Abstract
Most patients who undergo surgery recover uneventfully and resume their normal daily activities within weeks. Nevertheless, chronic postsurgical pain develops in an alarming proportion of patients. The prevailing approach of focusing on established chronic pain implicitly assumes that information generated during the acute injury phase is not important to the subsequent development of chronic pain. However, a rarely appreciated fact is that every chronic pain was once acute. Here, we argue that a focus on the transition from acute to chronic pain may reveal important cues that will help us to predict who will go on to develop chronic pain and who will not. Unlike other injuries, surgery presents a unique set of circumstances in which the precise timing of the physical insult and ensuing pain are known in advance. This provides an opportunity, before surgery, to identify the risk factors and protective factors that predict the course of recovery. In this paper, the epidemiology of chronic postsurgical pain is reviewed. The surgical, psychosocial, socio–environmental and patient-related factors that appear to confer a greater risk of developing chronic postsurgical pain are described. The genetics of chronic postsurgical pain are discussed with emphasis on known polymorphisms in human genes associated with chronic pain, genetic studies of rodent models of pain involving surgical approaches, the importance of developing accurate human chronic postsurgical pain phenotypes and the expected gains for chronic postsurgical pain medicine in the post-genomic era. Evidence is then reviewed for a preventive multimodal analgesic approach to surgery. While there is some evidence that chronic postsurgical pain can be minimized or prevented by an analgesic approach involving aggressive perioperative multimodal treatment, other studies fail to show this benefit. The transition of acute postoperative pain to chronic postsurgical pain is a complex and poorly understood developmental process, involving biological, psychological and social–environmental factors.
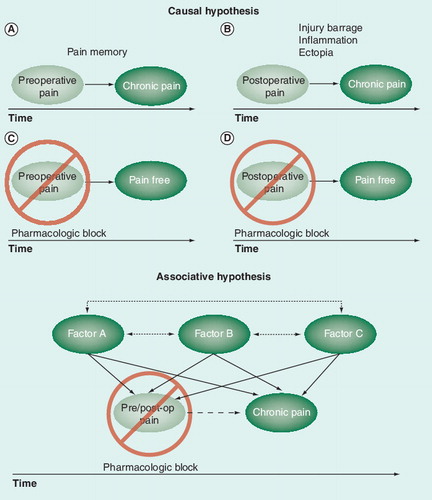
Top: transition to chronicity (A,B) may be prevented by pharmacological blockade of preoperative pain (C) and/or acute postoperative pain (D) assuming preoperative or acute postoperative pain are the causes of chronic postsurgcial pain. Bottom: transition to chronicity will not be prevented if pains are merely associated and caused by one or more inter-related factors. post-op: Postoperation.
EMLA: Eutectic mixture of local anesthetics; G: Group; PACU: Post-anesthetic care unit; PO: per os; post-op: Postoperation.
![Figure 2. Illustration of the study by Fassoulaki et al.Citation[181] examining the preventive effect of gabapentin (a Cavα2-δ ligand) in women undergoing breast cancer surgery.EMLA: Eutectic mixture of local anesthetics; G: Group; PACU: Post-anesthetic care unit; PO: per os; post-op: Postoperation.](/cms/asset/0cd4622a-3443-4ab1-b8e5-6369eb859225/iern_a_11214171_f0002_b.jpg)
Lines with double arrows between variables show associative relationships reported in the literature. Lines with a single arrowhead show causal relationships based on randomized controlled trials of preventive analgesia.
The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” Citation[1]. There is, however, no one accepted definition of chronic postsurgical pain and it is debatable whether, at the present time, a single definition would be useful Citation[2]. Most attempts at defining chronic postsurgical pain, whether general Citation[3] or specific to a surgical procedure Citation[4,5], are descriptive in nature and offer a time frame for chronicity beginning 2 or 3 months after surgery. Macrae and Davies propose the following four-point definition of chronic postsurgical pain: “the pain developed after a surgical procedure; the pain is of at least 2 months duration; other causes for the pain have been excluded; the possibility that the pain is continuing from a pre-existing problem should be explored and exclusion attempted” Citation[2].
Most patients who undergo surgery recover uneventfully and resume their normal daily activities within weeks. However, chronic postsurgical pain develops in an alarming proportion of patients. The magnitude of the problem is evidenced by recent epidemiological data documented later. A fact that is rarely appreciated by pain clinicians and researchers is that every chronic postinjury or postsurgical pain was once acute; the development of chronic postsurgical pain involves a transitional process. The traditional, dominant focus on studying established chronic pain misses important cues that may help us to predict who will go on to develop chronic postsurgical pain and who will recover uneventfully. Emphasis on identifying the processes that underlie the transition to chronicity has been a neglected topic of investigation. The aim of this review is to critically examine the factors that are associated with the development of chronic postsurgical pain. We first review available data on the epidemiology and course of chronic postsurgical pain following various surgical procedures. We then describe the surgical, psychosocial, social–environmental and patient-related factors that appear to confer a greater risk of developing chronic postsurgical pain. We also discuss the genetics of chronic postsurgical pain, with an emphasis on known polymorphisms in human genes associated with chronic pain in general, pain genetic studies of rodent models involving surgical approaches, the importance of developing accurate human pain phenotypes and the expected gains for chronic postsurgical pain medicine in the postgenomic era. Finally we review the evidence suggesting that a preventive analgesic approach can reduce the incidence and intensity of chronic postsurgical pain. We conclude with key issues, an expert commentary and a 5-year view into the future.
Epidemiology of chronic postsurgical pain
shows the incidence of chronic postsurgical pain following a variety of surgical procedures, including limb amputation, thoracotomy, mastectomy, hernia repair, open cholecystectomy, cesarean section, hip replacement and median sternotomy for coronary artery bypass graft surgery. This table illustrates the growing awareness of chronic postsurgical pain Citation[6–9]. For example, a prospective study of approximately 5000 patients estimated the incidence of acute neuropathic pain in the days after surgery to be between 1 and 3% Citation[10]. A 1-year follow-up showed that 56% of the patients with acute neuropathic pain continued to have pain Citation[10]. Other reports suggest the incidence of acute postsurgical neuropathic pain is considerably higher Citation[11] and, given the 56% rate of conversion to chronicity Citation[11], support the view that up to 10% of patients report severe, intractable chronic postsurgical pain 1 year after surgery. These statistics are staggering, especially when one considers the total number of patients worldwide who undergo surgery each year. It is not at all surprising, then, to see that almost 25% of more than 5000 patients referred to chronic pain treatment centers have chronic postsurgical pain Citation[12].
We know next to nothing about pain beyond the 1-year mark. Most studies followed patients for approximately 1 year after surgery. The data show the 1-year incidence to be highly variable and surgery specific, ranging from a low of approximately 10–15% following modified radical mastectomy Citation[13] to a high of 61–70% for thoracotomy Citation[14] and amputation Citation[15]. Follow-up beyond the 1-year mark is not common but there are two studies of patients followed for up to 5 or 6 years after hernia repair and two studies of lower limb amputees followed for 2 years. The longer term hernia repair follow-up data indicate that pain persists in 8.1–19% of patients for up to 6 years, with severe or very severe pain occurring in 1.8%. A total of 2 years after amputation approximately 60% of amputees report phantom limb pain (PLP) and 21–57% report stump (residual limb) pain Citation[16,17]. These studies indicate that even the lowest incidences are unacceptably high.
Some of the variability in these estimates can be accounted for by the criterion used to classify patients as having chronic pain; the more stringent the criterion, the lower the estimate. For example, studies that measure the intensity of pain or the impact of pain on daily activities show that the incidence of chronic postsurgical pain is lower when the cut-off for severity and impact of pain is high Citation[14–16,18–21]. Other reasons for the variability in estimates for a given surgical procedure include lack of precision in defining/diagnosing chronic postsurgical pain, sampling method, source of patients, their genetic background and whether the classification of the pain as chronic was by self-report. Despite this variability, even the most conservative estimates are a cause for concern.
Factors associated with chronic postsurgical pain
Surgical factors associated with chronic postsurgical pain
The following surgical factors are associated with an increased likelihood of developing chronic postsurgical pain: increased duration of surgery Citation[22,23], low (vs high) volume surgical unit Citation[13], open (vs laparoscopic) approach Citation[24], pericostal (vs intracostal) stitches Citation[25], conventional hernia repair Citation[24] and intraoperative nerve damage Citation[10,24]. Whether these factors are causally related to the development of chronic pain is not known. However, these factors appear to have in common greater surgical trauma and, in particular, they point to intraoperative nerve injury as a likely causal mechanism. Nerve damage produces acute and lasting changes in the injured nerves, and even in their intact neighboring nerves, pain pathways in the CNS, and motor and sympathetic outputs (reviewed by Devor and Seltzer Citation[26]). These changes are probably the main culprit in producing both acute and chronic neuropathic pain.
More than 30 years of basic science research has shown that nerve injury produces neuropathic conditions and behaviors in rodents that resemble symptoms of chronic neuropathic pain in humans (for reviews see Citation[27,28]). In fact, the most commonly used animal models of neuropathic pain involve intentional damage to peripheral nerves Citation[29–32] or spinal nerves Citation[33], by total nerve section Citation[29,32,33], or partial nerve ligation Citation[31] or constriction Citation[30]. Using these models enabled investigators to identify a number of intraoperative methodological factors. The type of knife that was used when cutting tissues and nerves affected neuropathic pain-related behavior in a rodent model of limb amputation pains and anesthesia dolorosa. Electrocautery was associated with significantly more chronic pain than was use of a laser Citation[34]. In a model of brachial plexus avulsion following hindpaw deafferentation, it was found that using ketamine and xylazine for general anesthesia significantly reduced the incidence of neuropathic pain when compared with anesthesia using a barbiturate, suggesting that improvements in the type of general anesthetic used during surgery may reduce pain chronicity Citation[35].
One of the triggers of pain chronicity is thought to be the amount of injury discharge produced intraoperatively and its impact on the CNS. When a peripheral nerve is transected or otherwise injured, it emits a long-lasting, high-frequency burst of activity Citation[36,37]. This injury discharge involves a train of impulses produced by freshly injured afferent nerve fibers (ruptured myelinated and unmyelinated axons) that uses glutamatergic neurotransmission via NMDA receptors to sensitize nociceptive pathways in the CNS. Injury discharge is under tonic endogenous inhibition by inhibitory interneurons in the spinal cord that use amino acids, such as GABA and glycine Citation[38]. The massive glutamatergic activation of postsynaptic NMDA receptors on interneurons in the spinal dorsal horn, many of which are inhibitory Citation[39], is believed to be excitotoxic, leading to their destruction and to disinhibition of pain pathways Citation[40–42]. Thus, one very useful preventive measure that can be taken when it comes to humans undergoing surgery is to avoid intraoperative nerve damage. Obviously, this is not possible for certain surgeries, such as limb amputation, that involve ligation and section of major nerve trunks. However, the practice of intentionally transecting nerves for surgical convenience should be avoided Citation[24] and doing so will, without doubt, contribute to a lower incidence of neuropathic pain Citation[6,43].
Note, however, that many operations do not result in transecting nerve trunks, but in stretching or crushing nerves during retraction of tissues when accessing deeper structures, or in cutting skin, fascia, muscles, joints, bony structures and viscera, all of which are innervated by sensory fibers. Thus, injuring such afferents by stretching the nerve trunk or the tissue it innervates, cauterizing or trapping it in a suture or clips, or cutting the tissue may be a trigger for increased acute postsurgical pain and possibly the transition to pain chronicity. In fact, there is a rodent model that mimics this type of injury by simply cutting skin and underlying muscle without damaging a nerve trunk. The incision proposed in the original model was cutting the thigh skin and underlying biceps femoris muscle Citation[44]. This injury produced small- to medium-sized, pyknotic and hyperchromatic neurons (‘dark neurons’) in the upper spinal dorsal horn laminae that process nociceptive input. It has been proposed that these changes are produced by an excitotoxic insult involving NMDA receptor activation subsequent to injury discharge, and that at least some of these neurons are inhibitory interneurons whose functional impairment or death contributes to a central state of hyperexcitability that underlies neuropathic hyperalgesia and allodynia Citation[44]. A few years later, this model was slightly modified by making the incision on the plantar surface of the hindpaw of the rodent, cutting through skin and muscle, thereby producing heat and mechanical allodynia and hyperalgesia, and guarding behavior that, in the rat, lasted 6 days Citation[45]. Subsequent work showed that the heat allodynia and spontaneous firing of impulses in C-fibers, innervating the skin adjacent to the incision, depended on Vanilloid 1 transient receptor potential (Trpv1) receptors, and the allodynia and firing of C-fibers were much reduced in Trpv1 gene knockout mice Citation[46]. Treating rats that had undergone the plantar postincision pain model with the Trpv1 antagonist AMG0347 decreased capsaicin-induced heat and mechanical hyperalgesia Citation[47]. This model is sensitive to opiate treatments, since a small dose of morphine affected guarding behavior and responses to noxious abnormal heat stimuli Citation[48]. NGF and TNF-α play a role in mediating the sensory abnormalities in this model, as they do in other models of neuropathic pain following nerve injury Citation[49,50]. However, neither gender nor strain (i.e., genetic variation) effects were noted across mice of the DBA2/J, C57BL/6J and 129X1/SvJ strains Citation[51]. Since the latter two are routine strain platforms for the study of gene knockout studies, this confirms that the plantar incision model can be used to detect gene effects on the sensory abnormalities that characterize it.
