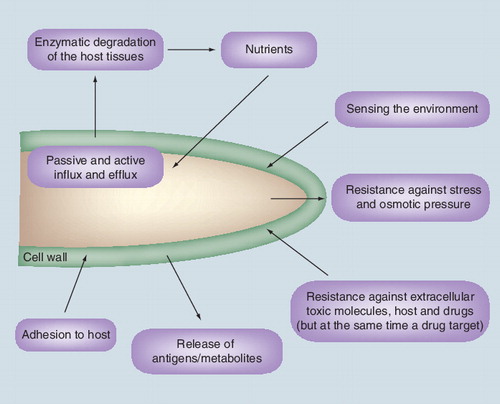
The cell wall is essential for the life of fungal pathogens. The major functions of the cell wall are detailed in . If the ‘job’ of the cell wall is to protect the fungus and allow it to survive in hostile environments, its unique composition also makes it an ideal target for the development of antifungal drugs Citation[1]. A total of 90% of the fungal cell wall is composed of specific polysaccharides that are not found in their human or plant host . Accordingly, any drug inhibiting polysaccharide synthesis should not have any secondary toxicity in humans, a recurrent drawback of antifungals such as amphotericin B. Indeed, antifungal drugs inhibiting cell wall biosynthesis have been launched commercially. However, in contrast to bacteria, where the peptidoglycan, an equivalent of the fungal cell wall, is the target of many classes of antibacterial agents Citation[2], only a single class of drugs has been developed that targets the fungal cell wall Citation[3]; these are the echinocandins that block β-(1,3)-glucan synthesis in a noncompetitive manner. Other inhibitors of chitin and mannan synthesis have been identified but none of these have yet reached the clinic Citation[4–6]. Even for the echinocandins that have demonstrated their activity against fungi, the precise mode of action of these compounds has not been elucidated Citation[7]. In this article, we will discuss some of the problems that may be responsible for the deficit in inhibitors targeting the cell wall, with a special emphasis on the Aspergillus fumigatus model studied in our laboratory, and we will also present future research lines that could lead to new antifungal drug developments.
Gene families
Many of the enzymatic activities that are necessary for cell wall construction are encoded by multigene families , however, the function of each member of the family is not always understood. Accordingly, inhibiting the enzyme activity of all members of the family by a single molecule is extremely difficult. This point can be illustrated by the chitin synthase (CHS) family of A. fumigatusCitation[1]. Based on sequence homologies and mutant phenotypes, the eight CHS that have been identified can be separated into two subfamilies: the first subfamily contains mutants that have a reduced CHS activity but not a reduced content of chitin in the cell wall, whereas the opposite is seen in the second subfamily. Moreover, inside each subfamily the phenotype of each chs mutant is different, suggesting a different function for each CHS. Under these conditions, identifying a single inhibitor that can block the synthesis of chitin known to be essential for fungi is a risky and laborious task. The only inhibitors reported to date in the literature are substrate analogs that are active in vitro in membrane preparations but poorly active in vivo in the fungus. Other inhibitors target only one class of CHS Citation[4,5]. Glycosylhydrolase and transglycosidase families often contain many genes, which are able to remodel the linear polysaccharides synthesized by the respective synthases . Members of a family can have the same enzymatic activity but may have a different biological function resulting from different cellular localization of proteins. This is seen in the members of the glucan-elongating protein/glycosylphospholipid-anchored surface protein family that display the same β-(1,3)-glucanosyltransferase activity but can be either dispensable or essential for the fungus Citation[8–10]. One of the possibilities for the control of the activity of all members of the same family is to block upstream regulators or activators of these activities. Rho1p and Rom2p have been the regulators identified for β-(1,3)-glucan synthase activity Citation[11]; regulators of other polysaccharide synthesis such as chitin or mannan have not yet been identified.
