Abstract
Bipolar disorder (BD) is a severe, chronic affective disorder, associated with significant disability, morbidity and premature mortality. Omega-3 polyunsaturated fatty acids (PUFAs) play several important roles in brain development and functioning. Evidence from animal models of dietary omega-3 (n-3) PUFA deficiency suggest that these fatty acids are relevant to promote brain development and to regulate behavioral and neurochemical aspects related to mood disorders, such as stress responses, depression and aggression, as well as dopaminergic content and function. Preclinical and clinical evidence suggests roles for PUFAs in BD. n-3 PUFAs seem to be an effective adjunctive treatment for unipolar and bipolar depression, but further large-scale, well-controlled trials are needed to examine its clinical utility in BD. The use of n-3 as a mood stabilizer among BD patients is discussed here. This article summarizes the molecular pathways related to the role of n-3 as a neuroprotective and neurogenic agent, with a specific focus on BDNF. It is proposed that the n-3–BDNF association is involved in the pathophysiology of BD and represents a promising target for developing a novel class of rationally devised therapies.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/expertneurothera; (4) view/print certificate.
Release date: July 1, 2011; Expiration date: July 1, 2012
Learning objectives
Upon completion of this activity, participants should be able to:
• Describe the role of n-3 PUFAs in brain development and functioning based on a review
• Describe potential roles for n-3 PUFAs in bipolar disorder
• Describe the role of BDNF in bipolar disorder
Financial & competing interests disclosure
EDITOR
Elisa Manzotti,Editorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD,Freelance writer and reviewer, Medscape, LLC
Disclosure:Laurie Barclay, MD, has disclosed no relevant financial relationships.
AUTHORS
Vicent Balanzá-Martínez,CIBERSAM University of Valencia Medical School, Valencia, Spain; and University Hospital Doctor Peset, Valencia, Spain; and Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Disclosure:Vicent Balanzá-Martínez has received research grants and has served as a consultant, advisor or speaker during the last 3 years for the following companies: AstraZeneca, Boehringer Ingelheim, Bristol-Myers-Squibb, Janssen-Cilag and Pfizer Inc.; he is supported by grants from Fundación Alicia Koplowitz and the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, CIBERSAM. He has disclosed no other relevant financial relationships.
Gabriel R Fries,Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Disclosure:Gabriel R Fries is supported by scholarships from Programa de Pós-Graduação em Ciências Biológicas: Bioquímica (CAPES), Brazil. He has disclosed no other relevant financial relationships.
Gabriela D Colpo,Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Disclosure:Gabriela D Colpo is supported by a scholarship from Programa de Pós Graduação em Ciências Médicas, Brazil, and from CNPq, Brazil. She has disclosed no other relevant financial relationships.
Patricia P Silveira,Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Disclosure:Patricia P Silveira has received grant/research support from CNPq, CAPES and Fundação de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS). She has disclosed no other relevant financial relationships.
André K Portella,Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Disclosure:André K Portella has received grant/research support from CNPq, CAPES and Fundação de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS). He has disclosed no other relevant financial relationships.
Rafael Tabarés-Seisdedos,CIBERSAM University of Valencia Medical School, Valencia, Spain
Disclosure:Rafael Tabarés-Seisdedos has received grants from or acted as a consultant for the following companies: AstraZeneca, Janssen, Eli-Lilly, Lundbeck, Novartis, Pfizer, Sanofi-Aventis and Wyeth, which were deposited into research accounts at the University of Valencia. He has disclosed no other relevant financial relationships.
Flávio Kapczinski,Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Disclosure:Flávio Kapczinski has received grant/research support from AstraZeneca, Eli Lilly, the Janssen-Cilag, Servier, CNPq, CAPES, NARSAD and the Stanley Medical Research Institute; has been a member of the speakers’ boards for AstraZeneca, Eli Lilly, Janssen and Servier; and has served as a consultant for Servier. He has disclosed no other relevant financial relationships.
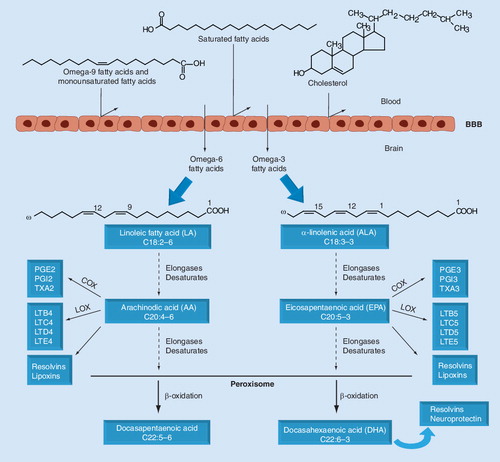
LA and ALA are converted to long-chain fatty acids through reactions of desaturation and elongation. The synthesis of DHA requires the passage of precursor fatty acids into the peroxisome, where they suffer one cycle of β-oxidation to produce DHA. The AA and EPA fatty acids synthesize prostanoids and leukotrienes by the enzymes COX and LOX, respectively. AA, EPA and DHA can also synthesize resolvins, proteins that have neuroprotective functions. These reactions occur primarily in the liver, but they can also take place in the brain, once omega-3 and omega-6 are transferred by the BBB.
AA: Arachinodic acid; ALA: α-linolenic acid; COX: Cyclooxygenase; DHA: Docasahexaenoic acid; EPA: Eicosapentaenoic acid; LA: Linoleic acid; LTB4: Leukotriene B4; LTB5: Leukotriene B5; LTC4: Leukotriene C4; LTC5: Leukotriene C5; LTD4: Leukotriene D4; LTD5: Leukotriene D5; LTE4: Leukotriene E4; LTE5: Leukotriene E5; LOX: Lipoxygenase; PGE2: Prostaglandin 2; PGE3: Prostaglandin 3; PGI2: Prostacyclin I2; PGI3: Prostacyclin I3; TXA2: Thromboxane A2; TXA3: Thromboxane A3.