Another variant of the skin and muscle incision model of Nachemson and Bennett Citation[44] was adapted for laparotomy, by having the incision performed in rats at a subcostal level accessing the peritoneal cavity Citation[52]. The surgical procedure produced decreased ambulation and rearing by approximately 50% 24 h after surgery, which was reversed by morphine (but not the rearing) and ketorolac. There were synergistic effects when the two analgesics were coadministered. This model mimics the behavioral aspects of postoperative pain seen clinically, including the reported analgesic efficacy and occurrence of side effects found with these agents in humans Citation[52].
Psychosocial factors & chronic postsurgical pain
Only recently have researchers begun to examine psychosocial risk factors associated with the development of chronic postsurgical pain. Several psychosocial predictors of chronic postsurgical pain Citation[23,53,54] or chronic postsurgical pain disability Citation[16,55] have been identified, including increased preoperative state anxiety Citation[56], an introverted personality Citation[53], less catastrophizing, social support and solicitous responding in the week after amputation Citation[16,55], higher concurrent emotional numbing scores at 6 and 12 months Citation[57], fear of surgery Citation[23], and ‘psychic vulnerability’ Citation[54], a construct similar to neuroticism Citation[9]. Solicitous responding includes behaviors on the part of significant others that unwittingly positively and/or negatively reinforce the patient’s pain behaviors thereby increasing their frequency of occurrence. For example, in response to a complaint of pain or a grimace from the patient when performing a household chore, an empathic spouse may tell the patient to rest while she performs the task herself. Such solicitous behaviors may, in some instances, have the unintended consequence of increasing the frequency of pain behaviors and thus contributing to pain-related disability.
Catastrophizing
Pain catastrophizing is characterized by unrealistic beliefs that the current situation will lead to the worst possible pain outcome Citation[58], negative thoughts about the future and self Citation[59] and “an exaggerated negative ‘mental set’ brought to bear during actual or anticipated pain experience” Citation[60]. Pain catastrophizing is a multi-dimensional construct comprising elements of rumination, magnification and helplessness Citation[60–62]. One of the few consistent findings in the pain literature is that chronic pain patients who do not catastrophize fare better than patients who do catastrophize Citation[63].
Jensen et al.Citation[55] and Hanley et al.Citation[16] evaluated a biopsychosocial model predicting pain interference (disability) and depression in a 2-year prospective study of 70 patients initially assessed 1 month after lower limb amputation. Patients completed a measure of PLP intensity and the following psychosocial measures 1 month, 6 months Citation[55], 1 year and 2 years Citation[16] after amputation: pain cognitions (catastrophizing, perceived control over pain), pain coping, social support, social environment (solicitous responding from a significant other), pain interference and depressive symptoms. Regression analyses showed that 1-month pain and psychosocial measures accounted for a total of 46% of the variance in pain interference change scores at the 2-year follow-up. Psychosocial measures accounted for 27% of the variance, with catastrophizing, social support and solicitous responses each accounting for a unique and statistically significant portion of the variance. Greater social support and less spousal solicitousness (indicating, for example, a lesser tendency for spouses to attend to complaints of pain and to take over the pain patient’s jobs and duties) and, counterintuitively, more frequent catastrophizing at 1 month were associated with improvements (greater decreases) in pain interference scores 2 years later. In addition, 1-month pain and psychosocial measures accounted for a total of 36% of the variance in change in depressive symptoms at the 2-year follow-up, with only catastrophizing contributing a unique and statistically significant portion of the variance.
More frequent catastrophizing predicting better outcome appears to be a counterintuitive finding, and may be, in part, accounted for by the use-change scores that complicate interpretation. The authors note that those patients who catastrophized most at the initial assessment likely had more room for improvement in pain interference and depressive symptoms at the 2-year follow-up, suggesting that the findings may be an artifact of using difference scores and from a regression to the mean. The authors offered an alternative interpretation, suggesting that there may be something beneficial about pain catastrophizing early after amputation, either because it mobilizes social support resources that ultimately assist in recovery and adjustment or because it is associated with an acute, adaptive grief reaction that contributes to earlier long-term resolution of issues concerning limb loss. Alternatively, catastrophizers may seek and receive medical treatments early on that have a lasting effect on impulse generators in the amputated nerves and/or the CNS.
In a more recent prospective, longitudinal study, 55 patients scheduled to undergo total knee arthroplasty were assessed before and at several time points up to 2 years after surgery Citation[64]. In contrast to McGill Pain Questionnaire (MPQ) pain scores, which decreased over time, catastrophizing scores remained relatively constant over the 2-year follow-up period. Regression analyses showed that preoperative catastrophizing of a ruminative nature significantly predicted MPQ pain ratings 2 years after surgery. It remains to be seen whether the relationship between preoperative catastrophizing and chronic postsurgical pain after total knee arthroplasty is causal or associative.
Social support & social–environmental factors
The relevance to chronic postsurgical pain of social support in general, and solicitous responding in particular, has been established in a 2-year prospective study of amputees with PLP Citation[16,55]. The relationship between solicitous responding from significant others and the patients’ chronic pain intensity and behaviors is explained by well established operant conditioning principles. The operant model proposes that in offering pain contingent help (e.g., taking over household jobs) in response to pain behaviors (e.g., guarding, limping) and verbal expressions of pain, well-intentioned spouses unwittingly negatively reinforce the patient’s pain behaviors, leading to an increase in their frequency of occurrence Citation[65,66]. As noted previously, greater social support and less spousal solicitousness 1 month after amputation were associated with improvement in pain interference scores 2 years later.
Chronic postsurgical pain & post-traumatic stress disorder
Post-traumatic stress disorder (PTSD) typically develops after exposure to an event or situation that is perceived to be threatening to the physical or emotional integrity of an individual. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision) diagnostic criteria Citation[67] for PTSD cover three symptom clusters, including re-experiencing the traumatic event (e.g., nightmares and ‘flashbacks’), emotional numbing (e.g., feeling detached from others), avoidance of thoughts, feelings and activities associated with the trauma and increased arousal (e.g., insomnia, exaggerated startle reflex and hypervigilance). Recent data show that chronic pain and PTSD are highly comorbid Citation[68,69].
Salomons et al. presented two case reports of patients who developed PTSD following an episode of awareness under anesthesia Citation[70]. For both patients, post-traumatic sequelae persisted for years and included pain symptoms that resembled, in quality and location, the very pain experienced during surgery. In addition to the similarity to the original pain, these pain symptoms were triggered by stimuli associated with the traumatic situation, suggesting that they were flashbacks to the episode of awareness under anesthesia. Both patients participated in individual, trauma focused, cognitive–behavioral therapy with successful resolution of the PTSD.
Katz et al. evaluated the relationship between the post-traumatic stress symptom (PTSS) emotional numbing and chronic postsurgical pain disability 6 and 12 months after lateral thoracotomy using a prospective, longitudinal design Citation[57]. A total of 54 patients who were scheduled to undergo posterolateral thoracotomy for intrathoracic malignancies were recruited 1 week before surgery and completed validated questionnaires assessing pain disability and PTSS. Pain intensity and total morphine consumption were recorded 24 and 48 h after surgery; 6 and 12 months after surgery patients were telephoned to determine chronic postsurgical pain incidence, pain disability and PTSS. The incidence of chronic post-thoracotomy pain was 68.1 and 61.1% at the 6- and 12-month follow-ups, respectively. Multiple regression analyses showed that neither preoperative factors nor acute movement-evoked postoperative pain predicted 6- or 12-month pain disability. However, concurrent pain intensity and emotional numbing, but not avoidance symptoms, made unique, significant contributions to the explanation of pain disability at each follow-up (total R2 = 76.3 and 63.9% at 6 and 12 months, respectively). Over time, the relative contribution of pain intensity decreased, while that of emotional numbing increased, indicating a progressive decoupling of pain intensity and disability and a concomitant strengthening of the link between emotional numbing and disability. This suggested that pain may serve as a traumatic stressor that causes increased emotional numbing. The source of the emotional numbing is not known; it may arise from living with pain, which serves as a reminder of the cancer or living with a diagnosis of cancer, as well as the threat of and/or a recurrence of cancer. It is also possible that the emotional numbing is, in part, due to a previous unrelated traumatic event. Further research is needed to determine the role (if any) of the endogenous opioid system in mediating this effect. It is interesting to add in this context that there is a growing body of literature indicating that living with chronic pain is associated with structural changes (usually thinning) of the cortex in parts of the brain typically associated with aversive-affective processing of pain in the several chronic pain syndromes Citation[71–75].
Kleiman et al. provided preliminary evidence for a construct termed sensitivity to pain traumatization (SPT), which describes the anxiety-related psychological and emotional reactions, as well as behavioral responses to pain that resemble symptoms of PTSD Citation[76]. They evaluated the relationships among commonly used pain-related measures of anxiety and fear in 372 patients approximately 1 week prior to major surgery. Exploratory factor analysis (unrotated) of the combined scale items (n = 79) supported a two-factor solution accounting for 30 and 9.9% of the variance, respectively, and consisting of 75 items. Since the first factor included all but one item in the second factor, a one-factor solution better approximated the simple structure. A rotated (Varimax) solution suggested the existence of 11 small independent factors (each accounting for between 2 and 11% of the variance) comprising the same 74 items as in factor one of the unrotated solution, and accounting for 57.6% of the shared variance. The pattern of findings suggested that several lower order factors loaded on a single higher order factor, which they called SPT. Evidence for an overlap between SPT and PTSD symptoms involved clusters of lower order SPT factors describing intrusive thoughts, behavioral avoidance and hyperarousal. Importantly, SPT showed a strong and significantly positive correlation (r = 0.52) with the total score on the PTSD Checklist – Civilian Version suggesting good preliminary construct validity for SPT.
Taken together, the results of these studies Citation[57,70,76] indicate that PTSSs are associated with concurrent Citation[57,76] and subsequent Citation[57,70] pain and pain disability, months to years later, but the nature of the mechanisms underlying these relationships remain to be determined. One possible reason for the high comorbidity of PTSD and chronic pain may be the substantial symptom overlap common to both disorders, including: anxiety and hyperarousal, attentional biases, avoidant behaviors, emotional lability, and elevated somatic focus Citation[68]. The overlap in symptoms suggests the two disorders may be mutually maintaining Citation[68,77]. The idea of mutual maintenance may be linked to a common or shared underlying psychological vulnerability that may depend on shared genetic determinants and which places at-risk individuals at greater risk of developing one or both disorders. Anxiety sensitivity has been identified as one of the trait vulnerability factors that predispose individuals to the development of chronic pain, PTSD or both Citation[68].
The intractability of the two disorders is not surprising when viewed in the context of mutual maintenance and shared vulnerability models. This underscores the importance of screening for both disorders when either one is present. This may be especially relevant in patients scheduled for major surgery given that diagnosis and surgery are highly stressful events and that many individuals scheduled for surgery also have chronic pain.
From a treatment perspective, there is very little published on comorbid PTSD and chronic pain Citation[78] and nothing published on patients scheduled for surgery. There is some evidence that a cognitive–behavioral perspective that addresses both disorders is essential; otherwise, treatment of one (and not the other) would be expected to lead to partial recovery or, if complete recovery occurred in one domain, recovery might be transient Citation[68,70,78]. The decoupling of pain and PTSD symptoms with time would argue for the need of an early initiation of treatment. However, until we know more about the source and nature of the traumatic stressors that patients experienced, it may be premature to make treatment recommendations.