Compensatory reactions
This is an emerging concept in cell wall biology and medical mycology, which results from the fact that the cell wall cannot be considered as an inert exoskeleton anymore, but rather as a living organelle, the composition of which changes continuously upon toxic environmental aggression such as the presence of an antifungal drug. A major and common response of the fungus when the cell wall is damaged is upregulation of chitin synthesis Citation[12]. Other responses have been seen such as upregulation of α-(1,3)-glucan synthesis in AspergillusCitation[13]. These responses are mostly under the control of the cell wall integrity pathways but also of other MAP kinase or calcineurin pathways that sense cell wall stress and activate the expression of cell wall genes Citation[7,14]. These compensatory reactions can even overcome the essentiality of a gene such as CHS1 of Candida albicansCitation[12]. The occurrence of these compensatory reactions has led to a new concept in antifungal drug treatment based on combinatorial therapy, which results in the use of two drugs together Citation[12,14–16]. These combinations either target two cell wall biosynthetic pathways or a MAP kinase or Calcineurin pathway and a cell wall biosynthetic pathway. In addition, monoclonal antibodies able to interfere with polysaccharide synthesis could be a substitute for one of the inhibitors Citation[17]. Although the therapeutic efficacy of drug combinations has been repeatedly shown in the literature, they are not used in the clinic, with the exception of the combination of commercial cell wall inhibitors and sterol synthesis inhibitors that are used in a desperate attempt to treat a patient rather than on a scientifically based approach. Two major obstacles to the commercial launch of antifungal drug combinations remain. First, the regulatory agencies will require new toxicity and efficacy tests for drug combinations even though each single molecule has already passed US FDA approval and, second, the industry is reluctant to establish a combination of drugs that does not come from its proprietary chemical library.
Identifying new targets & revisiting old targets
Drug targets are either essential genes or essential enzymatic activities. Based on the Saccharomyces cerevisiae genome data, it is estimated that a fifth of the genes in a fungal genome are essential. However, most of them are common to eukaryotic machineries, including humans, and are not, for that reason, priority targets for the pharmaceutical industry because of putative problems of toxicity. However, scrutinizing essential pathways present in all eukaryotic cells is worthwhile because it can lead to the discovery of new antifungal targets. For example, the glycosylphosphatidylinositol (GPI) machinery is essential in all eukaryotes and all genes involved in GPI biosynthesis are conserved both in fungi and mammals. However, in fungi, the addition of a fourth mannose in the GPI anchor has led to the discovery of a new gene SMP3that is essential in fungi but not in mammals Citation[18]. In A. fumigatus, a fifth mannose is added to the anchor by an unknown protein Citation[19]. The search of inhibitors for such GPI biosynthetic enzymes has, however, been hampered by the difficulty of developing an easy high-throughput screening (HTS) tool, another requirement of the industry to undertake a search for any putative antifungal molecule.
As mentioned previously, glucan synthase inhibitors have been identified but the search for new inhibitors should continue, especially since echinocandins are noncompetitive inhibitors of glucan synthase with an unknown target. In spite of the difficulties mentioned earlier, the search for inhibitors of chitin synthesis must go on. Insecticides based on CHS inhibitors have been developed, giving hope for new developments in the fungal armamentarium Citation[20]. Original HTS must be developed for the identification of molecules blocking the synthesis and incorporation of chitin into the cell wall. Searching for an inhibitor of chitin synthesis cannot rely on the inhibition of the incorporation of radiolabeled UDP-GlcNAc into neosynthesized chitin by membrane preparations since genetic studies have shown that a reduction in CHS activity may not be associated with a reduction in chitin level of the cell wall.
The third most important component of the cell wall are mannans. They are very heterogeneous in nature and are either present as a peptidomannan in yeast or as part of the constructive polysaccharides of the cell wall, such as the galactomannan in A. fumigatusCitation[21]. Long fungal mannan polymers have very specific structures that are different from the N-glycan core of the eukaryote glycosylated proteins. Mannosyltransferases responsible for the elongation of mannans, such as Och1p and the mannosyltransferase complexes including Hoch1p, Mnn9p, Van1p, Anp1p, Mnn10 and Mnn11, are fungal specific Citation[22]. However, deletion of these mannosyltransferases is not essential in yeast. One of the reasons for this could be the lack of substrate specificity of the mannosyltransferases that will lead to compensatory reactions if one of these genes is deleted [Bussey H, Pers. Comm.]. In filamentous fungi, the transferases responsible for the synthesis and cross-linking of mannan to the branched β-(1,3)-glucan have not yet been identified. Although no inhibitor of N-mannan synthesis has been developed, mannan-binding inhibitors such as pradimicin or benanomycin have been identified Citation[6]. These inhibitors were very potent antifungals but their mode of action was not completely understood and their commercialization was stopped because of toxicity issues.