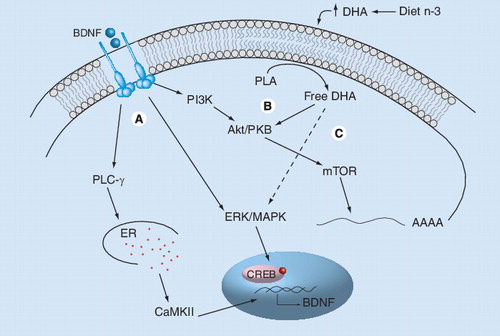
(A) Mature BDNF binds to the TrkB receptor and activates three main intracellular signaling pathways involving PLC-γ, ERK/MAPK and Akt/PKB. Activation of PLC-γ leads to the release of calcium from the ER and to the activation of CaMKII, leading to the phosphorilation of CREB and activation of gene transcription. Activation of the ERK/MAPK pathway can also regulate transcription through the phosphorylation of CREB, whereas PI3K phosphorylates and activates Akt/PKB and mTOR, regulating translation initiation. (B) DHA increases neurotrophic signaling by activating one branch of the classical BDNF signaling via PI3-K/Akt pathways. (C) DHA increases BDNF synthesis by activating MAPK signaling. Activated MAPK phosphorylates CREB, which translocates into the nucleus and activates BDNF gene transcription.
BDNF: Brain-derived neurotrophic factor; CaMKII: Calcium–calmodulin kinase II; CREB: cAMP response element-binding protein; DHA: Docosahexaenoic acid; ER: Endoplasmic reticulum; n-3: Omega-3 fatty acids; PLA: Phospholipase A2; PLC-γ: Phospholipase C γ; TrkB: Tyrosine kinase receptor B.
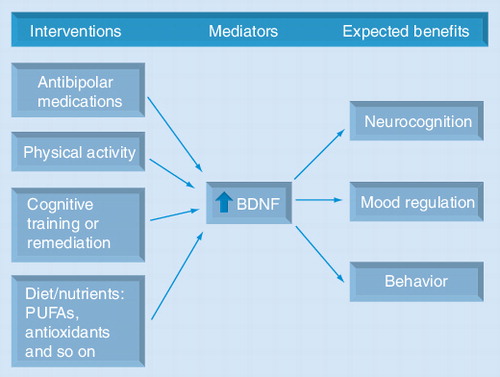
Several pharmacological agents used to treat bipolar disorders, such as mood stabilizers, antidepressants and atypical antipsychotics, increase BDNF levels. Interventions on lifestyle-related factors, such as regular physical exercise and diet/nutrition, also increase neurotrophins. In addition, the neurocognitive benefits derived from psychosocial interventions, such as cognitive training or remediation, are thought to be mediated by neurotrophins. These strategies may have synergistic effects. Multitargeted interventions that increase BDNF and other neutrophins may maximize neurogenesis/neuroprotection, and as a result potentially improve mood, neurocognitive functioning and mental health. The expected benefits from each intervention may be different, but in all instances BDNF would be a putative key mediator.
BDNF: Brain-derived neurotrophic factor; PUFA: Polyunsaturated fatty acid.
Bipolar disorder (BD) is a chronic, recurrent affective disorder characterized by cyclic episodes of mania/hypomania and depression, interspersed with periods of clinical remission or euthymia. BD is a complex syndrome characterized by the dysfunction of multiple neurobehavioral domains. Beyond the core dysfunction in mood regulation, the broad phenotypic expression of BD includes anxiety, psychosis, impulsivity, disturbances in cognition and circadian rhythm (e.g., sleep–wake cycle), as well as high rates of medical and psychiatric comorbidities Citation[1,2].
Moreover, BD is a severe condition that is often associated with significant disability, morbidity and premature mortality Citation[3,4]. All of this seems to be due to cognitive, as well as physical health, deterioration Citation[2,5,6]. Persistent cognitive dysfunctions may predict a poorer long-term functional outcome among BD patients Citation[7,8]. In addition, BD carries a higher risk for a wide range of medical conditions, including cardiovascular disease (CVD), cerebrovascular diseases and neurological disorders, such as migraine or epilepsy, as well as metabolic abnormalities, such as obesity/overweight and Type 2 diabetes mellitus Citation[9–11]. These comorbidities frequently complicate the clinical presentation and management of BD and worsen treatment response, course, outcome and quality of life Citation[12,13].
The omega-3 (n-3 or ω-3) and omega-6 (n-6 or ω-6) polyunsaturated fatty acids are either obtained from the diet or synthesized from the essential fatty acids α-linolenic acid (ALA) and linoleic acid (LA), respectively. Fatty acids play different physiological roles in the organism, are important in the structure of cellular membranes and are essential for brain functions and nerve impulse transmission Citation[14]. The main n-3 long-chain polyunsaturated fatty acid (LC-PUFA or simply PUFA, hereafter) in the neural membrane is docosahexaenoic acid (DHA). DHA is necessary for the structure of brain cellular membranes and influences signaling events that are essential to neuron differentiation and survival Citation[15,16]. n-3 PUFAs have been suggested as a treatment for clinical depression Citation[17–19]. Recent evidence suggests an involvement of n-3 PUFAs with the BDNF/tyrosine kinase receptor B (TrkB) signaling pathway Citation[20,21], which may in part explain some of the neuroprotective effects of n-3 in experimental models.
BDNF is a protein of the neurotrophin family, which is involved in neuroprotection, including neuronal survival, dendritic arborization, synaptic plasticity and neurodevelopment Citation[22]. A growing body of evidence has suggested that BDNF is involved in the pathophysiology of BD Citation[23]. Several agents that have positive effects in mood, including antidepressants and mood stabilizers, enhance BDNF levels Citation[24–26], whereas acute episodes of BD have been associated with a decrease of its serum levels Citation[27]. Therefore, it seems that drugs with a BDNF-enhancing capacity may have therapeutic effects on BD.
