Abstract
Stroke is a leading cause of cardiovascular morbidity and mortality worldwide. Approximately, 795,000 strokes occur in the USA each year, 610,000 of which are first events, and 185,000 of which are recurrent events. Of all strokes, 87% are ischemic strokes. Novel anticoagulants serve as an alternative antithrombotic intervention in patients with ischemic cerebrovascular disease. This paper reviews the role of the novel anticoagulants, dabigatran, rivaroxaban and apixaban, in stroke prevention among patients with nonvalvular atrial fibrillation.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/expertneurothera; (4) view/print certificate.
Release date: January 30, 2012; Expiration date: January 30, 2013
Learning objectives
Upon completion of this activity, participants should be able to:
• Describe the role of dabigatran in stroke prevention among patients with NVAF
• Describe the role of rivaroxaban in stroke prevention among patients with NVAF
• Describe the role of apixaban in stroke prevention among patients with NVAF
Financial & competing interests disclosure
EDITOR
Elisa Manzotti,Editorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay,Freelance writer and reviewer, Medscape, LLC
Disclosure:Laurie Barclay, MD, has disclosed no relevant financial relationships.
AUTHORS
Sarkis Morales-Vidal,Department of Neurology, Stroke Program, Loyola University Chicago, Stritch School of Medicine, Maywood, IL 60153, USA
Disclosure:Sarkis Morales-Vidal has disclosed no relevant financial relationships.
Michael J Schneck,Department of Neurology, Stroke Program, Loyola University Chicago, Stritch School of Medicine, Maywood, IL 60153, USA
Disclosure:Michael J Schneck has disclosed no relevant financial relationships.
Murray Flaster,Department of Neurology, Stroke Program, Loyola University Chicago, Stritch School of Medicine, Maywood, IL 60153, USA
Disclosure:Murray Flaster has disclosed no relevant financial relationships.
José Biller,Department of Neurology, Stroke Program, Loyola University Chicago, Stritch School of Medicine, Maywood, IL 60153, USA
Disclosure:José Biller has disclosed no relevant financial relationships.
Examples of specific drugs are cited.
Note: this is not meant to be an exhaustive list of all agents.
LMWH: Low-molecular-weight heparin.
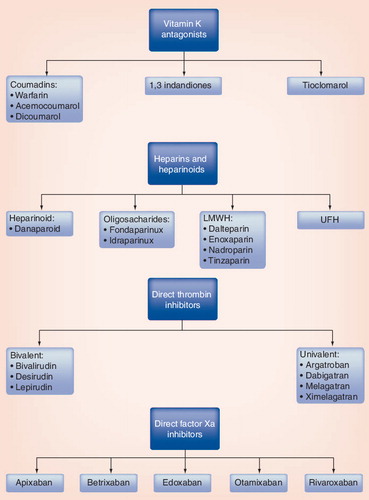
Classical antithrombotic agents include vitamin K antagonists (VKAs) and heparin products. Long-term anticoagulation is mostly provided with VKA in clinical practice. Disadvantages of VKA include delayed onset of action, need for close monitoring of the international normalized ratio (INR) and diet restriction. New direct thrombin inhibitors (DTIs) and factor Xa inhibitors do not share the disadvantages of VKAs and may provide equal or superior thromboembolic prevention. The main disadvantages of DTIs and direct factor Xa inhibitors are the limited clinical experience with these agents. This article reviews the mechanisms of action and current role of DTIs and factor Xa inhibitors in patients with cerebrovascular disease with a focus on dabigatran, rivaroxaban and apixaban for which Phase III trials have been completed.
Mechanism of action
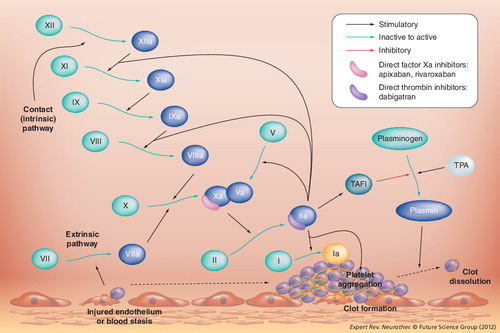
Thrombus formation requires platelet endothelial adhesion, platelet aggregation, clot formation and stabilization. The coagulation cascade has three main pathways: the intrinsic (contact activation pathway), the extrinsic (tissue factor pathway) and a final common pathway (thrombin pathway). The common end point of the intrinsic and extrinsic pathways is the activation of factor X, also known as ‘prothrombinase’ or ‘Stuart–Power factor’. Factor Xa along with factor Va (prothrombinase complex) activates prothrombin (factor II) into thrombin (factor IIa). The main role of thrombin is the conversion of fibrinogen into fibrin and the activation of factor XIII. Factor XIIIa crosslinks fibrin polymers, stabilizing the hemostatic clot. Other functions of thrombin include activation of factor VIII and factor V. In addition, thrombin activates protein C. summarizes the classification of anticoagulant drugs. A summary of the coagulation cascade and the site of action of factor Xa inhibitors and DTIs is illustrated in . compares pharmacological differences of apixaban, rivaroxaban, dabigatran and warfarin.