O-mannosyltransferase seems to be a better target than N-mannosyltransferase. Three families of PMT genes (PMT1, -2 and -4) were identified in the fungal kingdom [Bussey H, Pers. Comm.]. O-mannosylation is essential in fungi. This was seen for a single PMT2 deletion or for double deletions PMT1 and PMT4 in A. fumigatus and Schizosaccharomyces pombeCitation[23]. To date, only Pmt1p activity inhibitors have been identified and the search for Pmtp inhibitors suffers from a lack of substrates and method to measure O-mannosyltransferase activity of all PMT members Citation[24]. We have recently discovered in our laboratories that pmt4Δ mutants of A. fumigatus are extremely sensitive to β-(1,3)-glucan synthase inhibitors (2–5 log depending on the echinocandin molecule) Citation[23]. This discovery suggests that echinocandins could be efficiently used in combination with Pmt4p inhibitors. Previously, it was thought that O-mannan was only present in fungi but it is now known to be present in other eukaryotes, including humans where mutation in O-mannosyltransferase PMT1 and PMT2 give rise to congenital muscular dystrophies Citation[25]. The occurrence of O-mannans in higher eukaryotes has, however, reduced the interest in a search for inhibitors of O-mannosyltransferases.
Instead of looking for single essential genes, another strategy in the search for fungal inhibitors is to look for essential enzymatic activities only found in the fungal kingdom. All of the steps of polysaccharide biosynthesis and remodeling in the cell wall during fungal growth are essential . Remodeling enzymes can, therefore, be considered essential targets. Most of the remodeling enzymes encountered to date are GPI anchored. Because of the occurrence of a GPI anchor, the enzyme bound to the plasma membrane is located in the cell wall space and can act on linear neosynthesized polysaccharides extruded from the plasma membrane. This localization in the cell wall space is a huge advantage when looking for an inhibitor because such an inhibitor will easily reach its target. By contrast, a competitive inhibitor for a β-(1,3)-glucan synthase will have to cross the plasma membrane, be transported to the active site and bind to the catalytic site, all steps that may lead to the selection of resistance against the inhibitor or putative degradation of the inhibitor. The GPI proteins putatively involved in cell wall modifications must be common to all fungal species since the composition of the cell wall core is identical. Six families have been identified and among these only two contain genes that are essential: the first one is GEL, which is a β-(1,3)-glucanosyltransferase Citation[26]. The second activity is encoded by members of the DFG5/DCW1 family that are synthetically lethal. No enzymatic activity has been associated with these last proteins. Inhibitors targeting polysaccharide branching or cross-linking have not been identified to date.
Is a universal target the right antifungal: a grail?
The pharmaceutical industry is looking for an active compound that is able to kill all human pathogens from yeast to filamentous fungi, from the Ascomycetes to the Zygomycetes and Basidiomycetes. The two fungal genera most commonly targeted are Candida and Aspergillus. This search may seem unrealistic since the organization of cell wall enzymes is species specific, the permeability of the cell wall and associated extracellular matrix to the antifungal drug varies with the fungal species and the sensitivity of the enzymes themselves to the drug is also species-dependent Citation[27]. For example, Candida neoformans glucan synthase is insensitive to caspofungin Citation[28] and echinocandins are not very efficient in treating Zygomycetes. The presence of more than 20 chitin synthase genes in Rhizopus will prevent the identification of a single molecule able to inhibit the total chitin synthase activity in this zygomecete. In addition, some of the cell wall polysaccharides are essential in one species and absent in others. For example, α-(1,3)-glucans are essential in A. fumigatus and absent in CandidaCitation[29]. Similarly, efficient β-(1,6)-glucan synthesis inhibitors have been identified Citation[30]. They are able to block the growth of Candida but are of course totally inefficient against A. fumigatus since this fungus does not have β-(1,6)-glucan in its cell wall Citation[21]. This yeast specificity has impeded the development of a compound effective against all fungi; is the search for such a compound justified today in a pharmaceutical industry where the numbers of chemical families with antifungal properties are lacking?