The BDNF/TrkB signaling pathway is one of the different neurobiological mechanisms of action that have been proposed to explain the mood-regulating effects of n-3 PUFAs in BD Citation[28–30], including the modulation of signal transduction pathways, the reduction of proinflammatory cytokines or the blockade of calcium channels Citation[31].
In this article, we will examine the available evidence of the possible therapeutic use of n-3 in BD. A possible role via the BDNF neuroprotection/neurogenesis system is described.
Omega-3 long-chain, polyunsaturated fatty acids
Fatty acids play different physiological roles in the organism, are extremely important for the structure of cell membranes and metabolic processes, and are essential for brain functions and nerve impulse transmission Citation[14].
Long-chain polyunsaturated fatty acids include omega-6 (n-6 or ω-6) and omega-3 (n-3 or ω-3) families of fatty acids, whose precursors are LA and ALA, respectively . Both of them have 18 carbon atoms, with a carboxylic group at one of the chain edges and a methylic group at the other, also known as omega (n) termination. LA presents two double bonds, and the first is located at the carbon 6 from the methylic group (n-6). ALA presents three double bonds, and the first is located at the third carbon from the methylic group (n-3).
The dietary precursors ALA and LA are rapidly absorbed and metabolized after their ingestion, mediated by a series of elongation and desaturation reactions. However, the conversion rates of n-3 and n-6 long-chain PUFAs from ALA and LA are very inefficient in humans and have been estimated to be approximately 1 and 3–6%, respectively Citation[32–34]. The liver is the most active tissue in converting LA–arachidonic acid (AA) and ALA–DHA, and has a key role in providing PUFAs for less active tissues, such as the brain Citation[34]. Elongases act by directing two carbon atoms into the initial part of the chain, whereas desaturases act by oxidizing two carbons of the chain and creating a double bond in a cis configuration Citation[35]. After going through these processes, LA and ALA produce long-chain fatty acids. LA is precursor of AA, while eicosapentaenoic acid (EPA) and DHA are synthesized from ALA .
Arachidonic acid, through the action of cyclooxygenase (COX) enzymes, produces prostanoids of the family 2 (which include prostaglandins and thromboxanes), whereas through the action of lipoxygenase (LOX) enzymes AA forms leukotrienes of the family 4. These molecules have proinflammatory actions and influence multiple physiological and pathological mechanisms in the organism Citation[36].
The EPA long-chain fatty acid goes under COX and LOX activities and forms prostanoids of the family 3 (including prostaglandins and thromboxanes) and leukotrienes of the series 5, both of which have anti-inflammatory properties Citation[14]. The fatty acids AA and EPA compete for the same enzymes and a greater affinity of the AA fatty acid for COX and LOX results in an excessive production of proinflammatory molecules . Unbalanced dietary intake of n-6 relative to n-3 PUFA may exacerbate inflammatory states and is thought to account for the increasing incidence of lifestyle-associated, chronic conditions, such as CVD, metabolic syndrome (MetS), autoimmune/inflammatory conditions and mental health problems Citation[37].
Docosahexaenoic acid is a long-chain fatty acid composed of 22 carbon atoms and 6 double bonds, the first being located at the carbon 3 from the methylic group. Most brain lipids are glycerophospholipids composed mainly of DHA and AA, and thus play important roles in the development and functioning of the CNS Citation[38]. DHA is necessary for the structure of brain cell membranes and influences signaling events that are essential to neuron differentiation and survival Citation[16,39].
Evidence suggests that PUFAs are capable of crossing the BBB Citation[40]. In humans, the majority of DHA accumulation in the brain occurs during the perinatal period, from the beginning of the third trimester of pregnancy to the second year of life Citation[41,42]. The ability to synthesize DHA from ALA is greater in the developing brain than in the mature brain, and therefore diet is considered to be the best way to maintain DHA levels in the adult brain Citation[43,44].
Behavioral & neurochemical effects of PUFAs
Large amounts of n-3 PUFAs in the brain suggest a major role of these compounds in brain structure and function. The involvement of PUFAs in CNS function can be assessed with the use of dietary manipulation in animal models. Chronic dietary deficiency in ALA in rodents greatly affects the fatty acid composition of cerebral membrane phospholipids Citation[45]. The balance between AA and DHA is a major determinant in the maturation of brain function Citation[46]. It has been shown in rodents and nonhuman primates that inadequate supplies of n-3 PUFAs during the perinatal period result in impaired learning capacity, neurotransmission processes and visual function Citation[47–50]. Therefore, the adequate ingestion of n-3 PUFAs is crucial for brain development, particularly at the time of neuronal migration, myelination, neurite growth and synaptogenesis Citation[51].
Supplementation with n-3 PUFAs in rats improves parameters in different memory and learning tasks Citation[52], while a restriction of these fatty acids in the diet leads to a worsening on these behavioral tests Citation[53]. The supplementation with DHA in the diet also plays a synergic role with exercise, increasing synaptic plasticity, memory and learning through an increase of calcium–calmodulin protein kinase II (CaMKII) levels, cAMP response element-binding protein (CREB) and BDNF in the hippocampus of animals Citation[54]. These proteins are essential to synaptic plasticity, memory consolidation and improvement of nerve impulse transmission Citation[55].
In addition, n-3 fatty acids have a neuroprotective and antioxidant capacity. Studies have shown that rats that had suffered brain injury and were treated with n-3 supplementation demonstrate a decreased oxidative damage (e.g., diminished levels of nitric oxide and protein carbonyl formation), normalized BDNF levels and a better performance in memory tests compared with animals receiving a standard diet Citation[20,56].