Vitamin K is essential for the hepatic carboxylation of glutamic acid residues of coagulation factors by γ-glutamyl carboxylase. Vitamin K-dependent factors are factor VII, factor IX, factor X, factor II, protein C, protein S and protein Z. Protein C (half-life 14 h) and protein S (half-life 48 h) are natural anticoagulants. The half-life of factor II, the main stimulant for clot formation, is 72 h. Activated vitamin K-dependent factors are not inhibited by VKA. This explains the delayed onset of action of VKA, requiring approximately 72 h to achieve a therapeutic INR.
Factor Xa inhibitors prevent the formation of thrombin. Factor Xa is part of the prothrombinase complex that also includes factor Va and requires the presence of calcium. Indirect factor Xa inhibitors bind to antithrombin. Fondaparinux and idraparinux are indirect factor Xa inhibitors. In contrast to heparin and other heparinoids, fondaparinux selectively inhibits factor Xa. Direct factor Xa inhibitors antagonize the active site of the free-form and prothrombinase-bound forms of factor Xa. Apixaban and rivaroxaban are examples of direct factor Xa inhibitors.
The action of direct thrombin inhibitors (DTIs), as opposed to heparin products, is independent of antithrombin. DTIs bind to both soluble and fibrin-bound thrombin but heparin only inhibits the soluble thrombin molecule. Thrombin has one catalytic site and two exosites. Direct thrombin inhibitors can be univalent or bivalent. Univalent DTIs inhibit the action of thrombin by binding to the catalytic site. Bivalent DTIs inhibit the action of thrombin by binding to both the catalytic site and exosite Citation[1]. Univalent DTIs include argatroban, ximelagatran and dabigatran. Bivalent DTIs include hirudin, lepirudin, desidurin and bivalirudin Citation[1].
Factor Xa inhibitors & direct thrombin inhibitors in atrial fibrillation-related strokes
Atrial fibrillation (AF) is a major cause of stroke in the elderly Citation[2]. The estimated prevalence is 4.5 million people in the EU and 3.03 million people in the USA. The 2050 projected prevalence is 7.56 million persons Citation[3]. The frequency of AF is predicted to increase 2.5-fold in the next 50 years Citation[4]. The risk of AF increases with age, affecting 9% of patients older than 80 years of age Citation[4]. AF is more common among women, and more common in whites than in blacks Citation[4]. Elderly patients (>75 years of age) on anticoagulation have higher bleeding event rates than younger anticoagulated patients Citation[5]. AF, whether paroxysmal, permanent or persistent, is an independent stroke risk factor. The presence of congestive heart failure, arterial hypertension, >75 years of age, diabetes mellitus, prior strokes or transient ischemic attacks (CHADS2 score) further increases the risk of stroke in patients with nonvalvular AF (NVAF) Citation[2]. The American College of Cardiology, American Heart Association and the Heart Rhythm Society 2006 practice guidelines recommend aspirin for a CHADS2 score of 0 (low risk), aspirin or warfarin for a CHADS2 score of 1 (intermediate risk), and warfarin for CHADS2 scores of ≥2 (high risk) Citation[2]. The major limitation of the CHADS2 scoring system is that many patients often fall in the intermediate risk category. A new scoring system, the congestive heart failure – hypertension – age >75 years – diabetes mellitus – stroke, transient ischemic attack or thromboembolism – vascular disease (previous myocardial infarction, peripheral arterial disease or aortic plaque) – age between 65 and 74 years – female Sex (CHA2DS2-VASc) score takes into account specific age groups (>75 years vs ages 65–74), the presence of vascular disease (history of myocardial infarction, peripheral arterial disease or aortic plaque) and female gender. The CHA2DS2-VASc scoring system has better predictive value when placing patients into high- or low-risk categories.
Patients with high CHADS2 and CHA2D2-VASc scores have a higher mortality risk from ischemic stroke Citation[6,7]. These additional risks must be taken into account when interpreting trials of new anticoagulant agents. In addition, factors contributing to bleeding risk should be considered. The hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (age over 65 years) and drugs/alcohol score (HAS-BLED) concomitantly had a better performance than any other contemporary scoring system in a large cohort study Citation[8]. Each component of HAS-BLED scores one point with a maximum score of nine. A HAS-BLED score of ≥ three indicates an increased risk of bleeding at 1 year with the use of anticoagulation.