Patient-related factors associated with chronic postsurgical pain
One of the most consistent patient-related factors found to be associated with the development of acute and chronic postsurgical pain is concurrent or past pain Citation[6,9,79,80]. This appears to be true across surgery types and regardless of time frame. The presence of preoperative pain, or its intensity or duration, is a risk factor for the development of severe early acute postoperative pain Citation[81], acute pain days Citation[13,17,82–84] and weeks Citation[85] after surgery, as well as long-term postsurgical pain Citation[17,24,57,64,82,85–90]. Not only does preoperative pain predict later pain, severity of acute postoperative pain in the days and weeks after surgery predicts pain after discharge Citation[85,91] and is also a risk factor for the development of chronic postsurgical pain Citation[10,89,92–100].
No other patient factor is as consistently related to the development of future pain problems as is pain itself. Female gender Citation[81,85] and younger age Citation[81,85,98] predict chronic postsurgical pain, but not with the consistency or magnitude with which pain predicts pain. What must be determined, however, is the aspect(s) of pain that is predictive. There are several possibilities for why pain predicts pain, including those that propose a causal or associative role. The following factors are not mutually exclusive:
• Intraoperative nerve damage and the injury discharge that it causes Citation[36–38];
• Sensitization of nociceptors in the surgical field Citation[93];
• Early postoperative ectopic activity in the regenerating sprouts of injured primary afferents and in somata of these fibers in dorsal root ganglia associated with the injured nerves Citation[101], and even in somata of intact neurons in dorsal root ganglia neighboring those associated with injured nerves Citation[102];
• Collateral sprouting from intact nociceptive Aδ afferents that neighbor the field innervated by injured afferents Citation[94,103];
• Central sensitization induced by the surgery and maintained by peripheral input Citation[40,104–106];
• Structural changes in the CNS induced by perioperative nociceptive activity (e.g., disappearance of antinociceptive inhibitory interneurons in the spinal dorsal horn Citation[41,42] centralization of pain and somatosensory pain ‘memories’ Citation[105,107,108]);
• Heretofore unidentified pain genes that confer increased risk of developing intense acute pain and chronic postsurgical pain Citation[109–114];
• Consistent response bias over time. Some individuals have a tendency to report more intense pain than other individuals Citation[79]. Thus, they would report intense pain immediately after surgery as well as in the long term. Excluding this possibility is important for establishing other plausible mechanisms for the pain (e.g., see Citation[94]);
• Psychological and emotional factors, including emotional numbing Citation[57] and catastrophizing Citation[16,55,64];
• Social from environmental factors, such as solicitous responding from significant others Citation[16,55] and social support Citation[16];
• Publication bias in which findings of a significant relationship between pain before and after surgery are published whereas negative findings do not make it into print.
Genetics of chronic postsurgical pain
The genetics of pain is a very young research field (for recent reviews see Citation[112,113]). Therefore, it should come as no surprise that, as of yet, there are no published reports about genes predisposing humans for the transition of acute pain after surgery to chronic postsurgical pain. There are only a few reports that have identified polymorphisms in human genes associated with chronic pain. These include polymorphisms in the following genes: 5-HTTLPR (the gene encoding the serotonin transporter) that were found to associate significantly with levels of migraine Citation[115], burning mouth syndrome Citation[116], irritable bowel syndrome Citation[117] and fibromyalgia Citation[118]; IL1B (encoding IL-1β) plays a role in burning mouth syndrome Citation[116]; IL1RN (encoding IL-1 receptor antagonist) and MC1R (encoding the melanocortin-1 receptor) in vulvodynia Citation[119]; IL23R (which encodes a subunit of the receptor for IL-23) in Crohn’s disease Citation[120]; GCH1 (encoding GTP cyclohydrolase, an enzyme catalyzing tetrahydrobiopterin, BH4, an essential cofactor for catecholamine, serotonin and nitric oxide production) has been implicated in persistent radiculopathic pain following surgical diskectomy (note that the sought association in this study was not with pain postsurgery but with surgical alleviation of an existing chronic lumbar root pain that was caused by disk herniation) Citation[121]; COMT (encoding catechol-O-methyl transferase, the enzyme that inactivates dopamine, epinephrine and norepinephrine in the nervous system) has been implicated in a number of chronic pain entities (for review see Citation[122]); and polymorphisms in CACNA1, ATP1A2, MTHFR, SCN1A and the female hormone genes ESR1, ESR2 and FSHR have been implicated in migraines (for review see Citation[123]). Note, however, that in many of these studies it has not been established whether the identified genetic determinants were associated with chronic pain indirectly, by producing a disease that carries a morbidity for chronic pain, or directly, by producing and maintaining chronic pain once having the disease.
Pain genetic studies that are relevant to chronic postsurgical pain in rodent models have yielded the following results. Note that these models were not explicitly developed to model chronic postsurgical pain per se, yet used surgical approaches to injure peripheral nerves, spinal nerves, dorsal roots and the spinal cord Citation[26–33,124]. Some of these models were used to determine the level of heritability for spontaneous and stimulus-induced neuropathic pain Citation[114] in evaluating genetic differences across lines and strains of mice and rats in traits relevant to chronic postsurgical pain in humans Citation[110,111,125–130], and the effect of genetic and environmental interactions on chronic postsurgical pain-related behaviors in these models Citation[131–133]. These models were also used to map chromosomal regions (termed quantitative trait loci) that harbor chronic postsurgical pain-related genes Citation[114,125,131,134,135]. These intervals were mapped using sets of recombinant inbred lines that were produced by repeatedly breeding two inbred parental lines Citation[125], or by genotyping F2 offspring of inbred lines that contrast on the chronic postsurgical pain strain under study Citation[134]. Using gene-expression and proteomic methods, hundreds of genes and proteins were identified in dorsal root ganglia and spinal cord (but none in supraspinal structures as of yet) of mice and rats in chronic postsurgical pain-related models Citation[136–143]. Furthermore, manipulations of individual genes by overexpression or deletion (in transgenic ‘knockout’ experiments), or by shutting off expression using siRNA or antisense oligodeoxynucleotide injections, have identified over 240 rodent genes that have some demonstrated role in various acute and chronic pain models, some of which are relevant to chronic postsurgical pain. The information about these genes and the models in which they were studied is accessible on the internet Citation[301].
Phenomics of chronic postsurgical pain
A gene for chronic postsurgical pain is identified when evidence is presented of a statistically significant ‘association’ between a pain trait characterizing chronic postsurgical pain and a genetic marker, usually a single nucleotide polymorphism in or near these genes. Thus, discovering such genes critically depends on the pain traits collected from participants in a given cohort. There is currently no consensus over what these pain traits should be; moreover, ‘pain phenomics’, the research field that constructs questionnaires and other tools to collect clinically-relevant phenotypic pain data for genetic studies, has not been born as of yet. Consequently, phenomic questionnaires for chronic pain are currently unavailable but critically important to the identification of genes for chronic postsurgical pain. Chronic postsurgical pain, like other chronic pain syndromes, is multifaceted, characterized by multiple sensory–discriminative, affective–emotive and cognitive–evaluative variables. In order to identify genes for these dimensions/aspects and solve clinical problems of chronic postsurgical pain by addressing the neural structures and networks that bring them about, one should faithfully characterize each patient in as much detail as is practical. It is not enough to collect from the participants a categorical answer as to whether or not he/she has chronic postsurgical pain, and it is also not enough to limit the description of chronic postsurgical pain to the intensity of pain on a 0–10 visual analog scale. This is because there may very well be different genes controlling the interindividual variability in pain intensity of a typical pain episode, different genes for the frequency of such episodes, other genes for the amount of suffering the pain causes, additional genes for the type of the pain (e.g., ‘burning’ vs ‘electrical shock-like’), yet different ones for the spread of pain beyond the surgical field where nerves were injured and so on. Therefore, if we are to provide a comprehensive solution to chronic postsurgical pain based on genetics, we must develop tools and questionnaires to collect from every participant in a genetic study a broad array of phenotypes (i.e., quantifiable pain traits).
Expected gains for chronic postsurgical pain medicine in the postgenomic era
It is expected that a replicated genome-wide association study of chronic postsurgical pain, if powered statistically to detect genetic variations of small effects, followed by gene validation experiments, will discover most genetic variations that affect the transition to pain chronicity Citation[113,144,145]. This knowledge will be translated to the development of novel diagnostic kits that assess the risk an individual carries for developing chronic pain (if undergoing a surgery that carries significant morbidity for chronic postsurgical pain). Similar kits are expected to provide prognostic predictions that would enable caregivers to ‘tailor’ the best treatment choices an individual is expected to benefit from, based on his/her ‘genetic fingerprints’. These kits could also be used to better select subjects for clinical trials, thereby minimizing costs and shortening drug development times. New compounds would be developed that provide effective pre-, intra- and post-operative care, thereby preventing the outbreak of chronic postsurgical pain. Tagging the product of such genes with antibodies and carrying out immunohistological studies in animal models of chronic postsurgical pain could uncover new pain mechanisms and identify neuronal and/or glial cell types expressing chronic postsurgical pain genes, and their networks, some of which are currently unknown to us. Better rodent models that carry chronic postsurgical pain genes, relevant to human pain, could be developed and used as a platform to test the efficacy of novel drugs for chronic postsurgical pain. Finally, gene therapy would make it possible to patch ‘bad’ gene variants that carry the risk for chronic postsurgical pain. Judging by the rapid advancements in the Genome Project, this futuristic scenario may become a reality in our lifetime.
Preventive analgesia
As depicted in , it is important to ascertain the precise mechanisms that underlie the relationship between pain at time one (e.g., preoperative pain or acute postoperative pain) and pain at time two (e.g., 1 year after surgery). The idea that pain is in some way etched into the CNS Citation[108,146,147] has been at the heart of efforts to halt the transition to chronicity by blocking noxious perioperative impulses from reaching the CNS using a pre-emptive or preventive pharmacological approach. However, if the relationship between acute postoperative pain and chronic pain is merely associative, and both are caused by one or more factors that themselves are inter-related, then no type or amount of blocking will prevent the development of chronic postsurgical pain.
Studies in a rodent model of PLP indicated that blocking afferent input from the surgical field at the time of nerve injury and shortly thereafter prevents chronic neuropathic pain-related behavior, while artificially prolonging it, especially in C-fibers, increases the chronic pain behavior Citation[36,37,38,129,148–150]. This line of basic science research continues to introduce novel compounds (e.g., ralfinamide Citation[150]) that show positive pre-emptive effects in rodent models of neuropathic pain and which could be tested as pre-emtpive analgesics in humans. These findings have taken a long time to impact clinical practice and, as such, the management of acute postoperative pain has been dominated by an outdated concept of pain. Pain was viewed as the end product of a passive system that faithfully transmits a peripheral pain signal from nociceptors to a pain center in the brain. This view has resulted in a strategy for managing postoperative pain that is inadequate, in part because it treats the patient only after the pain is well established. Patients arrive in the postanesthetic care unit (recovery room) after surgery, often in extreme pain, where they receive multiple doses of analgesics in a short period of time in an effort to bring the pain down to a tolerable level Citation[151,152]. However, basic science and clinical data show that brief, noxious inputs or frank injury that activates A- and mainly C-fibers (e.g., cutting skin, muscle or nerve fibers) induce long-lasting changes in central neural function that persist well after the offending stimulus has been removed or the injury has healed Citation[40–42,44,104–107]. This view of pain, involving a dynamic relationship between peripheral and central mechanisms, is inconsistent with the archaic notion that pain results from transmission of impulses along a straight through, feed-forward pathway from a site of injury to the brain Citation[153].
The practice of treating pain only after it has become well entrenched is slowly being replaced by a preventive approach that aims to block the transmission of the primary afferent injury discharge, the inflammatory response, and ensuing ectopic activity Citation[154–157]. The intent behind this approach is not simply that it reduces nociception and stress during surgery – although these are also worthwhile objectives. The idea that acute postoperative pain might be intensified by a state of central neural hyperexcitability induced by surgery can be traced to Crile Citation[158] and later to Wall Citation[159], who suggested that ‘pre-emptive preoperative analgesia’ would block the induction of central neural sensitization brought about by incision and thus reduce acute postoperative pain intensity. Since its introduction, this concept has been refined, based in part on confirmatory and contradictory clinical evidence, developments in basic science and critical thought. The suggestion that surgical incision triggers central sensitization Citation[159] has been expanded to include the sensitizing effects of preoperative noxious inputs and pain, other noxious intraoperative stimuli, as well as perioperative peripheral and central inflammatory mediators and ectopic neural activity Citation[160].