Studies using a single-generational n-3 PUFA-deprived rat model have reported that an n-3 PUFA-deficient diet induces long-lasting hyperactive locomotion independent of stress or exploratory behavior in rodents Citation[53,57–60]. The development and expression of amphetamine-induced sensitization is significantly increased in DHA-deficient rodents Citation[61]. n-3 PUFA-deprived rats also have an increased score on the Porsolt forced swim test for depression, and increased scores on the isolation-induced resident intruder test for aggression Citation[62], suggesting that n-3 PUFAs may be involved in the development of behaviors commonly found in some psychiatric disorders, such as depression and aggression Citation[63]. DHA supplementation completely reverses the anxiety-like behavior induced by an n-3 PUFA-deficient diet and attenuates the freezing behavior in conditioned-fear stress responses, which suggests that DHA is involved in the modulation of stress response in rats Citation[64]. Fedorova et al. showed that n-3 PUFA deficiency differently affects anxiety levels in mice maintained under stressful conditions Citation[16].
At a neurochemical level, long-term dietary deficiency in n-3 PUFAs induces a reduction in the amount of dopamine (DA) and DA D2 receptors in the frontal cortex Citation[65]. Following an amphetamine challenge, greater DA and DA metabolite concentrations were observed in the ventral striatum, but not in the prefrontal cortex in these animals Citation[61]. In addition, using microdialysis, it was shown that there was a decrease in cortical DA release accompanied by an increase in metabolite release, suggesting modifications in DA turnover and metabolism in these rats Citation[66,67]. Extracellular DA is increased in the nucleus accumbens of awakened n-3 PUFA-deficient rats Citation[68]. These results suggest that the mesolimbic DA pathway is more active, whereas the mesocortical pathway is less active in n-3 PUFA-deficient rats than in control rats Citation[69].
When offered during rodent gestation, n-3-deficient diets impact both the pregnant female and the fetus. Specific brain regions of the pregnant female rat are differentially depleted of DHA, namely the frontal cortex and the temporal lobe, regions involved in cognition and affect Citation[70]. In addition, maternal dietary n-3 fatty acid deprivation impairs fetal brain DHA accretion and phosphatidylserine metabolism Citation[71,72], which may change the release of lipid mediators and neurotransmitter precursors important to brain function Citation[73,74]. In n-3-deficient animals, glucose utilization and glucose transporter GLUT1 immunolabelling are significantly altered in cortical brain areas Citation[75,76], suggesting an altered central energetic metabolism, which is essential to cognitive performance and neuronal activity.
These effects are not limited to the fetal period. Other systems implicated in certain psychiatric disorders seem to be persistently altered following a chronic deficiency in n-3 PUFAs from conception. For instance, immunohistochemical studies reveal an increase in the D2 receptor in discrete regions of the mesolimbic and mesocortical pathways, as well as in a large number of brain areas from the n-3 PUFA-deficient pups at 2 weeks of age, possibly to compensate for low levels of DA in synaptic clefts during brain development Citation[77]. In addition, a decrease of the DA-synthesizing enzyme tyrosine hydroxylase accompanied by a downregulation of the vesicular monoamine transporter (VMAT-2) and a depletion of VMAT-associated vesicles in the hippocampus were observed in deficient offspring compared with adequately fed controls Citation[78]. In adulthood, rats raised from conception on diets containing low amounts of n-3 PUFAs have a decreased number of tyrosine hydroxylase-positive cells in the substantia nigra pars compacta and ventral tegmental area, with dendritic depletion and isolation of tyrosine hydroxylase-positive cells Citation[79]. These findings support a role for n-3 PUFAs in the survival of DA neurons and suggest that altered DA cell number, as well as function, may play a role in the behavioral effects observed in rats raised on n-3 PUFA-deficient diets. Moreover, the n-3 PUFA-deficient diets can affect cholinergic neurotransmission, in which a higher basal acetylcholine release in the hippocampus and a reduction in muscarinic receptor binding was observed in deficient rats compared with controls Citation[47].
Deficiency in n-3 PUFAs during fetal life is also associated with metabolic disturbances. The ingestive behavior in the maintenance of body fluid and metabolite homeostasis is affected by limiting the perinatal supply of dietary n-3 PUFA, with an exaggerated salt appetite caused by n-3 PUFA deficiency Citation[80]. Blood pressure is elevated in n-3 PUFA-deficient animals Citation[81,82]. It has been suggested that n-3 PUFA deficiency causes an enhanced activation of the renin–angiotensin system Citation[83], a system involved in both the control of sodium appetite and blood pressure. Studies in rodents have shown that insulin resistance can be improved when fat-rich diets contain n-3 PUFAs Citation[84–86]. These diets have been shown to promote changes in the fatty acid profile of membrane lipids of endothelial cells, as well as to modulate inflammatory cytokines, reducing the atherogenic lipid profile Citation[87,88]. They also reduce the weight gain and improve postprandial lipemia in the obese JCR:LA-cp rat Citation[89].
The chronic n-3 deficiency also seems to interact with early life stressors to predict vulnerability to behavioral alterations. For instance, the early maternal separation paradigm is a valid model for early life stress and development of a depression-like syndrome in rats Citation[90]. Using this paradigm in chronically dietary n-3 PUFA-depleted dams, behavioral impulsivity and changes in the reward response were shown in the adult offspring Citation[91]. The n-3 PUFA-deficient status and the maternal separation stress acted synergistically to increase sucrose consumption used as marker of reward sensitivity. Furthermore, n-3 PUFA-deficient rats showed increased reactivity to novelty in the open field test.
Researchers were able to demonstrate that a reversal diet with adequate n-6 and n-3 PUFAs given during the lactating period to rats originating from ALA-deficient dams is able to restore both the fatty acid composition of brain membranes and several parameters of dopaminergic neurotransmission. On the other hand, when given from weaning, there was only a partial recovery of biochemical parameters, such as fatty acid content, and no recovery of neurochemical factors, such as DA. Therefore, profound n-3 PUFA deficiency during the lactating period is suggested to be an environmental insult leading to irreversible damage to specific brain functions, mainly the ones related to the dopaminergic function. This could be linked to the emergence of critical neurodevelopmental processes during this period Citation[92].