For over 30 years, warfarin has been the only anticoagulant for primary and secondary stroke prevention in patients with AF. Disadvantages of warfarin therapy include the need for frequent monitoring of INR, a narrow therapeutic index and extensive dietary and drug–drug interactions. Because of these difficulties, up to one third of patients on warfarin for chronic anticoagulation are not within the therapeutic window Citation[9,10]. Additionally, there is a 2% annual risk of major bleeding. The combination of aspirin and clopidogrel has a similar risk of bleeding with less effective stroke prevention Citation[11].
Regarding AF, the major aim of newer anticoagulant therapies is to provide equal or better secondary stroke prevention with a lower risk of major hemorrhage when compared with warfarin therapy. Initial clinical trials of newer anticoagulant drugs typically involve prevention of deep vein thrombosis and pulmonary embolism following hip or knee replacement. Ximelagatran was initially approved in Europe for venous thromboembolism prophylaxis following hip or knee replacement surgery based on the results of the METHRO III trial Citation[101]. Subsequently, the SPORTIF III and SPORTIF V trials showed better efficacy of ximelagatran over warfarin for secondary stroke prevention Citation[12,13]. However, the US FDA did not approve ximelagatran for stroke or deep venous thromboembolism prevention because of an unacceptably high risk of hepatotoxicity Citation[102,103].
PETRO was a Phase II clinical trial that was the first trial to investigate effects of dabigatran etexilate for stroke prevention in patients with AF Citation[14]. In this trial, 502 patients were randomized to either dabigatran etexilate (50, 150 or 300 mg twice daily [b.i.d.], alone or combined with aspirin) or warfarin (INR 2.0–3.0). There was a 6% rate of major bleeding in the dabigatran 300 mg b.i.d. plus aspirin group. Elevation of liver enzymes occurred in 0.9% of dabigatran recipients. Dose ranging data from PETRO and an extension (PETRO-EX) trial, suggested that dabigatran doses of up to 150 mg daily were inadequate Citation[14].
The Phase III RE-LY trial randomized 18,113 patients with AF to either blinded-use dabigatran etexilate at dosages of 110 or 150 mg b.i.d. versus open-label use of warfarin with a target INR of 2.0–3.0 Citation[15]. The RE-LY trial design was prospective, randomized and open-label with blinded end point adjudication. The primary objective was to establish the noninferiority of dabigatran etexilate compared with adjusted warfarin (INR 2.0–3.0). Eligible patients had NVAF with a moderate-to-high risk for thromboembolic events. Inclusion and exclusion criteria of the RELY trial are summarized in Box 1. The primary outcome was pooled stroke (ischemic or hemorrhagic) and systemic embolism. Major bleeding was the primary safety outcome. summarizes main results of the RE-LY trial. The primary outcome was similar in the dabigatran 110 mg b.i.d. and warfarin groups. The primary outcome was less frequently seen in the dabigatran 150 mg b.i.d. compared with the warfarin group.
Hemorrhagic stroke was less frequent with both dosages of dabigatran as compared with warfarin. Major bleeding, excluding hemorrhagic strokes, was more frequent with dabigatran 150 mg b.i.d. and with warfarin rather than with dabigatran 110 mg b.i.d. Gastrointestinal bleeding was higher with both doses of dabigatran compared with warfarin. There was a nonstatistically significant decrease in all-cause mortality in the dabigatran 150 mg b.i.d. group but a statistically significant decrease in vascular mortality was observed in the dabigatran 150 mg b.i.d. group. There was a small but statistically significant increased risk of myocardial infarction with dabigatran 150 mg b.i.d.. The most commonly reported adverse events with dabigatran were dyspepsia, dizziness, dyspnea and peripheral edema. Moreover, amiodarone increased the serum concentration of dabigatran Citation[16].
The quality control of INR levels with warfarin therapy related to the influence of the relative effects of dabigatran (110 and 150 mg b.i.d.) was evaluated in a post-hoc analysis Citation[17]. Outcomes were evaluated with center-based INR control. Quartiles were evaluated based on the time in treatment range (TTR) defined as INR 2.0–3.0. The average TTR for warfarin in RE-LY was 64%. For stroke prevention, dabigatran 150 mg b.i.d. was superior and dabigatran 110 mg b.i.d. was noninferior to warfarin irrespective of INR control. Regarding the secondary outcomes of mortality and all vascular events, dabigatran (110 and 150 mg b.i.d.) was superior to warfarin with poor INR control but similar in those with good INR control.