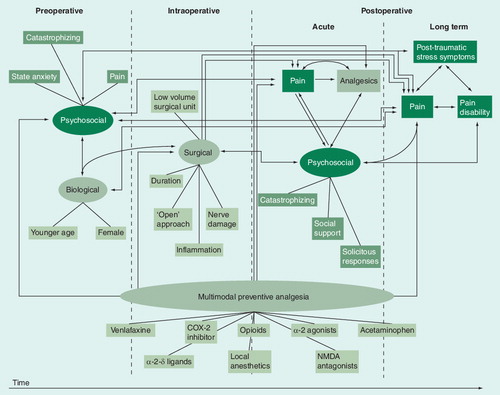
The perioperative period can be divided into three distinct phases: pre-, intra- and post-operative. Specific factors within these phases contribute to the development of acute postoperative pain. These factors include: preoperative noxious inputs and pain; A- and C-fiber injury barrage resulting from the cutting of skin, muscle, nerve and bone, or wound retraction; and postoperative peripheral nociceptive input and input in low-threshold afferents, including that arising from the inflammatory response and ectopic neural activity in the case of nerve injury. Each of these factors can contribute to peripheral and central sensitization and each, therefore, can be targeted using a preventive approach. We propose that the relative contribution of these factors to acute postoperative pain is dependent on the surgical procedure, extent and nature of tissue damage, duration of surgery, timing of treatments relative to incision, pharmacokinetics of the agent(s) used preoperatively, presence or absence of additional analgesia intraoperatively, nature of postoperative analgesia and many other variables, including the genetic background that contributes a predisposition to be affected by any one of the parameters mentioned earlier. Minimizing the negative impact of as many as possible of these factors across the three perioperative phases will increase the likelihood of preventing the induction and maintenance of peripheral and central sensitization. Preventing sensitization will reduce pain and analgesic requirements Citation[160].
In contrast to pre-emptive analgesia, which requires two groups of patients to receive identical treatment before or after the same type of incision or surgery Citation[161], the focus of preventive analgesia is not on the relative timing of analgesic or anesthetic interventions, but on attenuating the impact of the peripheral nociceptive barrage associated with noxious preoperative, intraoperative and/or postoperative events/stimuli. The rationale for preventive analgesia is to capitalize on the combined effects of several analgesic agents, administered across the preoperative, intraoperative and postoperative periods, in reducing peripheral and central sensitization Citation[160]. Therapeutic benefits associated with multimodal regimens include improved efficacy, lower doses and fewer adverse effects Citation[162].
Preventive analgesia is demonstrated when postoperative pain and/or analgesic use are reduced beyond the clinical duration of action of the target agent, which has been defined as more than 5.5 half-lifes of the target agent Citation[160,163]. This requirement ensures that the observed effects are not simply analgesic effects. A recent review of the preventive analgesia literature indicates that, across a variety of classes of agents, preventive analgesia reduces pain, analgesic consumption or both at a point in time that exceeds 5.5 half-lifes of the target agent Citation[160]. Although the evidence favors a preventive approach, relatively few studies have been designed to examine the possibility that chronic postsurgical pain can be prevented or attenuated, as reviewed later.
Postamputation PLP
A series of studies, beginning with the promising results of the nonrandomized study by Bach et al.Citation[164], have examined the long-term benefits of perioperative epidural analgesia in reducing the incidence and intensity of PLP after limb amputation Citation[165]. The initial promise, however, has not materialized. In what is perhaps the most methodologically sound study conducted on the topic to date, Nikolajsen et al. evaluated the long-term effects on phantom limb and stump pain of continuous epidural morphine and bupivacaine administered 18 h before, during and for approximately 1 week after lower limb amputation Citation[166]. The control group received epidural saline before and throughout the surgical procedure followed by epidural morphine and bupivacaine postoperatively. There were no significant differences between the groups in pain incidence, intensity or opioid consumption at any time up to 12 months after surgery. Subsequent randomized, controlled studies using perioperative epidural bupivacaine and diamorphine Citation[167], intrathecal bupivacaine and ketamine Citation[168], intravenous ketamine for 3 days Citation[169], epidural bupivacaine and ketamine for up to 3 days Citation[168] have failed to find a significant long-term reduction in PLP incidence or intensity compared with placebo control conditions. Moreover, only one Citation[170] of the three randomized, double-blind studies Citation[170–172] conducted to evaluate the efficacy of oral gabapentin on already established PLP has shown a superiority of gabapentin over a placebo control condition.
The one recent positive study by Schley et al. compared two groups that underwent unilateral amputation ranging from removal of one finger to the entire forearm Citation[173]. All amputees received postoperative analgesia by continuous brachial plexus anesthesia (ropivacaine 0.375% 5 ml/h) for 7 days. Thereafter, all participants could receive additional boli of ropivacaine 0.375% upon request. The main variable of difference between the groups was that for 4 weeks postamputation, one group received a daily dose of the NMDA receptor blocker memantine (20–30 mg) and the other group received placebo. During the first week postamputation, the memantine-treated group needed significantly fewer local anesthetic blocks of the brachial plexus to relieve their pain. Moreover, at 4 weeks and 6 months after surgery, but not at 12 months, the memantine-treated group had significantly lower rates of PLP, and those with PLP reported lower pain intensity compared with the placebo group.
Several related issues require further study with regard to prevention of postamputation PLP:
It is important to distinguish between the effects of preamputation pain location, duration, frequency and intensity Citation[108], and those of the surgical procedure (i.e., cutting of skin, muscle, bone, and ligation and transection of major nerve trunks) on the development of chronic PLP and stump pain Citation[108]. To date, studies have not separately evaluated the nature, quality and intensity of preamputation pain versus acute postamputation pain. Other, more recent studies in nonamputee populations, have examined this issue as it pertains to the development of acute, as opposed to chronic, postsurgical pain. The data suggest that in the presence of presurgical pain, preoperative administration of analgesics does not lead to the anticipated lessening of postoperative pain or analgesic consumption, perhaps because central sensitization has already been established and the treatment could not reverse it. Postoperative pain and analgesic consumption were significantly reduced by pre- and intra-operative epidural morphine, but not saline, for patients who did not report presurgical pain Citation[174]. However, among patients with presurgical pain, pre- and intra-operative epidural morphine was no more effective than saline Citation[174]. This raises the important issue of what effect blocking preoperative pain would have on the intensity of acute postoperative pain. A recent study of patients undergoing total hip arthroplasty showed that relief of preoperative pain by epidural ropivacaine for at least 12 h before surgery, followed by intraoperative epidural ropivacaine, reduced patient-controlled epidural anesthesia ropivacaine consumption 48 h after surgery compared with preoperative epidural saline and intraoperative epidural ropivacaine Citation[175]. Based on these interesting and controversial findings, future studies should report on the presence (and duration) or absence of presurgical pain.
An important factor that has not received much attention is the distinction between preoperative pain related to the surgical procedure (i.e., in the anatomical region of the surgery, such as preoperative pain in the limb to be amputated) and pain that is unrelated to the surgical procedure (e.g., chronic lower back pain in a patient scheduled for posterolateral thoracotomy). A recent study of patients following radical prostatectomy found that patients were significantly at risk of developing chronic postsurgical pain whether or not the preoperative pain was related to the surgical site Citation[90]. This distinction is important, not only to PLP after limb amputation, but to all surgeries, since these two situations may implicate different mechanism(s) underlying the development of chronic postsurgical pain.
A coexisting disease that affects peripheral nerve function may have contributed to the general lack of significant findings Citation[176]. Likewise, such disease could have a deleterious effect on the neuroprotective capacity of the CNS to resist the excitotoxic impact of injury discharge. The vast majority of negative studies have recruited patients with diabetes and/or peripheral vascular disease, in contrast to the positive results found by Schley et al., who studied individuals with acute trauma of the upper extremity requiring amputation Citation[173].
Finally, further study is needed to improve the effectiveness of the perioperative blockade by using local anesthesia of peripheral nerves plus intrathecal analgesia. To date, the vast majority of negative studies have used epidural analgesia, which may not provide a sufficiently dense afferent blockade Citation[177] to prevent central sensitization and rostral transmission of afferent input. Even a clinically effective blockade with intrathecal bupivacaine does not completely block nociceptive impulses from reaching the brain Citation[178], suggesting that central sensitization might take place even with subclinical nociceptive inputs.
Postmastectomy pain
Recent evidence from well-controlled studies indicates that gabapentin, which binds to the α-2-δ subunit of the calcium channel (Cavα2-δ Citation[179]), provides profound pain relief and opioid-sparing effects in the acute postoperative setting Citation[180]. Two studies have focused on prevention of chronic postsurgical pain following breast surgery. Fassoulaki et al. randomized 50 patients undergoing breast cancer surgery to one of two groups using a triple-dummy, double-blind design Citation[181]. Patients in the multimodal treatment group received gabapentin (400 mg q6 h) starting the evening before surgery, transdermal eutectic mixture of local anesthetics (EMLA cream) beginning the day of surgery, and intraoperative ropivacaine irrigation of the brachial plexus and several intercostal spaces. Patients in the control group received placebos in place of the three active agents. After surgery, patients continued to receive gabapentin (400 mg) or placebo every 6 h for 8 days in addition to transdermal eutetic mixtures of local anesthetic cream or placebo cream daily for 3 days. At 3, but not 6, months after surgery, patients in the multimodal treatment group had a significantly lower incidence of axilla pain (14 vs 45%), arm pain (23 vs 59%) and analgesic use (0 vs 23%) compared with the placebo control patients.
These results contrast with an earlier randomized controlled trial by the same group using the same patient population Citation[182]. Patients scheduled for breast surgery for cancer were randomly assigned to receive gabapentin (200 mg), mexiletine (400 mg) or placebo capsules three-times daily beginning the evening before surgery and continuing for 10 days after surgery. Although gabapentin proved to be significantly more effective than mexiletine or placebo in reducing acute movement-evoked pain intensity in the first 5 days after surgery, the 3-month follow-up failed to demonstrate a difference in the incidence or intensity of chronic pain. However, the incidence of burning pain was significantly lower in the two treatment groups compared with the placebo control group Citation[182]. The failure to find a significant reduction in pain incidence or intensity at the follow-up may have been owing to an insufficient dose of gabapentin.
Iohom et al. compared the efficacy of a comprehensive, preventive multimodal analgesic regimen to a standard treatment in a randomized, nonblinded trial of 29 women undergoing surgery for breast cancer (mastectomy or breast tumor resection with axillary node clearance) Citation[100]. Patients in the standard treatment group (Group S) received intramuscular morphine (0.1 mg/kg every 4 h), diclofenac suppositories (100 mg every 12 h) and dextropropoxyphene hydrochloride 32.5 mg plus oral acetaminophen 650 mg every 6 h for 48 h. Beginning 12 h before surgery, patients in Group N received intravenous parecoxib (a COX-2 inhibitor) 40 mg every 12 h followed by oral celecoxib (a COX-2 inhibitor) 200 mg until day 5 after surgery. In addition, before surgery, a paravertebral catheter was inserted and a continuous block established for up to 48 h after surgery with bupivacaine 0.25% (10 ml 12 hourly). Finally, patients in Group N also received oral acetaminophen 1 g every 6 h for 48 h. All patients received a general anesthetic for surgery. Pain intensity after movement was significantly lower in Group S compared with Group N across the 48-h study period. A telephone follow-up 2–3 months later, conducted by a blinded interviewer, showed a significantly lower incidence (and intensity) of chronic postoperative breast surgery pain in Group N (0%) than Group S (85%). While it is not possible to attribute the long-term prevention of pain specifically to COX-2 inhibition, these results do suggest that parecoxib and celecoxib contributed to the reduced incidence of chronic pain, consistent with evidence and predictions from the basic sciences Citation[183]. Notwithstanding these positive effects, there have been serious concerns raised regarding the potential increased risk of cardiovascular thrombotic and myocardial events associated with the administration of COX-2 inhibitors Citation[184–186] and, more generally, with nonselective NSAIDs Citation[184].