This evidence shows that n-3 PUFAs are relevant to the promotion of brain development and function, and are important to regulate behavioral and neurochemical aspects related to mood disorders, such as stress responses, depression and aggression, as well as dopaminergic content and function. In addition, these fatty acids also play important physiological roles as modulators of the metabolic status and physical health, decreasing CVD and other chronic diseases.
The role of n-3 PUFAs in BD
The role of n-3 PUFAs in mood disorders has been extensively reviewed (the interested reader can consult Citation[17–19,93–96]). The focus here is on the potential role of n-3 PUFAs as therapeutic targets specifically in BD. First, we review the observational evidence on the relationship between BD and n-3 PUFA intake (epidemiological research) and n-3 PUFA status (biochemical studies). Second, human intervention studies that support the therapeutic efficacy of n-3 in BD are summarized.
An ecological study found that lower per capita fish/seafood consumption, a surrogate for n-3 dietary intake, was associated with higher prevalence rates of bipolar spectrum disorders, across ten countries Citation[97]. In other cross-national epidemiological analyses, Hibbeln and colleagues found similar inverse correlations with prevalence rates of major depression Citation[98] and postpartum depression Citation[99]. Although this does not prove causality, these data suggest that a greater intake of seafood may account for a lower prevalence of mood disorders. However, most epidemiological research has been performed at the individual level, including cross-sectional studies of the association between depressive symptoms and fish/n-3 PUFA dietary intake. Most (e.g., Citation[100,101]), although not all Citation[102], studies have reported a negative association between fish or n-3 PUFA intake and self-reported major depressive disorder (MDD) or depressive symptoms, hence supporting a benefit of consumption of n-3 in affective disorders. For example, a large epidemiological study found that individuals who ate fish at least twice a week are less likely to report depressive symptoms Citation[100].
Biomarkers of n-3 status are thought to reflect exposure better than estimates of dietary intake. Since the CNS is very difficult to examine in vivo, PUFA levels may be assessed in other cell types or peripheral tissues. Blood PUFA content is positively correlated with PUFA intake Citation[103] and may be a suitable index of PUFA composition in brain cell membranes, although not identical Citation[104]. The erythrocyte membrane phospholipid (fatty acid) content is the standard method to assess n-3 PUFA status in clinical practice. Several case–control, biochemical studies have found significantly decreased levels of DHA Citation[105,106] or ALA and EPA Citation[107] in erythrocytes of BD patients compared with healthy controls, although not all have Citation[108]. In addition, healthy first-degree relatives of BD patients showed a tendency towards reduced n-3 fatty acids in blood phospholipids Citation[109]. Still, blood PUFA levels have been negatively correlated with the severity of affective symptoms in some Citation[108,110], although not all Citation[106], studies. Patients with MDD also show lower levels of n-3 PUFAs, DHA and EPA, and higher ratios of n-6:n-3 PUFAs than controls (for a meta-analysis, see Citation[111]), as well as a negative association between n-3 PUFA status and the severity of affective symptoms Citation[96,112]. Moreover, DHA concentrations in the post-mortem orbitofrontal cortex of bipolar patients are significantly lower than those of healthy controls Citation[113], whereas Schwarz and colleagues found lipid abnormalities in gray and white matter, and erythrocyte membranes of drug-naive BD patients Citation[114].
The therapeutic effects of n-3 PUFAs have been tested in several neuropsychiatric disorders, mostly in MDD and Alzheimer’s disease, but also in schizophrenia, attention-deficit/hyperactivity disorder and anxiety disorders (for reviews, see Citation[94,95,115–117]). Few double-blind, placebo-controlled, randomized clinical trials (RCTs) to date have investigated the therapeutic role of n-3 PUFAs (fish oil, ethyl-EPA, EPA or DHA) in patients with BD.
In a pioneer prophylaxis trial, Stoll and colleagues showed that n-3 PUFAs improved the short-term course of BD Citation[118]. During this 4-month study, patients randomized to adjunctive treatment with high doses of fish oil (9.6 g/day of DHA plus EPA; n = 14) had significantly longer remission and significantly greater improvements of depressive symptoms, bipolar symptoms and global functioning than the placebo group (n = 16). However, no positive effect on manic symptoms was observed.
In a subsequent 12-week RCT, Frangou et al. compared the efficacy of two doses of ethyl-EPA (1 g/day and 2 g/day) versus placebo as add-on treatment in 75 patients with bipolar depression Citation[119]. Both doses of EPA significantly improved depressive symptoms, as well as global bipolar symptoms, compared with the placebo group, with no difference in manic symptoms. Moreover, a dose–response effect was not observed. By contrast, a multicenter collaborative trial of the Stanley Foundation did not find any evidence of efficacy over placebo of ethyl-EPA 6 g/day administered as adjunctive therapy for 4 months to outpatients with bipolar depression (n = 59) or rapid cycling BD (n = 62) Citation[120]. The first RCT of pediatric BD has been published recently Citation[121]. Augmentation with flax oil containing ALA did not confer better mood stabilization than placebo (olive oil). At study intake, 51 symptomatic children and adolescents were enrolled, but less than 50% completed this 16-week study.
Additional data from four small, open-label trials suggest that adjunctive n-3 fatty acids may reduce symptoms of bipolar depression Citation[122], irritability associated with BD Citation[123] and manic and depressive symptoms of children and adolescents with pediatric BD Citation[124]. Moreover, monotherapy with EPA plus DHA was associated with modest improvements in manic symptoms in an 8-week study of juvenile BD Citation[125].