In October 2010, dabigatran etexilate received US FDA approval for stroke prevention in patients with NVAF Citation[104]. The FDA approved the 150-mg b.i.d. dose, but not the 110-mg b.i.d. dose owing to higher stroke rates observed with the lower dose. In addition, patients with major bleeding who resumed the use of dabigatran were not at higher risk of recurrent bleeding with dabigatran 150 mg b.i.d. as compared with 110 mg b.i.d. Citation[18]. There are ongoing concerns about bleeding risk, especially in trauma patients, and the FDA is engaged in ongoing surveillance about the bleeding risks of dabigatran outside of clinical trials Citation[19]. Based on pharmacokinetic modeling and pharmacokinetic data from a substudy of RE-LY in patients with impaired renal function, the FDA also approved dabigatran 75 mg b.i.d. The 75-mg b.i.d. dose of dabigatran was approved for patients with an estimated creatinine clearance of 15–30 ml/min Citation[16]. Patients with major bleeding who resumed dabigatran were not at higher risk of recurrent bleeding with dabigatran 150 mg b.i.d. compared with 110 mg b.i.d. Citation[18].
ROCKET-AF was a randomized, ‘double-dummy’ and double-blind trial of 14,269 patients Citation[20]. summarizes the trial results. Eligible patients had a history of stroke, transient ischemic attacks or systemic embolism, and at least two risk factors (heart failure, >75 years of age, hypertension or diabetes mellitus). Patients were randomized to receive either rivaroxaban 20 mg once daily (15 mg daily if creatinine clearance was 30–49 ml/min) or warfarin (target INR of 2.0–3.0). The primary end point was stroke or systemic embolism. The main safety outcome was major bleeding and clinically relevant bleeding. The primary aim of this study was to establish noninferiority of rivaroxaban compared with warfarin. Results showed that rivaroxaban was noninferior to warfarin, but not superior to warfarin in a subsequent intention-to-treat analysis. Nonetheless, on-treatment analysis showed a 21% risk reduction with rivaroxaban in comparison to warfarin. Difference between the intention-to-treat and the on-treatment analysis may be explained by poorer adherence to rivaroxaban. Major bleeding was similar among the groups. Although there was a statistically significant reduction in intracranial and fatal bleeding in the rivaroxaban group, no difference in mortality was observed.
ROCKET-AF, in contrast to RE-LY, had a double-blind and double-dummy (sham INR adjustment) instead of an open-label design with blinded adjudication. In the ROCKET-AF trial, 55% of patients had a history of prior stroke and 90% of patients had a CHADS2 score of >3. On November 4, 2011, the FDA approved rivaroxaban to reduce the risk of stroke and systemic embolism in patients with NVAF. One concern was that in the 4 weeks after completion of ROCKET AF study drug, as the patients were transitioned to other anticoagulants at the end of the clinical trial, there was an increased risk of stroke in those in the rivaroxaban arm, presumably related to the short half life of rivaroxaban and the resultant lack of anticoagulation during the transition to warfarin Citation[2].
The Phase III study of the AVERROES trial randomized 5599 patients with AF who were not candidates for warfarin therapy to either apixaban or to aspirin Citation[21]. Patients had AF and at least one additional risk factor for stroke. The study end point was met prematurely; the study was stopped after a mean follow-up of only 1 year. summarizes the key findings of this trial. The composite outcome of stroke and systemic embolism was significantly lower in the apixaban compared with the aspirin group, while the primary safety outcome of major bleeding was similar in both groups. Mortality did not differ among the two groups.
The ARISTOTLE, a Phase III, randomized, double-blind, double-dummy control clinical trial comparing apixaban 5 mg b.i.d. (or 2.5 mg b.i.d. in vulnerable patients) to warfarin with an (INR target of 2.0–3.0) Citation[22,23]. The vulnerable population included patients 80 years of age or older, with bodyweight ≤ 60kg, or a serum creatinine ≥1.5 mg/dl. The primary efficacy outcome was stroke and systemic embolism. A total of 18,201 patients were enrolled. summarizes the results of this trial. The stroke rate and systemic embolism were lower in the apixaban group (1.27% per year) compared with warfarin (1.6% per year). The rate of major bleeding was also noted to be lower with apixaban (2.13% per year with apixaban vs 3.09% per year with warfarin). Likewise, hemorrhagic stroke rate was lower with apixaban. Mortality rates were also lowered with apixaban (3.52 vs 3.94% with heparin). The investigators concluded that apixaban was superior to warfarin in stroke or systemic embolism prevention.
