The problem of pediatric burns
On average, 4300 children require admission to hospital following a burn injury in England and Wales each year, of which 55% are scalds Citation[101]. Most are small in area, 80% are in children under 5 years of age and the majority are due to hot drink spillages Citation[102]. One of the primary problems in the treatment of burns is bacterial infection, which can delay healing, increase pain, increase the risk of scarring and, in some cases, cause death. In recent years there have been great improvements in the treatment of burns, particularly with biologically-derived dressings, which actively promote cell growth. However, the problem of infection has not gone away, and there is evidence that silver-treated antimicrobial dressings can delay burn healing.
Infection: the clinical context
In current clinical situations, burns in adults or children are cleaned, dead tissue/debris removed and a dressing or tissue graft (as required) is applied Citation[1]. Application of such dressings requires clean wounds best achieved by surgical cleaning under anesthesia in children. Following clinical intervention, the burn area is effectively sterile. However, bacteria can still enter the burn area by ingress under the dressing from surrounding nonsterile skin. The most important pathogenic bacteria in burns infections are Staphylococcus aureus, Streptococcuspyogenes, Pseudomonas aeruginosa and Klebsiella pneumoniae.
At the present time, the only way to diagnose infection in a child with a burn injury is by clinical condition or by direct wound assessment. This assessment commonly requires removal of the adherent dressing often under anesthesia with a need for repeated dressing changes and re-admission to hospital (and the costs involved with this). This will remove the benefits of the dressing for the child and may ultimately be proven unnecessary.
Young children with burns are at particular risk of infection. However, the clinical signs of burn-related infection in children are nonspecific. The child is likely to have a fever (pyrexia), a general deterioration in clinical condition, with a raised or lowered white blood count and/or raised C-reactive protein. All of these can also be indicative of an inflammatory response to the burn injury, deep untreated burn as well as sepsis. The following diagnoses must be considered in a child who has deteriorated nonspecifically or who is pyrexial after burn injury: toxic shock syndrome (TSS); burn wound infection; deep burn requiring surgical management; or sepsis from another cause. Currently it is very difficult to distinguish between these in a child with a scald covered with an adherent dressing. In all cases of presumed sepsis, the wound should be examined directly. Biological dressings should therefore be removed or at least inspected carefully.
One particular infection seen after burn injury in children and associated with a high mortality if undiagnosed is TSS. TSS was originally described in children with burn injuries in 1985 by Frame et al. Of the seven children followed, three subsequently died Citation[2]. TSS is caused by exotoxins commonly produced by S. aureus and less often by S. pyogenes and P. aeruginosa. The responsible exotoxin is most commonly TSS toxin-1 (TSST-1). Typical cases present within 2 days of thermal injury in a child under 2 years of age with a burn of less than 10% of body surface area Citation[3]. Children of less than 4 years of age have lower levels of anti-TSST-1 antibodies, rendering them more susceptible to infection. Children of this age range are unfortunately the most likely to suffer a scald injury.
The clinical features of TSS are a ‘prodromal period’ lasting 1–2 days with pyrexia, diarrhea, vomiting and general malaise. A rash is often present at this stage and the burn may be small and ‘appear’ clean. Shock will subsequently develop in untreated cases. Once shock has developed the mortality is approximately 50%, but this can be reduced by early diagnosis and treatment. The diagnosis of TSS is difficult because in the early stages the signs and symptoms resemble other common childhood illnesses. Management is supportive with specific strategies including intravenous antibiotics and the anti-toxin, most commonly administered via a blood product such as fresh frozen plasma or immunoglobin Citation[4]. All of these are expensive and will require a repeated and extended hospital stay. A means of diagnosis of wound colonization by S. aureus without requiring the removal of an adherent dressing would be a significant advance in the management of children with suspected TSS.
Current state-of-the-art dressings for burns treatment
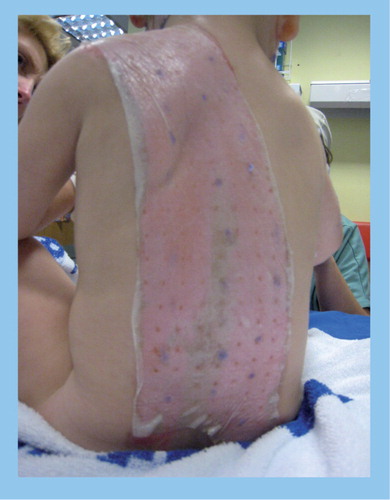
When a child presents with a scald or burn injury, management depends upon the burn depth. Burns are classified into superficial, partial thickness and full thickness. Partial thickness scalds are the most common injuries in children, affecting the epidermis and part of the dermis. Most partial thickness scalds will heal without further surgery, but a percentage will require skin grafting to achieve healing. Traditionally these were managed with simple non-adherent dressings. The current state-of-the-art dressings for the management of partial thickness burns where skin grafting is not predicted is focused on dressings that encourage and support epithelial cell growth and are designed to prevent microbial infection. An example is given in . This young child, treated at Frenchay hospital, UK, suffered a scald injury (cup of tea) and 30% burns. He was treated with the biologically-derived dressing Biobrane™ (Smith and Nephew, Hull, UK) and made a full recovery with virtually no scarring [Young A, Pers. Comm.].