Putative mechanisms of action
Several mechanistic pathways have been suggested to biologically explain the link between n-3 and BD, including: alterations in membrane function (membrane fluidity, receptor function, neurotransmission, membrane-related enzyme and ion channel activity, glucose transport and signal transduction) reviewed in Citation[126]; mood stabilization by targeting the AA cascade Citation[127]; BDNF enhancement Citation[28] via several mechanisms (see below); inflammation; changes in the synthesis of eicosanoid (prostaglandins, leukotrienes and thromboxanes) and docosatriene (resolvins and neuroprotectins) families of lipid mediators Citation[128,129]; and changes in the expression of many CNS genes Citation[130]; EPA seems to increase oxygen and glucose supply to the brain Citation[131]; and protection against oxidative stress Citation[115]. The DHA fatty acid incorporation into neuron cell membranes increases its order, thereby leading to a better binding of neurotransmitters with their receptors Citation[132]. Moreover, it eases signal transduction pathways Citation[133,134]. DHA and EPA can also modulate brain function by changing the production and function of neurotransmitters, such as serotonin and DA Citation[135,136].
Moreover, several neuroprotective effects of DHA have been reported in preclinical models of Alzheimer’s disease, including anti-inflammatory activity, antioxidant effects, neuroprotective metabolites (neuroprotectin D1), enhanced glucose transport and improved synaptic and membrane fluidity Citation[137]. These mechanisms result in reduced inflammation and oxidative stress, enhanced neuroprotective/neurogenic pathways, increased glucose utilization and neuron and synaptic function. PUFAs protect neurons directly by preventing neuronal apoptosis and by suppressing production of proinflammatory cytokines Citation[138]. Most of these neuroprotective mechanisms are relevant for BD. For example, n-3 supplementation increased cortical concentrations of N-acetyl-aspartate, a putative marker of neuronal density and integrity, in a small sample of female BD patients Citation[139], thereby protecting against excitotoxic apoptosis. In addition, n-3 PUFAs also increased glutathione, the major endogenous antioxidant defence Citation[140]. Altogether, n-3 and medications used to treat BD seem to share several mechanisms of action (see previously). It has been suggested that they both may work in a synergistic fashion and that n-3 may augment the therapeutic effects of standard antibipolar medications by means of those mechanisms Citation[117,118,127,141]. This synergistic action could improve patients’ mental health.
Discussion
This article shows the molecular pathways related to the role of n-3 as a neuroprotective and neurogenic agent. The relationship of n-3 with BDNF is the focus of this article. The use of n-3 as a mood stabilizer among BD patients is discussed.
A growing body of evidence has suggested that BDNF plays an important role in the pathophysiology of MDD and BD. BDNF is a member of the growth factor family, which is involved in synaptic efficacy, neuronal connectivity, dendritic arborization and neuroplasticity Citation[22]. Different stimuli can induce BDNF synthesis in neuron cells. The human BDNF gene has an extremely complex structure composed of 11 exons and nine promoters, which can be differentially activated Citation[142]. Such a complex set of genomic promoters and exons is thought to mediate an accurate control of the gene transcription. Evidence indicates that transcripts are differentially distributed across brain regions, in different cell types and even within different parts of the neuron Citation[143]. BDNF transcription is regulated mainly by the transcription factor CREB Citation[144]. CREB must be phosphorylated to pCREB in order to transcribe CREB-regulated genes, including BDNFCitation[145]. Once synthesized and processed, the mRNA is translated into a precursor form of the protein named proBDNF. ProBDNF is either proteolytically cleaved intracellularly by enzymes and secreted as the mature BDNF, or secreted as proBDNF and then cleaved by extracellular proteases to mature BDNF (for a review, see Citation[146]).
BDNF is highly expressed in the cerebral cortex and hippocampus, brain areas known to regulate complex brain functions, such as memory and emotion Citation[147]. Furthermore, it has been consistently associated with cognitive function in different species. Several learning tasks are associated with increased BDNF mRNA levels in rats Citation[148], and it seems that BDNF plays an important role in the late phase of long-term potentiation. Hippocampal BDNF is required for short-term memory formation of an aversively motivated learning task in one-trial inhibitory avoidance training in rats Citation[149], and evidence suggests that there is a BDNF-dependent phase in memory persistence processes Citation[150]. Furthermore, a significant positive association has been shown between serum BDNF levels and a test of verbal fluency in humans, once again suggesting the importance of BDNF in neurocognitive processes Citation[151].
Such a cognitive impairment and functional decline has been shown in BD patients Citation[152,153]. A single nucleotide polymorphism that leads to a valine-to-methionine substitution at the codon 66 in the BDNF gene is associated with impaired episodic memory in healthy subjects Citation[154] and significantly worse performance in the Wisconsin Card Sorting Test (WCST) in Val/Met BD patients compared with patients with the Val/Val genotype Citation[155]. In addition, a prospective study has demonstrated that BD patients that are carriers of the Met allele display greater temporal lobe reductions compared with Val/Val patients over a 4-year follow-up period Citation[156]. Altogether, it seems that BDNF may be associated with the cognitive decline associated with the length and progression of BD.
Serum BDNF levels are decreased in BD patients during manic and depressive episodes compared with controls Citation[27,157]. Moreover, Kauer-Sant’Anna and colleagues compared first-episode versus multi-episode BD patients, showing that BDNF levels are decreased after multiple episodes Citation[158]. This has led to the hypothesis that episode-related changes in neurotrophins may explain some of the brain structural changes observed in BD patients. Serum BDNF levels have been negatively correlated with length of illness Citation[158], and are thus suggested to play an important role in the pathophysiology of BD Citation[23].
Accumulated data suggest that BDNF may be associated with the remission of symptoms in BD, thus emphasizing the potential therapeutic use of BDNF-enhancing drugs in their treatment. There is evidence for a normalization of BDNF levels after treatment and remission of acute manic symptoms in BD patients Citation[159]. For instance, a twofold increase in the plasma levels of BDNF has been shown after 6 months of treatment in patients with a first episode of psychosis, including those with BD Citation[160]. Such data have also been confirmed in a recent meta-analysis Citation[161]. BDNF levels may be associated with improvement of acute symptoms in BD and may also offer a biological marker of clinical response to treatment in acute mania and psychosis.