The ENGAGE AF-TIMI 48 trial is a Phase III, double-blind and multinational randomized clinical trial comparing edoxaban (DU-176b) versus warfarin in patients with NVAF Citation[24]. Estimated enrollment is 20,500 patients. Patients will be randomized to receive either edoxaban 60 mg daily, edoxaban 30 mg daily or warfarin with a target INR of 2.0–3.0. Required CHADS2 score is ≥2. The primary goal is to test the noninferiority of edoxaban as compared with warfarin for the prevention of thromboembolic events in patients The primary end point is the composite of stroke and systemic embolic events. The primary safety outcome is major bleeding, including intracerebral hemorrhage.
Betrixaban (Portola Pharmaceuticals/Merck) is an oral, direct factor Xa inhibitor shown to be safe in the Phase II EXPLORE-Xa study Citation[25,26]. The study enrolled 508 patients with NVAF and at least one additional stroke risk factor. Dosages of betrixaban were 40, 60 and 80 mg daily and were compared with adjusted-dose warfarin (INR: 2.0–3.0). There was a reduction in major bleeds, particularly with the 40-mg betrixaban daily dose, as compared with adjusted-dose warfarin. Pharmacological advantages of betrixaban include its elimination as an unchanged molecule and its lack of interactions with other molecules metabolized by the cytochrome P450 enzyme. Moreover, betrixaban is being developed with an intravenous antidote (PRT064445). There are no ongoing Phase III clinical trials of betrixaban for stroke prevention.
As previously discussed RE-LY and ROCKET AF had a noninferiority to warfarin as a primary end point with an INR target of 2.0–3.0. ROCKET AF study warfarin was given in a blinded fashion, whereas warfarin administration was unblinded in RE-LY. Furthermore, patients in ROCKET AF had a CHADS2 score of 3 whereas RE-LY enrolled patients with a CHADS2 score of 1.
Comparison of the RE-LY and ROCKET AF studies both showed that dabigatran (hazard ratio [HR]: 0.26; p < 0.001) and rivaroxaban (HR: 0.59; p = 0.024) offered a reduced risk of hemorrhagic stroke compared with warfarin. Both drugs were noninferior to warfarin in reducing the primary end point of stroke and systemic embolism. In an intent-to-treat analysis, dabigatran 150 mg was superior to warfarin while rivaroxaban was not, although in a prespecified secondary on-treatment analysis, rivaroxaban was superior to warfarin. Dabigatran 150 mg also reduced the risk of ischemic stroke (HR: 0.76; p = 0.03) whereas rivaroxaban did not (p = 0.58). Furthermore, in the intent-to treat analysis there was an observed robust trend for reduction in mortality with dabigatran (p = 0.051), and only a modest trend for reduction in mortality with rivaroxaban (p = 0.152). However, the validity of comparing mortality rates is limited, because ROCKET AF enrolled subjects with higher CHADS2 scores.
Lack of studies assessing factor Xa inhibitors & direct thrombin inhibitors in other ischemic stroke syndromes
The WASID trial showed no evidence of the previously ‘assumed’ (by some experts) superiority of warfarin over aspirin for preventing strokes among symptomatic patients with large vessel intracranial atherosclerotic cerebrovascular disease. Moreover, warfarin was less safe than aspirin Citation[27,28]. The WARSS study showed no differences in the rate of stroke prevention between aspirin and warfarin therapy. However, warfarin was associated with a greater benefit among patients with posterior circulation strokes without brainstem infarction Citation[29,30]. Warfarin had a higher risk of adverse outcomes compared with aspirin. Thus, it is possible that DTIs or factor Xa inhibitors may offer greater benefit for secondary stroke prevention in symptomatic patients with intracranial arterial stenosis.
Whether anticoagulation is superior to antiplatelet therapy for stroke prevention in cervical arterial dissections (CADs) is not known. The CADISS is an exploratory open-label, randomized controlled clinical trial for patients with recent (within the past 7 days) ischemic strokes due to extracranial internal carotid artery or vertebral artery dissection Citation[31]. Subjects are randomized to receive either antiplatelet therapy or warfarin (target INR of 2.0–3.0). There are no studies evaluating the efficacy of either DTI or factor Xa inhibitors in patients with CAD.
The ISCVT showed the potential benefit of anticoagulation in patients with cerebral venous sinus thrombosis Citation[32]. Low-molecular-weight heparin appeared to be more effective than unfractionated heparin in a subgroup analysis of the ISCVT study Citation[33]. However, there are no randomized controlled trials evaluating the potential benefit of anticoagulation versus other interventions in cerebral venous thrombosis. Whether novel anticoagulants may offer advantages as alternative anticoagulant treatment in patients remains untested.
Although anticoagulation is indicated for cerebral venous thrombosis in patients with antiphospholipid antibody syndrome, anticoagulation has not been proven to be superior to aspirin for arterial ischemic stroke prevention in these patients Citation[34]. Trials evaluating the efficacy of novel DTIs or factor Xa inhibitors for arterial stroke prevention in patients with APAS are clearly needed.