Postradical prostatectomy pain
Gottschalk et al. conducted a randomized, double-blind study of 100 men undergoing radical retropubic prostatectomy Citation[187]. Patients were assigned to one of the three groups to receive either preoperative epidural bupivacaine, epidural fentanyl or epidural saline followed by postoperative patient-controlled epidural analgesia with morphine and bupivacaine for all three groups. Acute pain intensity while in hospital was significantly lower in the two groups treated before incision compared with the saline control condition. Patients were followed up by telephone interview 3.5, 5.5 and 9.5 weeks after surgery. Activity scores 3.5, but not 5.5 or 9.5, weeks after surgery, were significantly higher than in the groups that received the epidural agents before and during surgery. The incidence of pain at 9.5, but not at 3.5 or 5.5, weeks after surgery was significantly lower in the groups that received the epidural agents before and during surgery compared with the control group. The authors concluded that the intraoperative administration of epidural fentanyl or bupivacaine significantly reduces the intensity of acute postoperative pain, pain incidence 9.5 weeks after surgery and is associated with higher activity levels postdischarge.
Katz et al. evaluated the short- and long-term effects of preoperative or postincisional intravenous fentanyl plus low-dose intravenous ketamine versus a standard treatment receiving intravenous fentanyl but not ketamine on postoperative pain and analgesic use in men undergoing radical prostatectomy under general anesthesia Citation[188]. Pain scores and von Frey pain thresholds did not differ significantly among groups at any point in time during the first 3 days after surgery. Although day 3 cumulative patient-controlled analgesia (PCA) did not differ significantly among groups, on the third day after surgery the hourly rate of morphine consumption was significantly lower in the pretreated group compared with the two other groups. Follow-ups at 2 weeks and 6 months did not reveal significant group differences in pain incidence, intensity, disability or mental health. The addition of low-dose intravenous ketamine to a standard general anesthetic did not result in a clinically meaningful reduction in short-term pain or morphine consumption. Given the ineffective intervention, it is not surprising that there were no differences in the longer term.
Post-thoracotomy pain
Three studies have attempted to prevent the incidence and/or intensity of chronic pain after posterolateral thoracotomy with preoperative thoracic epidural analgesia Citation[95,189,190]. Obata et al. compared the pre-emptive effects of administering a 72-h epidural infusion of a local anesthetic, mepivacaine, beginning before incision versus after completion of surgery in 70 patients scheduled to undergo thoracotomy Citation[189]. Follow-up at 3 and 6 months showed a significant reduction in the incidence of chronic post-thoracotomy pain in the group of patients that received the epidural before surgery. These results were not confirmed by Ochroch et al. who randomly assigned 175 patients to receive thoracic epidural bupivacaine and fentanyl before incision or after rib approximation Citation[190]. The incidence of chronic post-thoracotomy pain at 48 weeks did not differ significantly between the groups treated before versus after incision. Similarly, Senturk et al. randomized 69 patients scheduled to undergo thoracotomy to one of three groups: preoperative thoracic epidural bupivacaine and morphine followed by postoperative epidural PCA, postoperative epidural PCA with bupivacaine and morphine or postoperative intravenous PCA with morphine Citation[95]. In contrast to the results of Obata et al., there were no significant differences 6 months after surgery between the two epidural groups, but both showed a significantly lower incidence and intensity of chronic post-thoracotomy pain than the intravenous PCA group Citation[189]. A more recent report examined the efficacy of gabapentin in reducing established post-thoracotomy pain Citation[191] based on preliminary findings from a case series of patients with severe pain at least 4 weeks after thoracic surgery or trauma Citation[192]. Patients were recruited into the trial if they had moderate-to-severe chronic post-thoracotomy pain (>5 on a 10 cm Visual Analogue Scale [VAS] and >12 on the Leeds Assessment of Neuropathic Symptoms and Signs [LANSS]) of at least 3 months duration that had proved refractory to earlier treatment and were assigned to receive either gabapentin or sodium naproxen (a nonselective NSAID) Citation[191]. At the end of the 60-day treatment period, the percentage of patients that still had severe pain was reduced significantly in the gabapentin group (15%) compared with the naproxen group (∼85%). Although these two studies were not randomized or blinded, they point to the next logical step of administering gabapentin before surgery and for several months following surgery in an attempt to prevent the development of chronic post-thoracotomy pain.
Chronic pain after iliac crest bone graft harvest
Iliac bone graft harvest for spinal fusion results in significant morbidity, including functional impairment, as well as severe pain at the harvest site in approximately 3% of patients Citation[193]. Two small scale, randomized, controlled, double-blind studies have attempted to prevent chronic bone graft harvest site pain with multiple injections of bupivacaine or bupivacaine and morphine into the harvest site beginning 10 min after the start of surgery Citation[194] and a 48-h continuous infusion of bupivacaine into the harvest site beginning at the time of wound closure after procurement of the graft Citation[195]. Both studies found a significant reduction in acute postoperative harvest-site pain and analgesic consumption, indicating that the interventions were efficacious. By 12 weeks after surgery the incidence of harvest-site pain was significantly lower in the bupivacaine plus morphine treated patients (none out of 15) than the saline control group (five out of 15) Citation[196]. In the other study, a 4-year follow-up showed that chronic pain was not present in any of the nine bupivacaine-treated patients and was present in seven out of the ten saline controls Citation[195]. Replication of these results with a larger sample size is required. These studies suggest that preventive analgesia can be achieved even when the analgesic intervention is started after incision and bone graft harvest (i.e., in the context of an unchecked peripheral nociceptive injury barrage during surgery).
Long-term pain after major abdominal–gynecological surgery
Katz et al. evaluated the short- and long-term preventive effect of perioperative administration of intravenous alfentanil for women scheduled for abdominal hysterectomy performed under general anesthesia Citation[196]. In total, 45 patients were randomly assigned to one of three groups in a double-blinded manner to receive no alfentanil, low-dose alfentanil (30 µg/kg at induction followed by intraoperative bolus doses of alfentanil 10–20 µg) or high-dose alfentanil (100 µg/kg at induction followed by an intraoperative infusion of alfentanil 1–2 µg/kg/min) before and during surgery. The results showed that, although a composite measure of pain and morphine consumption was significantly lower in the high- versus low-dose group up to 6 h after surgery, and significantly lower than the no dose group up to 12 h, these results were not clinically meaningful. A 6-month telephone follow-up did not reveal any significant differences among groups in pain incidence or severity. The authors conclude that surgical procedures carried out under general anesthesia alone using standard doses of opioids intraoperatively provide suboptimal protection from the injury barrage brought about by incision and subsequent noxious surgical events.
In another randomized, controlled, double-blind, study of 141 women undergoing abdominal gynecological surgery by laparotomy, Katz et al. found that preincisional, but not postincisional, administration of epidural lidocaine and fentanyl was associated with a significantly lower rate of morphine and reduced secondary mechanical hyperalgesia 48 h after surgery compared with a standard treatment group that received a sham epidural Citation[197]. Follow-up at 3 weeks showed that pain disability ratings were significantly lower in the two groups that received the active epidural when compared with the standard treatment group, but no intergroup differences in pain, pain disability or general mental health were found at the 6-month follow-up Citation[198].
Long-term pain after thyroidectomy
Brogly et al. evaluated the preventive effect of a single preoperative dose of gabapentin 1200 mg versus placebo in a randomized, controlled, double-blind trial of 50 patients undergoing thyroidectomy under general anesthesia Citation[199]. All patients received a bilateral superficial cervical plexus block with ropivacaine and clonidine after induction of the general anesthetic. Neither acute pain scores at rest, nor after swallowing, nor analgesic consumption, differed significantly between the groups across the 24-h study period. However, 6 months after surgery, in a follow-up mail survey, the authors found that the incidence of neuropathic pain complaints was significantly lower in the patients that received preoperative gabapentin (4.3%) than placebo (29.2%). The delayed effect of gabapentin is difficult to explain in the absence of a similar effect in the acute postoperative period when the drug was active. The authors suggest that the absence of an early effect of gabapentin may have been masked by the bilateral superficial cervical plexus block.
Long-term pain after major digestive surgery
Lavand’homme et al. randomly assigned 85 patients undergoing major digestive surgery to one of four groups to assess the preventive analgesic effects of administering a local anesthetic (lidocaine or bupivacaine), an opioid (sufentanil or morphine) and an α2 adrenergic agonist (clonidine) by the intravenous or epidural route, before and/or after surgery under general anesthesia Citation[200]. Patients received the three agents by the intravenous (Group 1) or epidural (Group 3) route before surgery and immediately after recovery for 72 h, the intravenous route followed the epidural route (Group 2) or the epidural route followed by the intravenous route (Group 4). All patients also received a bolus dose plus infusion of low dose ketamine, which was started before incision and maintained throughout the procedure. Postoperative analgesics were delivered by an intravenous or epidural PCA pump. The results showed that across the 72-h study period, the number of patient-controlled requests for postoperative analgesia and pain scores at rest, after movement and after coughing were significantly higher in Group 1 compared with the three other groups. The area of secondary mechanical hyperalgesia to punctuate stimulation surrounding the incision showed the same pattern of results: the groups that received the epidural, whether before, after, or before and after surgery, showed a smaller area of hyperalgesia than the group that received the agents by the intravenous route before and after surgery. Patients were followed up by telephone and mail survey 2 weeks, 1, 6 and 12 months after surgery. The incidence of chronic postsurgical pain in Group 1 at the 6- (48%) and 12- (28%) month follow-ups was significantly greater than the zero incidence in Groups 3 and 4 at both time points. The 12-month results suggest a greater contribution to chronic pain of noxious intraoperative versus postoperative afferent input given the findings that the incidence of chronic pain in Group 2 was neither different from that of Group 4 nor Group 1. Overall, the long-term results point to the importance of perioperative nociceptive blockade by the epidural route in preventing chronic postsurgical pain after major digestive surgery.
Taken together, the results from the studies of preventive multimodal analgesia are equivocal. While there is some evidence that chronic postsurgical pain can be minimized or prevented by an analgesic approach involving aggressive perioperative multimodal treatment, other studies fail to show this benefit. A careful examination of these results raises several related issues that we must address, which are now discussed.
The results show significant reductions in the incidence and/or mean intensity of long-term pain problems in some cases. However, a preventive analgesic approach does not work for everyone and, at present, we do not know for whom such an approach is effective. One might assume that the mechanisms underlying the interindividual differences in efficacy of preventive analgesia is controlled genetically. When these determinants are identified, it might be possible to extend the efficacy of preventive analgesia for certain individuals.
We also do not know the mechanism(s) by which chronic postsurgical pain is reduced when preventive analgesia is effective. The early pain relieving effects can be attributed to the pharmacological action of the agents used preventively; however, by definition Citation[155,160,163] for studies that compare an active agent with a placebo control condition, preventive analgesia requires that the reduction in analgesic consumption and pain be observed at a point in time that exceeds the clinical duration of action of the target agent used preventively. This is meant to rule out the possibility that the observed effect is simply an analgesic effect. The typical explanation for a prolonged effect is that the agent(s) prevented (obtunded) the injury discharge, and/or peripheral and/or central sensitization and, thereby, reduced long-term pain. However, there really is very little good evidence that this is in fact the case, since we do not have accurate measures of sensitization in humans, and even if we did, this still would not indicate that reduction in sensitization is responsible for the long-term reductions in pain incidence and/or intensity. The longer the time from the administration of the analgesic agent(s), the greater the probability that other factors contribute to the long-term effects. For example, in discussing the effectiveness of perioperative epidural analgesia versus a sham epidural in reducing pain disability scores, but not pain intensity scores, 3 weeks after surgery Citation[197], Katz and Cohen suggested that the reduced hyperalgesia and rate of morphine consumption within the first 2 days after surgery afforded the epidural groups a ‘head start’ in terms of comfort level and recovery compared with the sham epidural group, possibly increasing their self-efficacy in dealing with pain or in mobilization Citation[198]. A similar finding has been reported with respect to activity levels 3.5 weeks after radical retropubic prostatectomy Citation[187]. In that study, activity levels but not pain intensity were significantly higher in patients who had received pre-emptive epidural bupivacaine or fentanyl. Thus, the mechanisms underlying preventive analgesia are probably more varied than currently acknowledged.
We do not know why preventive analgesia does not work in some patients. One explanation that has not been investigated is that for some patients, the drug dose simply may not be large enough or changes in the kinetics or dynamics of the drug under investigation may have an impact on the results. Another possibility is that preoperative pain interferes with the effectiveness of preventive analgesia, perhaps because central sensitization has already been established Citation[15,164,174,175]. Finally, genetic differences are also likely to play a role, in that study groups may have included a variable mixture of individuals susceptible or resistant to the potential pre-emptive effect of the studied treatment, thereby masking a real effect.