Antibipolar medications, such as mood stabilizers and antidepressants, act in signaling pathways that enhance neurotrophic and neuroprotective effects Citation[25,26,162,163]. Such enhancement has been partially explained by an increased activity of the transcription factor CREB via the adenylate cyclase pathway – that is, through increased PKA activity. n-3 PUFAs may play similar roles in BD, as previously mentioned Citation[21,137,141,164]. Interestingly, lithium can increase 17-hydroxy-DHA, a metabolite of DHA with neuroprotective properties, in a rat model of neuroinflammation Citation[165].
If both n-3 PUFAs and BDNF have been implicated in the pathophysiology of BD, one could ask whether any connection exists between n-3 PUFAs and BDNF, and ultimately whether such a connection, if it exists, is of any relevance for BD. The neuroprotective/neurotrophic effects of n-3 PUFAs have been reviewed elsewhere Citation[166]. Here, the focus will be on neurogenesis and regulation of BDNF gene expression by n-3 PUFAs.
Mounting preclinical evidence suggests that n-3 PUFAs may promote hippocampal neurogenesis in adult animals Citation[167–169]. Animals with n-3 PUFA supplementation showed an increase of 5-bromo-2´-deoxyuridine (a timidine analogue) in neurons of the hippocampal dentate gyrus Citation[167]. These positive effects of n-3 PUFA on neurogenesis may be explained by several mechanisms Citation[168], including processes associated with the structural and functional roles of PUFAs in the neuronal membranes (i.e., membrane order), such as enhanced neurotransmission and cell signaling; and/or immunomodulatory effects via the inhibition of proinflammatory cytokines Citation[170]. Apart from these indirect effects, n-3 PUFAs may enhance neurogenesis via directly controlling the transcription of key genes in the brain Citation[130].
It is well-documented that n-3 PUFAs may regulate the transcription of many genes Citation[171–173]. Specifically, microarray studies made it clear that n-3 PUFAs modulate the expression of genes involved not only in lipid metabolism, but also in the pathways of interest here, such as oxidative stress response and antioxidant capacity, cell proliferation, cell growth and apoptosis, and cell signaling Citation[173]. Moreover, the effects of n-3 PUFAs on gene expression seem to show some tissue specificity and this would subserve the pleiotropic effects of these essential nutrients. Interestingly, PUFA-enriched diets lead to significant gene-expression changes in the rat brain Citation[130] and this may help to better understand how n-3 PUFAs modulate the affective, neurocognitive and behavioral responses of the human brain.
BDNF expression can be enhanced by exercise, learning activities and dietary nutrients, such as vitamin E Citation[174,175], whereas a diet high in saturated fat and sucrose inhibits its expression Citation[174]. As previously discussed, medications used to treat BD are also associated with increased levels of BDNF Citation[24,25,176]. Hence, a shift paradigm in the treatment of BD has been recently proposed Citation[23]. Specifically, substances or interventions that increase BDNF levels/expression in the brain may exert mood-stabilizing effects and deserve further research.
In 2003, Logan proposed a novel mechanism of action “involving omega-3 modulation of CREB and BDNF” Citation[28]. Recent evidence supports that n-3 PUFAs may modulate neurotrophins Citation[20,21,30,177–179]. As a first confirmation of the original hypothesis Citation[28], n-3 PUFAs normalized hippocampal BDNF levels and counteracted the learning disability after traumatic brain injury in rats Citation[20]. Moreover, in a rat model of depression, a clinically relevant reduction in brain DHA content was associated with several neurobiological changes, including reduced hippocampal BDNF expression Citation[30]. Consistent with this, n-3 PUFA deprivation led to decreased levels of DHA, BDNF, pCREB and p38 MAPK in the rat prefrontal cortex, whereas the addition of DHA induced BDNF protein expression in rat cortical astrocytes Citation[21]. Hence, increasing BDNF gene expression would be a direct mechanism that may mediate at least in part the enhancing effects of n-3 PUFAs on neurogenesis Citation[177]. In addition, it seems that not only do n-3 PUFAs increase BDNF levels, but they also increase neurotrophin signaling by activating one branch of classical neurotrophin signaling via the PI3K/Akt pathway Citation[166].
Collectively, these data suggest that reversing abnormal BDNF/CREB-related processes would be one of the potential mechanisms by which n-3 PUFAs may represent a particularly relevant molecular and therapeutic target in BD. However, other mechanisms, such as fatty acid composition of cellular membranes, modulation of the dopaminergic systems, and the role of n-3 PUFAs on neurodevelopment and oxidative stress Citation[180], may also be essential to explain the potential beneficial effects of n-3 in BD.
Expert commentary
Epidemiological and biochemical research provides persuasive evidence for an association between BD and decreased n-3 PUFA intake/status. Overall, lower levels of n-3 PUFAs have been found in blood and post-mortem brain tissues of BD patients. The reason is not yet clear, but deficient intake/status has been invoked as a preventable risk factor for recurrent affective disorders Citation[96]. Since observational research is useful to estimate the prevalence of this putative deficiency in BD, future studies need to use larger samples and better control for several confounders, such as socioeconomic status, which may be linked to more protective lifestyles and healthier diets Citation[181].
So far, two studies found improvements in depressive and bipolar symptoms following supplementation with EPA plus DHA or ethyl-EPA, compared with placebo Citation[118,119], although neither of the studies found similar improvements in mania. Conversely, two other studies found no benefit of supplementation with ethyl-EPA or flax oil Citation[120,121] for depressive or manic symptoms. This evidence is also difficult to interpret owing to marked study differences in terms of dosage, composition and duration of interventions; inclusion criteria; comparators; and outcome measures, to name a few. Moreover, adequate, biologically inert placebos must be used in future RCTs. For example, olive oil may also have some neurotrophic/neuroprotective effects Citation[182].