The use of warfarin for stroke prevention in patients with systolic heart failure with a low ejection fraction (<35%) remains controversial. The WARCEF trial is currently recruiting patients, and is estimated to be further completed by February 2012 Citation[35]. Results of WARCEF may fuel further interest in novel anticoagulants for stroke prevention in that setting. The safety of intravenous (iv.) tissue plasminogen activation for acute ischemic stroke in patients receiving dabigatran, apixaban or rivaroxaban is unknown.
Comparison, cost–effectiveness & future perspectives of DTI versus factor Xa inhibitors
Direct comparisons of DTIs and direct factor Xa inhibitors are needed. At present, the only novel agent approved by the FDA for stroke prevention in patients with NVAF is dabigatran. For apixaban and rivaroxaban, FDA approval is still pending. In contrast to apixaban and rivaroxaban, dabigatran has an initial slow absorption during the postoperative period and food may delay absorption for up to 2 h. Freeman et al. performed a Markovian decision analysis for a hypothetical cohort, 60-year-old patients with NVAF based on current prices of warfarin and dabigatran in the UK and reported outcomes based on quality-adjusted life years (QALYs) Citation[36]. The investigators suggested that incremental cost–effectiveness ratio for high-dose dabigatran was superior to low-dose dabigatran. The investigators estimated a cost of US$ 45,372 per QALY for the high-dose dabigatran versus warfarin which is generally considered to be within the acceptable cost-effective range. Moreover, there was a gain in QALY of the high-dose dabigatran compared with warfarin. Patients at higher risk of ischemic stroke or intracerebral hemorrhage, including those with high CHADS2 score had particular incremental benefit Citation[36].
Future opportunities exist for testing these new agents among patients with an array of cerebrovascular disorder including intracranial arterial atherosclerotic steno-occlusive disease, cervicocephalic arterial dissections, mobile aortic arch atheroma, free-floating thrombus of the carotid artery, vertebrobasilar dolichoectasia artery and potential high-risk cardioembolic disorders (e.g., prosthetic heart valves).
Conclusion
Dabigatran is an effective alternative to warfarin for stroke prevention in patients with NVAF. Rivaroxaban is another promising alternative to warfarin in these patients. Apixaban appears superior to aspirin for stroke prevention in patients with NVAF that are not candidates for warfarin therapy.
Expert commentary
DTIs and factor Xa inhibitors will probably serve as alternatives to long-term anticoagulation with warfarin for patients with NVAF. The main advantage of dabigatran over warfarin is its lower bleeding risk. These novel agents should also be studied among patients with intracranial atherosclerotic arterial disease, cervicocephalic arterial dissections and the antiphospholipid antibody syndrome. These agents have a lower bleeding risk than warfarin. However, the current lack of available antidotes may limit their immediate rapid acceptance into general practice.
Five-year view
Within the next few years, direct thrombin inhibitors and factor Xa inhibitors will likely supplant warfarin for long-term anticoagulation in selective patients with NVAF.
Table 1. Pharmacological comparison of apixaban, rivaroxaban, dabigatran and warfarin.
Table 2. Summary of key findings of the RE-LY trial.
Table 3. Summary of the ROCKET-AF trial.
Table 4. Summary of the results of the AVERROES trial.
Table 5. Summary of the ARISTOTLE trial.
Box 1. Inclusion and exclusion criteria of the RE-LY trial.
Exclusion criteria
• Cardiac valvulopathy
• Any stroke within 14 days prior to possible randomization
• Disabling stroke
• Conditions associated with high bleeding risk
• Contraindications to warfarin therapy
• Reversible causes of atrial fibrillation
• Planned ablative or surgical treatment for atrial fibrillation
• Creatinine clearance ≤30 mg/dl
• Active endocarditis
• Active liver disease
• Pregnant women
• Women of child-bearing age not willing to use oral contraception
Inclusion criteria
• Two symptomatic episodes, at least 24 h apart, of atrial fibrillation occurring within 6 months
• Two symptomatic episodes, at least 24 h apart, of atrial fibrillation occurring within 6 months of randomization
• ≥18 years of age
• One of the following:
– History of stroke, transient ischemic attack or systemic embolism
– Ejection fraction <40% documented within 6 months of randomization
– Symptomatic heart failure within 6 months of randomization
– ≥75 years of age
– ≥65 years of age with history of diabetes mellitus, coronary artery disease or hypertension
Key issues
• RE-LY showed dabigatran to be as safe as warfarin with less intracranial bleeding.
• In a substudy of RE-LY, stroke rates were lower when patients were treated with dabigatran.