Dressings designed to promote epithelization are typically termed biological dressings. Biological dressings have improved healing in partial thickness scalds in children resulting in shorter lengths of stay and a reduced need for skin grafting. However, these dressings neither detect nor treat burn wound infection. One advanced system still in development is ICX-SKN™ made by Intercytex (Manchester, UK) Citation[5], designed to assist the regeneration of sub-epithelial cells (the dermis). Biobrane is currently in use at specialist burns centers. Biobrane consists of a 3D matrix of collagen-coated nylon and porcine-derived cell growth factors, providing an environment for rapid tissue regrowth. Dressings such as Biobrane have helped reduce the need for skin grafting, especially in the more superficial burn injuries Citation[6].
Unfortunately, dressings that encourage cell growth can also assist microbial growth. To this end, dressings such as Acticoat™, which contain silver to suppress microbial growth, have been developed for a range of wound situations. Unfortunately, silver-containing dressings may be partially cytotoxic, reducing epithelialization and tissue growth, which in turn may lead to a significant increase in hospitalization time and healthcare costs. Moreover, silver-containing dressings continually expose the wound to the antimicrobial agent, which may increase the rate of bacteria evolving resistance Citation[7].
At the present time, dressings for burn injuries can either promote tissue growth or stop/slow infection. There is a need for a technology that can be used with current biological dressings, responds to the microbiological environment of the wound, can alert clinicians to the presence of infection via a simple color change and will release antimicrobials only when required. There are currently no dressing systems on the market that actively and directly signal that the wound has become infected with pathogenic bacteria.
The concept to be realized: a ‘smart dressing’ for pathogenic bacteria
A pan-European team of researchers, Bacteriosafe, funded by the European Commission’s 7th Framework have commenced work on creating an advanced wound dressing technology that will detect wound infection and colonization by pathogenic bacteria giving a color change, and then, and only then, automatically releasing antimicrobials /antibiotics into the wound area.
The basis of the smart dressing is the premise that pathogenic bacteria responsible for wound infection secrete virulence factors that alter their host environment such as lipases, hyaluronidase, which degrade healthy tissue, and toxins such as α-hemolysin, leukocidins and leukotoxins, cytotoxins which actively kill healthy cells. This technology seeks to use this effect against the bacteria producing the toxin. The dressing will be impregnated with nanocapsules just a few hundred nanometers in diameter, which will contain an antibiotic/antimicrobial and a dye that on release changes color. The fundamental basis of the technology has been successfully investigated and published in the Journal of the American Chemical SocietyCitation[8]. Research is currently focused on stabilizing the nanocapsules whilst retaining their sensitivity to bacterial virulence factors and investigating a range of methodologies for attachment into wound dressings or wound dressing components such as non-woven polypropylene. The aim is to have a working prototype system by summer 2012, with a further 4 years or more expected for developing engineering solutions to allow manufacture and safety/compliance testing. It is important to note that the technology could in principle be integrated with existing burn/wound dressing systems, combining much of the exciting developments in biological or semi-biological dressings with a ‘smart’ detection and response system for infection.
Applications of such a system beyond pediatric burns
Once this technology is realized as a manufacturable product, applications beyond pediatric burns beckon. One obvious application is in adult burn treatment, especially for military personnel in battlefield situations. Hard to heal wounds which are prone to infection, such as diabetic leg ulcers, are another obvious application of the technology.
In this brief editorial we have attempted to briefly describe the complex clinical problem of pediatric burn management, and briefly describe a new technology which, if realized, could represent a step change in the way burns, and maybe wounds more generally, are treated.
Financial & competing interests disclosure
The authors would like to thank the European Commission’s 7th Framework programme, grant number 245500. Neither author has financial links with any manufacturer of burns dressings. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Wasiak J, Cleland H, Campbell F. Dressings for superficial and partial thickness burns. Cochrane Database Syst. Rev.2, 1–51 (2009).
- Frame JD, Eve MD, Hackett ME et al. The toxic shock syndrome in burned children. Burns11, 234–241 (1985).
- Thornton KL, Young AER. The recognition and treatment of toxic shock syndrome. Arch. Dis. Child.92, 4 (2007).
- White MC, Thornton K, Young AER. Early diagnosis and treatment of toxic shock syndrome in paediatric burns. Burns31, 193–197 (2005).
- Flasza M, Kemp P, Shering D et al. Development and manufacture of an investigational human living dermal equivalent (ICX-SKN). Regen. Med.2, 903–918 (2007).
- Whitaker IS, Worthington S, Jivan S, Phipps A. The use of Biobrane by burn units in the United Kingdom: a national study. Burns33, 1015–1020 (2007).
- Poon VKM, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns30, 140–147 (2004).
- Zhou J, Loftus AL, Mulley GJ, Jenkins ATA. A thin film detection/response system for pathogenic bacteria. J. Amer. Chem. Soc.132, 6566–6570 (2010).
Websites
- UK Burn Injury Data 1986–2007. First Report of the iBID www.ibid.org
- National Burn Care Review. Standards and Strategies for Burn Care 2001 www.nbcg.nhs.uk/national-burn-care-review