Antifungals are designed to be fungicidal. However, many of the azoles are fungistatic, and when the drug is removed from the medium, the fungus grows again. Similarly, caspofungin does not kill Aspergillusin vitro and a paradoxical effect is seen where the inhibitory effect is reduced when the amount of drug is increased Citation[31]. Despite this, these drugs have been used successfully in the clinic for many years. A question then arises: is it necessary for an antifungal drug to kill the fungus? Data have already shown that death of the fungus does not seem to be necessary. For example, blocking the synthesis of the Cryptococcus neoformans capsule will not kill the fungus but will block its development in the host Citation[32]. Immunotherapeutic interventions are based on the use of monoclonal antibodies that fungistically block fungal growth Citation[17,33]. Indeed, a major function of currently used antifungals is to halt the development of the fungus without killing it. Clearance of the fungus will result from the recovery of an active immune system. Will any pharmaceutical company accept the development of an antifungal drug formulation that is only able to restrict fungal growth for a limited period?
Conclusion
The number of antifungal drug families remains limited to three: polyenes, azoles and echinocandins. Among these, only one class of drugs, the echinocandins, is directed towards the cell wall, even though this organelle is an extremely rich source of putative antifungal targets. It is estimated that approximately a third of the fungal genome is involved in cell wall biosynthesis and organization. More work should be performed in this area, especially since strains resistant to azoles have been documented among patients (such as AIDS or cystic fibrosis patients) receiving long-term antifungal therapy Citation[34]. Resistance to echinocandins starts to appear in clinical isolates of C. albicans and A. fumigatusCitation[35,36]. Luckily, to date, this resistance has not been transferred to clinics, although reasons for this are unknown (but would be worth studying), and resistant strains do not grow as fast as wild-type strains in vivo. This has demonstrated a posteriori that the concept of searching for drugs that only experimentally slow down fungal multiplication is funded. However, if any of these drug-resistant strains maintains its pathogenicity potential, it will be a clinical disaster in the medical mycology arena. In phytopathology, fungicide-resistant strains are as virulent as the sensitive parental strains. In agriculture, the average duration for the exploitation of a new fungicide is 10 years at most. This corresponds with the time interval between the commercial launch of the product and the spread of resistant mutants in the field all over the world. Medical mycologists and drug companies should think about the time lapse between the spread of resistant mutants and the exploitation of a new fungicide.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Latgé JP. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol.66, 279–290 (2007).
- Schneider T, Sahl HG. An oldie but a goodie – cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol.300(2–3), 161–169 (2009).
- Douglas CM, D’Ippolito JA, Shei GJ et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-D-glucan synthase inhibitors. Antimicrob. Agents Chemother.41, 2471–2479 (1997).
- Sudoh M, Yamazaki T, Masubuchi K et al. Identification of a novel inhibitor specific to the fungal chitin synthase. Inhibition of chitin synthase 1 arrests the cell growth, but inhibition of chitin synthase 1 and 2 is lethal in the pathogenic fungus Candida albicans. J. Biol. Chem.275, 32901–32905 (2000).
- Hiramoto F, Nomura N, Furumai T, Igarashi Y, Oki T. Pradimicin resistance of yeast is caused by a mutation of the putative N-glycosylation sites of osmosensor protein Sln1. Biosci. Biotechnol. Biochem.69, 238–241 (2005).
- Yeager AR, Finney NS. The first direct evaluation of the two-active site mechanism for chitin synthase. J. Org. Chem.69, 613–618 (2004).
- Walker LA, Gow NA, Munro CA. Fungal echinocandin resistance. Fungal Genet. Biol.47(2), 117–126 (2010).
- Rolli E, Ragni E, Calderon J, Porello S, Fascio U, Popolo L. Immobilization of the glycosylphosphatidylinositol-anchored Gas1 protein into the chitin ring and septum is required for proper morphogenesis in yeast. Mol. Biol. Cell20, 4856–4870 (2009).
- Gastebois A. Protéines ancrées à la membrane plasmique par l’intermédiare d’un glycosylphosphatidylinositol (GPI) et modification des β (1,3) glucans chez Aspergillus fumigatus. PhD thesis. Université Paris VI, Pierre et Marie Curie, Ecole Doctorale B2M, Paris, France (2009).
- Mouyna I, Morelle W, Vai M et al. Deletion of GEL2 encoding for a β (1–3) glucanosyltransferase affects morphogenesis and virulence in Aspergillus fumigatus. Mol. Microbiol.56, 1675–1688 (2005).
- Sekiya-Kawasaki M, Abe M, Saka A et al. Dissection of upstream regulatory components of the Rho1p effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics162, 663–676 (2002).
- Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog.4, e1000040 (2008).
- Damveld RA, Franken A, Arentshorst M et al. A novel screening method for cell wall mutants in Aspergillus niger identifies UDP-galactopyranose mutase as an important protein in fungal cell wall biosynthesis. Genetics178, 873–88 (2008)
- Fortwendel JR, Juvvadi PR, Pinchai N et al. Differential effects of inhibiting chitin and 1,3-{β}-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother.53, 476–482 (2009).
- Stevens DA. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob. Agents Chemother.44, 547–548 (2000).
- Steinbach WJ, Cramer RA Jr, Perfect BZ et al. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother.51, 2979–2981 (2007).
- Cassone A. Fungal vaccines: real progress from real challenges. Lancet Infect. Dis.8, 114–124 (2008).
- Grimme SJ, Colussi PA, Taron CH, Orlean P. Deficiencies in the essential Smp3 mannosyltransferase block glycosylphosphatidylinositol assembly and lead to defects in growth and cell wall biogenesis in Candida albicans. Microbiology150, 3115–3128 (2004).
- Fontaine T, Magnin T, Melhert A, Lamont D, Latgé JP, Ferguson MA. Structures of the glycosylphosphatidylinositol membrane anchors from Aspergillus fumigatus membrane proteins. Glycobiology13, 169–177 (2003).
- Mommaerts V, Sterk G, Smagghe G. Hazards and uptake of chitin synthesis inhibitors in bumblebees Bombus terrestris. Pest Manag. Sci.62, 752–758 (2006).
- Fontaine T, Simenel C, Dubreucq C et al. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem.275, 41528 (2000).
- Mora-Montes HM, Ponce-Noyola P, Villagomez-Castro JC, Gow NA, Flores-Carreon A, Lopez-Romero E. Protein glycosylation in Candida. Future Microbiol.4, 1167–1183 (2009).
- Willer T, Brandl M, Sipiczki M, Strahl S. Protein O-mannosylation is crucial for cell wall integrity, septation and viability in fission yeast. Mol. Microbiol.57, 156–170 (2005).
- Orchard MG, Neuss JC, Galley CM et al. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT1). Bioorg. Med. Chem. Lett.14, 3975–3978 (2004).
- Yanagisawa A, Bouchet C, Quijano-Roy S et al. POMT2 intragenic deletions and splicing abnormalities causing congenital muscular dystrophy with mental retardation. Eur. J. Med. Genet.52, 201–206 (2009).
- Kitagaki H, Wu H, Shimoi H, Ito K. Two homologous genes, DCW1 (YKL046c) and DFG5, are essential for cell growth and encode glycosylphosphatidylinositol (GPI)-anchored membrane proteins required for cell wall biogenesis in Saccharomyces cerevisiae. Mol. Microbiol.46, 1011–1022 (2002).
- Maligie MA, Selitrennikoff CP. Cryptococcus neoformans resistance to echinocandins: (1,3)β-glucan synthase activity is sensitive to echinocandins. Antimicrob. Agents Chemother.49, 2851–2856 (2005).
- Cornely OA. Aspergillus to Zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections. Infection36, 296–313 (2008).
- Denning DW. Echinocandin antifungal drugs. Lancet362, 1142–1151 (2003).
- Beauvais A, Maubon D, Park S et al. Two α (1–3) glucan synthases with different functions in Aspergillus fumigatus. Appl. Environ. Microbiol.71, 1531–1538 (2005).
- Kitamura A, Someya K, Hata M, Nakajima R, Takemura M. Discovery of a small-molecule inhibitor of {β}-1,6-glucan synthesis. Antimicrob. Agents Chemother.53, 670–677 (2009).
- Wiederhold NP. Paradoxical echinocandin activity: a limited in vitro phenomenon? Med. Mycol.47(Suppl. 1), S369–S375 (2009).
- Doering TL. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol.63, 223–247 (2009).
- Larsen RA, Pappas PG, Perfect J et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother.49, 952–958 (2005).
- Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res.9, 1029–1050 (2009).
- Arendrup MC, Perkhofer S, Howard SJ et al. Establishing in vitro – in vivo correlations for Aspergillus fumigatus: the challenge of azoles versus echinocandins. Antimicrob. Agents Chemother.52, 3504–3511 (2008).