• Dabigatran has a higher gastrointestinal bleeding risk and a small but statistically significant increased risk of myocardial infarction compared with warfarin.
• Apixaban was superior to aspirin in patients with nonvalvular atrial fibrillation who could not tolerate warfarin.
• Disadvantages of these novel anticoagulant agents are current unavailability of antidotes, and limited long-term safety data.
• ROCKET-AF showed rivaroxaban to be noninferior to warfarin for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
References
- Di Nisio M, Middeldorp S, Buller HR. Direct thrombin inhibitors. N. Engl. J. Med.353, 1028–1040 (2005).
- Fuster V, Rydén LE, Cannom DS et al. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol.57(11), e101–e98 (2011).
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrialfibrillation and flutter in the United States. Am. J. Cardiol.104(11), 1534–1539 (2009).
- Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA285(18), 2370–2375 (2001).
- Pérez-Gómez F, Iriarte JA, Zumalde J et al. Antithrombotic therapy in elderly patients with atrial fibrillation: effects and bleeding complications: a stratified analysis of the NASPEAF randomized trial. Eur. Heart J.28(8), 996–1003 (2007).
- Kim YD, Cha MJ, Kim J et al. Ischaemic cardiovascular mortality in patients with non-valvular atrial fibrillation according to CHADS2 score. Thromb. Haemost.105(4), 712–720 (2011).
- Marini C, De Santis F, Sacco S et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke36, 1115–1119 (2005).
- Pisters R, Lane DA, Nieuwelaat R et al. A novel, user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest138, 1093–1100 (2010).
- Connolly SJ, Pogue J, Eikelboom J et al. ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation118(17), 2029–2037 (2008).
- Matchar DB, Samsa GP, Cohen SJ et al. Improving the quality of anticoagulation of patients with atrial fibrillation in managed care organizations: results of the managing anticoagulation services trial. Am. J. Med.113(1), 42–51 (2002).
- Connolly S, Pogue J, Hart R et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W), a randomized controlled trial. Lancet367, 1903–1912 (2006).
- Francis CW, Berkowitz SD, Comp PC et al. The EXULT A Study Group. Comparison of ximelagatran with warfarin the prevention of venous thrombo embolism after total knee replacement. N. Engl. J. Med.349, 1703–1712 (2003).
- Olsson SB. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III), randomized controlled trial. Lancet362, 1691–1698 (2003).
- Ezekowitz MD, Reilly PA, Nehmiz G et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am. J. Cardiol.100(9), 1419–1426 (2007).
- Connolly SJ, Ezekowitz MD, Yusuf S et al.; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med.361(12), 1139–1151 (2009); erratum in: N. Engl. J. Med.363(19), 1877 (2010).
- Pradaxa (dabigatran etexilate), prescribing information. Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA (2010).
- Wallentin L, Yusuf S, Ezekowitz MD et al. RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalized ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet376(9745), 975–983 (2010).
- Beasley BN, Unger EF, Temple R. Anticoagulant options – why the FDA approved a higher but not a lower dose of dabigatran. N. Engl. J. Med.364(19), 1788–1790 (2011).
- Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N. Engl. J. Med.365, 2039–2040 (2011).
- Patel MR, Mahaffey KW, Garg J et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med.365(10), 883–891 (2011).
- Connolly SJ, Eikelboom J, Joyner C et al. AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N. Engl. J. Med.364(9), 806–817 (2011).
- Lopes RD, Alexander JH, Al-Khatib SM et al. ARISTOTLE Investigators. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am. Heart J.159(3), 331–339 (2010).
- Granger CB, Alexander JH, McMurray JJ et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med.365(11), 981–992 (2011).
- Ruff CT, Giugliano RP, Antman EM et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective Anticoagulation with Factor Xa Next Generation in Atrialfibrillation-Thrombolysis in Myocardial Infarction Study 48 (ENGAGE AF-TIMI 48). Am. Heart J.160(4), 635–641 (2010).
- Ezekowitz M, Diaz R, Dorian P et al. Randomized clinical trial of three doses of a long-acting oral factor Xa inhibitor betrixaban, in patients with atrial fibrillation. Presented at: The American College of Cardiology’s 59th Annual Scientific Session. Atlanta, Georgia, USA, 14–16 March 2010.
- Gorina E, Connolly S, Diaz R et al. Phase II randomized, parallel group, dose-finding, multicenter, multinational study of the safety, tolerability and pilot efficacy of three blinded doses of the oral factor Xa inhibitor betrixaban compared with warfarin in AF. EXPLORE-Xa. Presented at: Presented at: The American College of Cardiology’s 59th Annual Scientific Session. Atlanta, Georgia, USA, 14–16 March 2010.