Chronic pain is a complex experience that manifests in several major domains, which include sensory–discriminative, affective–aversive and cognitive–evaluative. Each domain comprises several subdomains, for example, the sensory–discriminative domain consists of pain intensity (ranging from the lowest to the maximal; typical intensity), pain location (is it felt in the surgical field and/or can spread ‘extraterritorially’ to areas not innervated by injured nerves, can be felt as ‘mirror image’ pain on the contralateral intact side as well), pain quality (e.g., burning, crushing and electrical shock-like) and temporal (episodic or constant; if episodic, the frequency and duration of episodes is essential to document) subdomains. Similar breakdown into subdomains can be demonstrated for the affective–emotive and cognitive–evaluative dimensions. It is well established that parts of the brain that process sensory–discriminative aspects of the pain are largely separate from those that process affective–aversive aspects (e.g., S1 and S2 cortices and limbic areas, respectively). Moreover, each aspect largely engages unique biochemistry and targets for drugs including, presumably, those relevant for preventive treatments. However, the main outcome measures in the vast majority of randomized controlled trials are simply pain intensity and/or presence/absence of pain and analgesic use. While there is some correlation between pain intensity and the aversive–affective aspects of the pain, this correlation is not very tight and the intensity cannot be taken as the sole parameter for the complex experience that pain is, especially when it has become chronic. It is rare to find a study that is more comprehensive in the outcome measures assessed. Of particular relevance to chronic postsurgical pain is the tendency for the anesthesia literature to focus on outcome measures of pain and analgesic use, and the psychological literature to focus on measures of pain disability or pain interference, making cross-study comparisons difficult. Recommendations for assessment of core measures and domains in clinical trials Citation[201] include relevant psychological, emotional and physical–functional variables, in addition to those routinely assessed (i.e., pain and analgesic use). Assessment of additional domains of functioning may help to shed light on the predictors of severe acute postoperative pain, the processes involved in recovery from surgery and the risk factors for developing chronic postsurgical pain Citation[198].
Expert commentary
As depicted in , the transition of acute postoperative pain to pathological chronic postsurgical pain is a complex developmental process involving biological, psychological and social-environmental factors across the three phases of the perioperative period (preoperative, intraoperative and postoperative). The noxious effects of surgery (e.g., arising from incision, retraction, inflammatory response, ectopic activity following nerve injury and CNS reorganization into a centrally sensitized state), in conjunction with the competing, beneficial effects of perioperative, preventive multimodal analgesia, interact with pre-existing and concurrent pain, psychological and emotional factors (e.g., catastrophizing and pain coping strategies), as well as the social environment (e.g., solicitousness and social support) to determine the nature, severity, frequency and duration of chronic postsurgical pain. Effectiveness of psychological management programs for other chronic pain problems is well established, but prevention and treatment efforts for chronic postsurgical pain have lagged behind. Development of a truly multimodal treatment regimen is essential, including not only modification of known surgical risk factors and protective pharmacotherapy, but also provision of perioperative psychological pain management programs aimed at preventing and treating chronic postsurgical pain. We are, at present, a long way from being able to predict with certainty who will recover uneventfully and who will go on to develop debilitating chronic pain. Finally, pain genetics carries the hope that such a reality can be achieved in our lifetime.
Five-year view
We do not expect significant gains to be made in the prevention of chronic postsurgical pain within the next 5 years. However, with the relatively recent introduction, and perioperative use, of the COX-2 inhibitors and the Cavα2-δ ligands, gabapentin and pregabalin, we may begin to see evidence of a reduction in the incidence and intensity of certain chronic postsurgical pains when used in combination with other agents in a balanced regimen of multimodal analgesia. Since these agents are available for oral administration, patients can be discharged with orders to continue to take them for several weeks after surgery, thus providing the opportunity for prolonged inhibition of the inflammatory cascade and suppression of ectopic activity. Both the COX-2 inhibitors and the Cavα2-δ ligands have been shown to produce clinically significant reductions in pain and opioid requirements in the acute postoperative setting and a logical extension would be to provide ongoing treatment with these agents to help minimize the transition to pain chronicity. We anticipate that some progress will be made in identifying heretofore unknown psychosocial predictors of chronic postsurgical pain and, hopefully, we will see the beginnings of psychological treatments aimed both at preventing chronic postsurgical pain and better managing it once it has developed. Clinically relevant behavioral mouse and rat models are available for the study of mechanisms underlying acute and chronic postsurgical pain and they can be used as platforms for research and development of the next generation of analgesics, such as ralfinamide, that shows pre-emptive analgesic efficacy in rodent models. A very promising new research field that lags behind research into other human diseases is pain genetics. Powerful genetic assays are available to identify the genes controlling the interindividual variability in developing such pain types, as well as the variance in the efficacy of preventive and palliative analgesia. In the coming 5 years, we should see the first signs of the ‘genetic revolution’ in pain medicine that will identify novel treatment targets.
Table 1. Incidence of chronic postsurgical pain following various surgical procedures.
Key issues
• Very few studies define chronic postsurgical pain. The absence of an operational definition of chronic postsurgical pain has hindered progress in epidemiological and clinical research, making cross-study comparisons difficult and partly explaining the large variability in estimates of chronic postsurgical pain and the efficacy of pre-emptive analgesia on this type of pain.
• Notwithstanding these obstacles, severe chronic postsurgical pain occurs in up to 10% of patients.
• Surgical procedures with the greatest incidence of chronic postsurgical pain are associated with intentional or unintentional nerve damage, such as limb amputation, mastectomy and posterolateral thoracotomy. The incidence of chronic pain after these surgical procedures ranges between approximately 60 and 70%.
• Avoiding nerve damage during surgery will undoubtedly reduce the incidence of chronic postsurgical pain.
• The most consistent risk factor for chronic postsurgical pain is the presence and/or intensity of prior pain, experienced either preoperatively or early postoperative pain experienced in the days and weeks after surgery. One of the most important issues is to determine whether the relationship between prior pain, early postoperative pain and chronic postsurgical pain is causal, associative or a combination of the two.
• Perioperative, multimodal preventive analgesia regimens have been shown to protect against the development of chronic postsurgical pain and to the incidence and/or the intensity of chronic postsurgical pain.
• Preliminary data indicate that psychological and social–environmental risk factors are associated with the development of chronic postsurgical pain, but we do not know whether these effects are associative or causal.
• Psychological prevention and treatment efforts for chronic postsurgical pain have lagged behind other chronic pain disorders. Development of perioperative psychological pain management programs aimed at preventing and treating chronic postsurgical pain is essential.
• A big hope for pain medicine lies in genetics. However, the genetics of chronic postsurgical pain cannot advance without the availability of cohorts for study, comprising DNA samples of individuals with a comprehensive characterization of their acute and chronic postsurgical pain phenotypes, previous life experiences with pain and a detailed medical case history. There are currently no such cohorts available for study, although the genetic methodologies are at hand. It is essential to create multicenter research teams to collect such cohorts.
Financial & competing interests disclosure
Joel Katz and Ze’ev Seltzer are supported by Canadian Institutes of Health Research, Canada Research Chairs (Tier 1) in Health Psychology at York University (for Joel Katz), and in Comparative Pain Genetics at the University of Toronto (for Ze’ev Seltzer). Preparation of this manuscript was made possible by infrastructure grants from the Canadian Foundation for Innovation and the Ontario Innovation Trust. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Merskey H, Bogduk N. Classification of Chronic Pain: Description of Chronic Pain Syndromes and Definition of Pain Terms. IASP Press, WA, USA (1994).
- Macrae WA, Davies HTO. Chronic postsurgical pain. In: Epidemiology of Pain. Crombie IK, Linton S, Croft P, Von Knorff M, LeResche L (Eds). IASP Press, WA, USA 125–142 (1999).
- Macrae WA. Chronic post-surgical pain: 10 years on. Br. J. Anaesth.101(1), 77–86 (2008).
- Gerner P. Postthoracotomy pain management problems. Anesthesiol. Clin.26(2), 355–367, vii (2008).
- Jenkins JT, O’Dwyer PJ. Inguinal hernias. BMJ336(7638), 269–272 (2008).
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet367(9522), 1618–1625 (2006).
- Macrae WA. Chronic pain after sternotomy. Acta Anaesthesiol. Scand.45(8), 927–928 (2001).
- Macrae WA. Chronic pain after surgery. Br. J. Anaesth.87(1), 88–98 (2001).
- Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology93(4), 1123–1133 (2000).
- Hayes C, Browne S, Lantry G, Burstal R. Neuropathic pain in the acute pain service: a prospective study. Acute Pain4, 45–48 (2002).
- Gray P. Acute neuropathic pain: diagnosis and treatment. Curr. Opin. Anaesthesiol.21(5), 590–595 (2008).
- Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain76(1–2), 167–171 (1998).
- Tasmuth T, Blomqvist C, Kalso E. Chronic post-treatment symptoms in patients with breast cancer operated in different surgical units. Eur. J. Surg. Oncol.25(1), 38–43 (1999).
- Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol. Scand.43(5), 563–567 (1999).
- Nikolajsen L, Ilkjaer S, Christensen JH, Kroner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet350(9088), 1353–1357 (1997).
- Hanley MA, Jensen MP, Ehde DM et al. Psychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb pain. Disabil. Rehabil.26(14–15), 882–893 (2004).
- Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain21(3), 267–278 (1985).
- Meyerson J, Thelin S, Gordh T, Karlsten R. The incidence of chronic post-sternotomy pain after cardiac surgery – a prospective study. Acta Anaesthesiol. Scand.45(8), 940–944 (2001).
- Gotoda Y, Kambara N, Sakai T et al. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur. J. Pain5(1), 89–96 (2001).
- Bay-Nielsen M, Perkins FM, Kehlet H. Pain and functional impairment 1 year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann. Surg.233(1), 1–7 (2001).
- Grant AM, Scott NW, O’Dwyer PJ. Five-year follow-up of a randomized trial to assess pain and numbness after laparoscopic or open repair of groin hernia. Br. J. Surg.91(12), 1570–1574 (2004).
- Kalso E, Mennander S, Tasmuth T, Nilsson E. Chronic post-sternotomy pain. Acta Anaesthesiol. Scand.45(8), 935–939 (2001).
- Peters ML, Sommer M, de Rijke JM et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann. Surg.245(3), 487–494 (2007).
- Liem MS, van Duyn EB, van der Graaf Y, van Vroonhoven TJ. Recurrences after conventional anterior and laparoscopic inguinal hernia repair: a randomized comparison. Ann. Surg.237(1), 136–141 (2003).
- Cerfolio RJ, Price TN, Bryant AS, Sale Bass C, Bartolucci AA. Intracostal sutures decrease the pain of thoracotomy. Ann. Thorac. Surg.76(2), 407–411; discussion 411–402 (2003).
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Textbook of Pain. Wall PD, Melzack R (Eds). Churchill Livingstone, Edinburgh, UK 129–164 (1999).
- Seltzer Z. The relevance of animal neuropathy models for chronic pain in humans. Semin. Neurosci.7(4), 211–219 (1995).
- Zeltser R, Seltzer Z. A practical guide for the use of animal models for neuropathic pain. In: Touch, Temperature and Pain. Boivie J, Hansson P, Lindblom U (Eds). IASP Press, WA, USA 337–379 (1994).
- Wall PD, Devor M, Inbal R et al. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain7(2), 103–111 (1979).
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain33(1), 87–107 (1988).
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain43(2), 205–218 (1990).
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain87(2), 149–158 (2000).
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain50(3), 355–363 (1992).
- Zeltser R, Beilin B, Zaslansky R, Seltzer Z. Comparison of autotomy behavior induced in rats by various clinically-used neurectomy methods. Pain89(1), 19–24 (2000).
- Vaculin S, Rokyta R. Effects of anesthesia and nociceptive stimulation in an experimental model of brachial plexus avulsion. Phys. Res.53(2), 209–214 (2004).
- Seltzer Z, Beilin BZ, Ginzburg R, Paran Y, Shimko T. The role of injury discharge in the induction of neuropathic pain behavior in rats. Pain46(3), 327–336 (1991).
- Wall PD, Waxman S, Basbaum AI. Ongoing activity in peripheral nerve: injury discharge. Exp. Neurol.45(3), 576–589 (1974).
- Seltzer Z, Cohn S, Ginzburg R, Beilin B. Modulation of neuropathic pain behavior in rats by spinal disinhibition and NMDA receptor blockade of injury discharge. Pain45(1), 69–75 (1991).
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr. Opin. Neurobiol.13(1), 42–49 (2003).
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci.15(3), 96–103 (1992).
- Azkue JJ, Zimmermann M, Hsieh TF, Herdegen T. Peripheral nerve insult induces NMDA receptor-mediated, delayed degeneration in spinal neurons. Eur. J. Neurosci.10(6), 2204–2206 (1998).