- Chimowitz MI, Lynn MJ, Howlett-Smith H et al. Warfarin–Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N. Engl. J. Med.352(13), 1305–1316 (2005).
- Kasner SE, Lynn MJ, Chimowitz MI et al. Warfarin Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Warfarin vs aspirin for symptomatic intracranial stenosis: subgroup analyses from WASID. Neurology67(7), 1275–1278 (2006).
- Hankey GJ. Warfarin–Aspirin Recurrent Stroke Study (WARSS) Trial: is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke? Stroke33(6), 1723–1726 (2002).
- Sacco RL, Prabhakaran S, Thompson JL et al. WARSS Investigators. Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin–Aspirin Recurrent Stroke Study. Cerebrovasc. Dis.22(1), 4–12 (2006).
- Cervical Artery Dissection in Stroke Study Trial Investigators. Antiplatelet therapy vs. anticoagulation in cervical artery dissection: rationale and design of the Cervical Artery Dissection in Stroke Study (CADISS). Int. J. Stroke2(4), 292–296 (2007).
- Ferro JM, Canhão P, Stam J et al. ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke35(3), 664–670 (2004).
- Coutinho JM, Ferro JM, Canhão P et al. ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke41(11), 2575–2580 (2010).
- Okuma H, Kitagawa Y, Yasuda T et al. Comparison between single antiplatelet therapy and combination of antiplatelet and anticoagulation therapy for secondary prevention in ischemic stroke patients with antiphospholipid syndrome. Int. J. Med. Sci.7(1), 15–18 (2009).
- Pullicino P, Thompson JL, Barton B et al. WARCEF Investigators. Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF), rationale, objectives, and design. J. Card. Fail.12(1), 39–46 (2006).
- Freeman JV, Zhu RP, Owens DK et al. Cost–effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann. Intern. Med.154(1), 1–11 (2011).
- Rhea JM, Molinaro RJ. Direct thrombin inhibitors: clinical uses, mechanism of action, and laboratory measurement. MLO Med. Lab. Obs.43(8), 20–24 (2011).
- Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy31(12), 1175–1191 (2011).
Websites
- AstraZeneca Pharmaceuticals. Successful outcome to the mutual recognition procedure for Exanta™ (ximelagatran) in Europe. www.astrazeneca.com/Media/Press-releases/Article/20040505--SUCCESSFUL-OUTCOME-OF-THE-MUTUAL-RECOGNITION-PROCEDUR
- FDA. Cardiovascular and Renal Drugs Advisory Committee testimony. www.fda.gov/ohrms/dockets/ac/04/transcripts/2004-4069T1.pdf
- AstraZeneca clinical trials. www.astrazenecaclinicaltrials.com/other-drug-products/discontinued-products/exanta/
- Sandy Walsh. FDA approves Pradaxa to prevent stroke in people with atrial fibrillation. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm
Direct thrombin inhibitors and factor Xa inhibitors in patients with cerebrovascular disease
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/expertneurothera. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are considering novel anticoagulant therapy for your patient, a 66-year-old woman with NVAF. On the basis of the review by Dr. Morales-Vidal and colleagues, which of the following statements about the role of dabigatran in stroke prevention is most likely correct?
□ A Dabigatran is a bivalent DTI
□ B Compared with warfarin, dabigatran has a lower gastrointestinal bleeding risk
□ C Compared with warfarin, dabigatran has a lower myocardial infarction risk
□ D The RE-LY study showed dabigatran to be as safe as warfarin, with lower stroke rates and less intracranial bleeding
2. You are considering treating the patient described above with rivaroxaban. On the basis of the review by Dr. Morales-Vidal and colleagues, which of the following statements about the role of rivaroxaban in stroke prevention among patients with NVAF is most likely correct?
□ A Rivaroxaban is a univalent DTI
□ B In ROCKET-AF, rivaroxaban was noninferior to warfarin for stroke prevention
□ C The FDA has not approved rivaroxaban for stroke prevention in patients with AF
□ D In ROCKET-AF, intention-to-treat analysis showed that rivaroxaban was superior to warfarin for stroke prevention
3. On the basis of the review by Dr. Morales-Vidal and colleagues, which of the following statements about the role of apixaban in stroke prevention among patients with NVAF is most likely correct?
□ A Apixaban appears to be superior to aspirin for stroke prevention in patients with NVAF who are not candidates for warfarin therapy
□ B Apixaban is a bivalent DTI
□ C In the AVERROES trial, the composite outcome of stroke and systemic embolism was not significantly lower with apixaban vs aspirin
□ D In the ARISTOTLE trial, apixaban was not superior to warfarin in stroke or systemic embolism prevention
Notes
Data taken from Citation[15].