- Coggeshall RE, Lekan HA, White FA, Woolf CJ. A-fiber sensory input induces neuronal cell death in the dorsal horn of the adult rat spinal cord. J. Comp. Neurol.435(3), 276–282 (2001).
- Rasmussen S, Kehlet H. Management of nerves during leg amputation – a neglected area in our understanding of the pathogenesis of phantom limb pain. Acta Anaesthesiol. Scand.51(8), 1115–1116 (2007).
- Nachemson AK, Bennett GJ. Does pain damage spinal cord neurons? Transsynaptic degeneration in rat following a surgical incision. Neurosci. Lett.162(1–2), 78–80 (1993).
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain64(3), 493–501 (1996).
- Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain141, 41–51 (2009).
- Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology108(6), 1100–1108 (2008).
- Zahn PK, Gysbers D, Brennan TJ. Effect of systemic and intrathecal morphine in a rat model of postoperative pain. Anesthesiology86(5), 1066–1077 (1997).
- Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of nerve growth factor and tumor necrosis factor on pain behaviors after plantar incision. J. Pain5(3), 157–163 (2004).
- Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain117(1–2), 68–76 (2005).
- Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology105(6), 1246–1253 (2006).
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology101(1), 191–203 (2004).
- Borly L, Anderson IB, Bardram L et al. Preoperative prediction model of outcome after cholecystectomy for symptomatic gallstones. Scand. J. Gastroenterol.34(11), 1144–1152 (1999).
- Jorgensen T, Teglbjerg JS, Wille-Jorgensen P, Bille T, Thorvaldsen P. Persisting pain after cholecystectomy. A prospective investigation. Scand. J. Gastroenterol.26(1), 124–128 (1991).
- Jensen MP, Ehde DM, Hoffman AJ et al. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain95(1–2), 133–142 (2002).
- Harden RN, Bruehl S, Stanos S et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain106(3), 393–400 (2003).
- Katz J, Asmundson GJ, McRae K, Halket E. Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomy. Eur. J. Pain (2008) (Epub ahead of print).
- Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain37, 51–56 (1989).
- Turk DC. A diathesis-stress model of chronic pain and disability following traumatic injury. Pain Res. Manage.7(1), 9–19 (2002).
- Sullivan MJL, Thorn B, Haythornthwaite JA et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain17(1), 52–64 (2001).
- Osman A, Barrios FX, Gutierrez PM et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J. Behav. Med.23(4), 351–365 (2000).
- Osman A, Barrios FX, Kopper BA et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J. Behav. Med.20(6), 589–605 (1997).
- Jensen MP, Turner JA, Romano JM, Karoly P. Coping with chronic pain: a critical review of the literature. Pain47(3), 249–283 (1991).
- Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res. Manag.13(4), 335–341 (2008).
- Fordyce WE. The cognitive/behavioral perspective on clinical pain. In: Managing the Chronic Pain Patient. Loeser JD, Egan KJ (Eds). Raven Press, NY, USA 51–64 (1989).
- Fordyce WE. Behavioral Methods for Chronic Pain and Illness. Mosby, MO, USA (1976).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Revised 4th Edition). American Psychiatric Association, Washington, DC, USA (2000).
- Asmundson GJG, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can. J. Psychiatry10, 903–937 (2002).
- Asmundson GJ, Katz J. Understanding pain and posttraumatic stress disorder comorbidity: do pathological responses to trauma alter the perception of pain? Pain138(2), 247–249 (2008).
- Salomons TV, Osterman JE, Gagliese L, Katz J. Pain flashbacks in posttraumatic stress disorder. Clin. J. Pain20(2), 83–87 (2004).
- DaSilva AF, Becerra L, Pendse G et al. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS ONE3(10), e3396 (2008).
- Apkarian AV, Sosa Y, Sonty S et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci.24(46), 10410–10415 (2004).
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA97(20), 11050–11055 (2000).
- DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology69(21), 1990–1995 (2007).
- Becerra L, Morris S, Bazes S et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J. Neurosci.26(42), 10646–10657 (2006).
- Kleiman V, Dzyuba M, Halket E, Asmundson GJG, Katz J. Pain Traumatization underlies pain-related anxiety in patients scheduled for major surgery: results of an exploratory factor analysis. J. Pain8(Suppl.), S68 (2007).
- Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin. Psychol. Rev.21(6), 857–877 (2001).
- Asmundson GJG, Taylor S. PTSD and chronic pain: cognitive–behavioral perspcetives and practical implications. In: Psychological Knowledge in Court. PTSD, Pain and TBI. Young G, Kane AW, Nicholson K (Eds). Springer, NY, USA 225–241 (2006).
- Katz J. Pain begets pain – predictors of long-term phantom limb pain and post-thoracotomy pain. Pain Forum6, 140–144 (1997).
- Dworkin RH. Which individuals with acute pain are most likely to develop a chronic pain syndrome? Pain Forum6, 127–136 (1997).
- Kalkman CJ, Visser K, Moen J et al. Preoperative prediction of severe postoperative pain. Pain105(3), 415–423 (2003).
- Nikolajsen L, Ilkjaer S, Kroner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain72(3), 393–405 (1997).
- Caumo W, Schmidt AP, Schneider CN et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol. Scand.46(10), 1265–1271 (2002).
- Scott LE, Clum GA, Peoples JB. Preoperative predictors of postoperative pain. Pain15(3), 283–293 (1983).
- Thomas T, Robinson C, Champion D, McKell M, Pell M. Prediction and assessment of the severity of post-operative pain and of satisfaction with management. Pain75(2–3), 177–185 (1998).
- Richardson C, Glenn S, Horgan M, Nurmikko T. A prospective study of factors associated with the presence of phantom limb pain six months after major lower limb amputation in patients with peripheral vascular disease. J. Pain8(10), 793–801 (2007).
- Brander VA, Stulberg SD, Adams AD et al. Predicting total knee replacement pain: a prospective, observational study. Clin. Orthop. (416), 27–36 (2003).
- Poobalan AS, Bruce J, King PM et al. Chronic pain and quality of life following open inguinal hernia repair. Br. J. Surg.88(8), 1122–1126 (2001).
- Hanley MA, Jensen MP, Smith DG et al. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J. Pain8(2), 102–109 (2007).
- Gerbershagen HJ, Ozgur E, Dagtekin O et al. Preoperative pain as a risk factor for chronic post-surgical pain – six month follow-up after radical prostatectomy. Eur. J. Pain (2009) (Epub ahead of print).
- Beauregard L, Pomp A, Choiniere M. Severity and impact of pain after day-surgery. Can. J. Anaesth.45(4), 304–311 (1998).
- Lau H, Patil NG, Yuen WK, Lee F. Prevalence and severity of chronic groin pain after endoscopic totally extraperitoneal inguinal hernioplasty. Surg. Endosc.17(10), 1620–1623 (2003).
- Callesen T, Bech K, Kehlet H. Prospective study of chronic pain after groin hernia repair. Br. J. Surg.86(12), 1528–1531 (1999).
- Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin. J. Pain12(1), 50–55 (1996).
- Senturk M, Ozcan PE, Talu GK et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth. Analg.94(1), 11–15 (2002).
- Tasmuth T, Kataja M, Blomqvist C, von Smitten K, Kalso E. Treatment-related factors predisposing to chronic pain in patients with breast cancer – a multivariate approach. Acta Oncol.36(6), 625–630 (1997).
- Romundstad L, Breivik H, Roald H et al. Chronic pain and sensory changes after augmentation mammoplasty: long term effects of preincisional administration of methylprednisolone. Pain124(1–2), 92–99 (2006).
- Poleshuck EL, Katz J, Andrus CH et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J. Pain7(9), 626–634 (2006).
- Gottschalk A, Ochroch EA. Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. Clin. J. Pain24, 708–716 (2008).
- Iohom G, Abdalla H, O’Brien J et al. The associations between severity of early postoperative pain, chronic postsurgical pain and plasma concentration of stable nitric oxide products after breast surgery. Anesth. Analg.103(4), 995–1000 (2006).
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain17(4), 321–339 (1983).
- Ringkamp M, Meyer RA. Injured versus uninjured afferents: who is to blame for neuropathic pain? Anesthesiology103(2), 221–223 (2005).
- Devor M, Schonfeld D, Seltzer Z, Wall PD. Two modes of cutaneous reinnervation following peripheral nerve injury. J. Comp. Neurol.185(1), 211–220 (1979).
- Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav. Brain Sci.20(3), 404–419; discussion 435–513 (1997).
- Devor M, Basbaum AI, Bennett GJ et al. Group report: mechanisms of neuropathic pain following peripheral injury. In: Towards a New Pharmacotherapy of Pain. Basbaum AI, Besson JM (Eds). John Wiley & Sons, NY, USA 417–440 (1991).
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain51(2), 175–194 (1992).
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science288(5472), 1765–1769 (2000).
- Katz J, Melzack R. Pain ‘memories’ in phantom limbs: review and clinical observations. Pain43(3), 319–336 (1990).
- Yukhananov R, Kissin I. Persistent changes in spinal cord gene expression after recovery from inflammatory hyperalgesia: a preliminary study on pain memory. BMC Neurosci.9, 32 (2008).
- Devor M, del Canho S, Raber P. Heritability of symptoms in the neuroma model of neuropathic pain: replication and complementation analysis. Pain116(3), 294–301 (2005).
- Devor M, Raber P. Heritability of symptoms in an experimental model of neuropathic pain. Pain42(1), 51–67 (1990).
- Foulkes T, Wood JN. Pain genes. PLoS Genet.4(7), e1000086 (2008).
- Seltzer Z, Mogil JS. Pain and genetics. In: Orofacial Pain: from Basic Science to Clinical Management. Sessle BJ, Lavigne G, Lund JP, Dubner R (Eds). Quintessence Publ., IL, USA 69–75 (2008).
- Mogil JS. The Genetics of Pain. IASP Press, WA, USA (2004).
- Borroni B, Brambilla C, Liberini P et al. Functional serotonin 5-HTTLPR polymorphism is a risk factor for migraine with aura. J. Headache Pain6(4), 182–184 (2005).
- Guimaraes AL, de Sa AR, Victoria JM et al. Interleukin-1β and serotonin transporter gene polymorphisms in burning mouth syndrome patients. J. Pain7(9), 654–658 (2006).
- Camilleri M, Busciglio I, Carlson P et al. Candidate genes and sensory functions in health and irritable bowel syndrome. Am. J. Physiol.295(2), G219–G225 (2008).
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum.46(3), 845–847 (2002).
- Foster DC, Sazenski TM, Stodgell CJ. Impact of genetic variation in interleukin-1 receptor antagonist and melanocortin-1 receptor genes on vulvar vestibulitis syndrome. J. Reprod. Med.49(7), 503–509 (2004).
- Duerr RH, Taylor KD, Brant SR et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science314(5804), 1461–1463 (2006).
- Tegeder I, Costigan M, Griffin RS et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat. Med.12(11), 1269–1277 (2006).
- Oertel B, Lotsch J. Genetic mutations that prevent pain: implications for future pain medication. Pharmacogenomics9(2), 179–194 (2008).
- De Vries B, Haan J, Frants RR, Van den Maagdenberg AM, Ferrari MD. Genetic biomarkers for migraine. Headache46(7), 1059–1068 (2006).
- Bennett GJ, Chung JM, Honore M, Seltzer Z. Models of neuropathic pain in the rat. In: Current Protocols in Neuroscience, Chapter 9: Preclinical Models of Neurologic and Psychiatric Disorders. Crawley JN, Gerfen CR, Rogawski MA et al. (Eds). John Wiley & Sons, Inc. NY, USA 1–16 (2003).
- Seltzer Z, Wu T, Max MB, Diehl SR. Mapping a gene for neuropathic pain-related behavior following peripheral neurectomy in the mouse. Pain93(2), 101–106 (2001).
- Xu XJ, Plesan A, Yu W, Hao JX, Wiesenfeld-Hallin Z. Possible impact of genetic differences on the development of neuropathic pain-like behaviors after unilateral sciatic nerve ischemic injury in rats. Pain89(2–3), 135–145 (2001).
- Benoliel R, Eliav E, Tal M. Strain-dependent modification of neuropathic pain behaviour in the rat hindpaw by a priming painful trigeminal nerve injury. Pain97(3), 203–212 (2002).
- Shir Y, Zeltser R, Vatine JJ et al. Correlation of intact sensibility and neuropathic pain-related behaviors in eight inbred and outbred rat strains and selection lines. Pain90(1–2), 75–82 (2001).
- Cohn S, Seltzer Z. Inherited propensity for neuropathic pain is mediated by sensitivity to injury discharge. Neuroreport2(11), 647–650 (1991).
- Liu CN, Raber P, Ziv-Sefer S, Devor M. Hyperexcitability in sensory neurons of rats selected for high versus low neuropathic pain phenotype. Neuroscience105(1), 265–275 (2001).
- Devor M, Gilad A, Arbilly M et al. Sex-specific variability and a ‘cage effect’ independently mask a neuropathic pain quantitative trait locus detected in a whole genome scan. Eur. J. Neurosci.26(3), 681–688 (2007).
- Mogil JS, Seltzer Z, Devor M. Social and environmental influences on pain: Implications for pain genetics. In: The Genetics of Pain. Mogil JS (Ed.). IASP Press, WA, USA 257–282 (2004).
- Shir Y, Seltzer Z. Heat hyperalgesia following partial sciatic ligation in rats: interacting nature and nurture. Neuroreport12(4), 809–813 (2001).
- Devor M, Gilad A, Arbilly M et al. pain1: a neuropathic pain QTL on mouse chromosome 15 in a C3HxC58 backcross. Pain116(3), 289–293 (2005).
- Nissenbaum J, Shpigler H, Pisante A et al. pain2: a neuropathic pain QTL identified on rat chromosome 2. Pain135(1–2), 92–97 (2008).
- Costigan M, Samad TA, Allchorne A et al. High basal expression and injury-induced down regulation of two regulator of G-protein signaling transcripts, RGS3 and RGS4 in primary sensory neurons. Mol. Cell. Neurosci.24(1), 106–116 (2003).
- Costigan M, Befort K, Karchewski L et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci.3, 16 (2002).
- Lacroix-Fralish ML, Tawfik VL, Tanga FY, Spratt KF, DeLeo JA. Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology104(6), 1283–1292 (2006).
- Mechaly I, Bourane S, Piquemal D et al. Gene profiling during development and after a peripheral nerve traumatism reveals genes specifically induced by injury in dorsal root ganglia. Mol. Cell. Neurosci.32(3), 217–229 (2006).
- Rodriguez Parkitna J, Korostynski M, Kaminska-Chowaniec D et al. Comparison of gene expression profiles in neuropathic and inflammatory pain. J. Physiol. Pharmacol.57(3), 401–414 (2006).
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J. Neurochem.87(3), 560–573 (2003).
- Xiao HS, Huang QH, Zhang FX et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl Acad. Sci. USA99(12), 8360–8365 (2002).
- Kunz S, Tegeder I, Coste O et al. Comparative proteomic analysis of the rat spinal cord in inflammatory and neuropathic pain models. Neurosci. Lett.381(3), 289–293 (2005).
- Seltzer Z, Dorfman R. Identifying genetic and environmental risk factors for chronic orofacial pain syndromes: human models. J. Orofac. Pain18(4), 311–317 (2004).
- Max MB, Stewart WF. The molecular epidemiology of pain: a new discipline for drug discovery. Nat. Rev.7(8), 647–658 (2008).
- Mitchell SW. Injuries of Nerves and their Consequences. JB Lippincott, PA, USA (1872).
- Nathan PW. Pain traces left in the central nervous system. In: The Assessment of Pain in Man and Animals. Keele CA, Smith R (Eds). Livingstone, Edinburgh, UK 129–134 (1962).
- Gonzalez-Darder JM, Barbera J, Abellan MJ. Effects of prior anaesthesia on autotomy following sciatic transection in rats. Pain24(1), 87–91 (1986).
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain52(3), 259–285 (1993).
- Zhang SH, Blech-Hermoni Y, Faravelli L, Seltzer Z. Ralfinamide administered orally before hindpaw neurectomy or postoperatively provided long-lasting suppression of spontaneous neuropathic pain-related behavior in the rat. Pain139(2), 293–305 (2008).
- Lentschener C, Tostivint P, White PF, Gentili ME, Ozier Y. Opioid-induced sedation in the postanesthesia care unit does not insure adequate pain relief: a case–control study. Anesth. Analg.105(4), 1143–1147 (2007).
- Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS J.4(1), 62–65 (2008).
- Melzack R, Katz J. A conceptual framework for understanding pain in the human. In: Pain Management. Waldman SJ (Ed.). Elsevier Science, NY, USA 2–9 (2007).
- Katz J. Pre-emptive analgesia: evidence, current status and future directions. Eur. J. Anaesthesiol. Suppl.10, 8–13 (1995).
- Katz J, McCartney CJL. Current status of pre-emptive analgesia. Curr. Opin. Anaesthesiol.15, 435–441 (2002).
- Kissin I. Preemptive analgesia: terminology and clinical relevance. Anesth. Analg.79, 809 (1994).
- Kissin I. Preemptive analgesia. Anesthesiology93(4), 1138–1143 (2000).
- Katz J. George Washington Crile, anoci-association, and pre-emptive analgesia. Pain53(3), 243–245 (1993).
- Wall PD. The prevention of post-operative pain. Pain33, 289–290 (1988).
- Katz J, Clarke H. Preventive analgesia and beyond: Current status, evidence, and future directions. In: Clinical Pain Management: Acute Pain. Macintyre PE, Rowbotham DJ, Howard R (Eds). Hodder Arnold Ltd, London, UK 154–198 (2008).
- McQuay HJ. Pre-emptive analgesia. Br. J. Anaesth.69(1), 1–3 (1992).
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Rev. Neurother.5(6), 823–830 (2005).
- McCartney CJ, Sinha A, Katz J. A qualitative systematic review of the role of N-methyl-D-aspartate receptor antagonists in preventive analgesia. Anesth. Analg.98(5), 1385–1400 (2004).
- Bach S, Noreng MF, Tjellden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain33(3), 297–301 (1988).
- Katz J. Phantom limb pain. Lancet350(9088), 1338–1339 (1997).
- Nikolajsen L, Brandsborg B, Lucht U, Jensen TS, Kehlet H. Chronic pain following total hip arthroplasty: a nationwide questionnaire study. Acta Anaesthesiol. Scand.50(4), 495–500 (2006).
- Lambert A, Dashfield A, Cosgrove C et al. Randomized prospective study comparing preoperative epidural and intraoperative perineural analgesia for the prevention of postoperative stump and phantom limb pain following major amputation. Reg. Anesth. Pain Med.26(4), 316–321 (2001).
- Wilson JA, Nimmo AF, Fleetwood-Walker SM, Colvin LA. A randomised double blind trial of the effect of pre-emptive epidural ketamine on persistent pain after lower limb amputation. Pain135(1–2), 108–118 (2008).
- Hayes C, Armstrong-Brown A, Burstal R. Perioperative intravenous ketamine infusion for the prevention of persistent post-amputation pain: a randomized, controlled trial. Anaesth. Intens. Care32(3), 330–338 (2004).
- Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Reg. Anesth. Pain Med.27(5), 481–486 (2002).
- Nikolajsen L, Finnerup NB, Kramp S et al. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology105(5), 1008–1015 (2006).
- Smith DG, Ehde DM, Hanley MA et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J. Rehabil. Res. Dev.42(5), 645–654 (2005).
- Schley M, Topfner S, Wiech K et al. Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. Eur. J. Pain11(3), 299–308 (2007).
- Aida S, Fujihara H, Taga K, Fukuda S, Shimoji K. Involvement of presurgical pain in preemptive analgesia for orthopedic surgery: a randomized double blind study. Pain84(2–3), 169–173 (2000).
- Klasen J, Haas M, Graf S et al. Impact on postoperative pain of long-lasting pre-emptive epidural analgesia before total hip replacement: a prospective, randomised, double-blind study. Anaesthesia60(2), 118–123 (2005).
- Hunter JP, Katz J, Davis KD. Stability of phantom limb phenomena after upper limb amputation: a longitudinal study. Neuroscience156(4), 939–949 (2008).
- Lund C, Selmar P, Hansen OB, Hjortso NC, Kehlet H. Effect of epidural bupivacaine on somatosensory evoked potentials after dermatomal stimulation. Anesth. Analg.66(1), 34–38 (1987).
- Lund C, Selmar P, Hansen OB, Kehlet H. Effect of intrathecal bupivacaine on somatosensory evoked potentials following dermatomal stimulation. Anesth. Analg.66(9), 809–813 (1987).
- Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin – calcium channel α(2)-δ [Ca(v)α(2)-δ] ligands. Pain142(1–2), 13–16 (2009).
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr. Opin. Anaesthesiol.20(5), 456–472 (2007).
- Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth. Analg.101(5), 1427–1432 (2005).
- Fassoulaki A, Patris K, Sarantopoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth. Analg.95(4), 985–991 (2002).
- Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol. Med.8(8), 390–396 (2002).
- Kearney PM, Baigent C, Godwin J et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ332(7553), 1302–1308 (2006).
- Bombardier C, Laine L, Reicin A et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N. Engl. J. Med.343(21), 1520–1528 (2000).
- Topol EJ. Rofecoxib, Merck, and the FDA. N. Engl. J. Med.351(27), 2877–2878 (2004).
- Gottschalk A, Smith DS, Jobes DR et al. Preemptive epidural analgesia and recovery from radical prostatectomy: a randomized controlled trial. JAMA279(14), 1076–1082 (1998).
- Katz J, Schmid R, Snijdelaar DG et al. Pre-emptive analgesia using intravenous fentanyl plus low-dose ketamine for radical prostatectomy under general anesthesia does not produce short-term or long-term reductions in pain or analgesic use. Pain110(3), 707–718 (2004).
- Obata H, Saito S, Fujita N et al. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can. J. Anaesth.46(12), 1127–1132 (1999).
- Ochroch EA, Gottschalk A, Augostides J et al. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology97(5), 1234–1244 (2002).
- Solak O, Metin M, Esme H et al. Effectiveness of gabapentin in the treatment of chronic post-thoracotomy pain. Eur. J. Cardiothorac. Surg.32(1), 9–12 (2007).
- Sihoe AD, Lee TW, Wan IY, Thung KH, Yim AP. The use of gabapentin for post-operative and post-traumatic pain in thoracic surgery patients. Eur. J. Cardiothorac. Surg.29(5), 795–799 (2006).
- Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery50(3), 510–516; discussion 516–517 (2002).
- Gundes H, Kilickan L, Gurkan Y, Sarlak A, Toker K. Short- and long-term effects of regional application of morphine and bupivacaine on the iliac crest donor site. Acta Orthop. Belg.66(4), 341–344 (2000).
- Singh K, Phillips FM, Kuo E, Campbell M. A prospective, randomized, double-blind study of the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after posterior spinal arthrodesis: a minimum of 4-year follow-up. Spine32(25), 2790–2796 (2007).
- Katz J, Clairoux M, Redahan C et al. High dose alfentanil pre-empts pain after abdominal hysterectomy. Pain68(1), 109–118 (1996).
- Katz J, Cohen L, Schmid R, Chan VW, Wowk A. Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology98(6), 1449–1460 (2003).
- Katz J, Cohen L. Preventive analgesia is associated with reduced pain disability 3 weeks but not 6 months after major gynecologic surgery by laparotomy. Anesthesiology101(1), 169–174 (2004).
- Brogly N, Wattier JM, Andrieu G et al. Gabapentin attenuates late but not early postoperative pain after thyroidectomy with superficial cervical plexus block. Anesth. Analg.107(5), 1720–1725 (2008).
- Lavand’homme P, De Kock M, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology103(4), 813–820 (2005).
- Dworkin RH, Turk DC, Farrar JT et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain113(1–2), 9–19 (2005).
- Aasvang EK, Mohl B, Bay-Nielsen M, Kehlet H. Pain related sexual dysfunction after inguinal herniorrhaphy. Pain122(3), 258–263 (2006).
- Aasvang EK, Bay-Nielsen M, Kehlet H. Pain and functional impairment 6 years after inguinal herniorrhaphy. Hernia10(4), 316–321 (2006).
- Nikolajsen L, Sorensen HC, Jensen TS, Kehlet H. Chronic pain following Caesarean section. Acta Anaesthesiol. Scand.48(1), 111–116 (2004).
- Richardson C, Glenn S, Nurmikko T, Horgan M. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin. J. Pain22(4), 353–358 (2006).
Website
- Pain genetics laboratory http://paingeneticslab.ca/4105/06_02_pain_genetics_database.asp