Overall, n-3 PUFAs seem to be more effective to improve depressive rather than manic symptoms Citation[183]. Consistent with this, several independent meta-analyses concluded that n-3 PUFAs are an effective adjunctive treatment for unipolar and bipolar depression Citation[17,18], and these antidepressant properties have been demonstrated in the forced swimming test in animal models Citation[179,184]. Modest benefits on manic symptoms were found in juvenile BD patients following n-3 supplementation, and this suggests that the value of n-3 PUFAs on BD might vary according to the patients’ age. However, this evidence stems from two small, open-label trials Citation[124–125] and needs to be confirmed.
Several aspects of mania and depression can be replicated by pharmacological manipulations of dopaminergic neurotransmission in humans, such as elevation of mood, reduction in the need for sleep, increased impulsivity and impaired cognitive function Citation[185]. There is evidence from structural Citation[186] and functional Citation[187] MRI studies that the brain areas innervated by DA may be abnormal in BD, although imaging studies still need to demonstrate consistent abnormalities. Analysis of the metabolites of DA indicates overactivity of DA in mania and decreased activity in depression Citation[188,189]. Actions of n-3 PUFAs on dopaminergic content and function may explain why they are effective to treat negative, but not positive, psychotic symptoms Citation[190] and this may also account for their higher efficacy in relatively hypodopaminergic states, such as depression, versus the excessive dopaminergic neurotransmission in mania.
Supplementation with n-3 PUFAs is a potential treatment for BD, but far more work is needed to clarify this relationship. Future studies must be sufficiently powered and have an adequate length because duration of most existing studies may be too short to reverse a putative chronic deficit of n-3 PUFAs. If long-term supplementation seems to be necessary to observe benefits in cardiovascular health Citation[191] and cognitive deterioration Citation[192], it is likely that similar periods of months to years are also needed to obtain maximum benefits on mental health. The most suitable timing, dosage and duration of interventions must be established. These will probably differ according to the clinical staging Citation[193,194], for example, primary prevention versus secondary prevention studies, early-stage versus late-stage BD patients. The prophylactic effects observed in the pioneer study of Stoll and colleagues Citation[118] require replication.
Most of the studies regarding the neuroprotective effects of n-3 are in vitro studies with cell culture or in vivo studies with animal models, and there is an obvious difficulty in translating such findings to the clinic. For instance, as previously discussed, there are no studies showing an association between BDNF and n-3 PUFAs in BD patients, even though both of these molecules have already been assessed in this disorder, and seem to share common intracellular signaling pathways. Such studies may help to clarify and further support the beneficial effects of n-3 PUFAs on neuroprotective signaling in BD.
Five-year view
Despite recent treatment progress, many BD patients still face several unmet needs, in terms of persistent affective and neurocognitive symptoms, and high rates of nonrecovery, as well as compromised quality of life and psychosocial functioning Citation[7,153]. Given the limitations of the available antibipolar medications and the increasing awareness that BD is a systemic disease Citation[5], interventions with pleiotropic effects are needed to obtain significant improvements in meaningful outcomes for BD patients. The similar actions of n-3 PUFAs and classical mood stabilizers modulating signal transduction systems, such as PKC, activity and phosphatidyl inositol, helped to unlock at least in part the pathophysiology of BD Citation[118,141]. It is proposed that the n-3–BDNF association is a promising target for hypothesis-driven, rational drug development for this severe, prevalent disorder. Should this progress be confirmed, n-3 supplementation must be offered to BD patients.
Individual differences in genetic background or baseline n-3 status, among other factors, may explain why not all patients respond to PUFA supplementation. On the one hand, intervention trials in the field of cognitive deterioration reveal that DHA only benefits those patients lacking the ApoE4 allele Citation[137]. Similarly, polymorphisms in genes regulating key enzymes of PUFA metabolism might modulate the effects of n-3 on mental health outcomes Citation[195]. Indeed, polymorphisms in genes coding for fatty acid desaturases may influence DHA status Citation[196] and treatment response Citation[197]. In this regard, pharmacogenomic studies will help to target BD patients most likely to benefit from adjuvant intervention. The fatty acid composition of serum phospholipids is genetically controlled by the FADS1 FADS2 gene cluster Citation[197] located in the chromosomal region 11q12–13.1, which in turn is a susceptibility locus for BD Citation[198]. Moreover, polymorphisms or mutations in the desaturase genes may account for PUFA dysregulation in BD Citation[199] and impaired fatty acid and phospholipid metabolism has been involved in the etiology of BD Citation[200]. On the other hand, it has been suggested that therapeutic effects from supplementation may be found only in PUFA-deficient patients Citation[201]. Conversely, subjects without baseline deficits are less likely to obtain benefits from additional supplementation Citation[51]. If that is the case, future intervention trials should use suboptimal baseline n-3 status as an inclusion criterion to select more homogenous samples of likely ‘responsive’ patients Citation[202].
Biochemical measures of PUFA levels are necessary to detect PUFA-deficient subjects and may represent clinically useful biomarkers. The n-3 index (EPA plus DHA% in erythrocyte membrane) has been suggested as a biomarker to flag coronary heart disease risk. An n-3 index of 8% or higher would confer maximum protection, whereas an index of 4% or less has been associated with the least cardioprotection Citation[203]. This represents a significant step ahead of psychiatry, but by the same token, a new avenue to be explored in mental health Citation[202]. Indexes of n-3 content may be useful for different purposes in clinical and research settings, such as monitoring patients’ adherence during intervention trials, stratifying patients according to risk levels or devising interventions aimed to optimize values of these indexes.
Currently, the erythrocyte membrane fatty acid content is the standard method to assess n-3 PUFA status in clinical practice. This process is time consuming Citation[204] and sometimes blood extraction may be uncomfortable or difficult to perform in certain patients. Measuring PUFA levels in cells of the oral mucosa is an easy, noninvasive assessment that may be useful for patients reluctant to consent because of blood sampling or for special populations, such as pediatric BD. In infants, this has been shown to reflect blood PUFA levels and dietary intake Citation[205], but not long-term exposure Citation[206]. In addition, desaturase expression in leukocytes has been suggested as a new diagnostic method to detect nutritional PUFA deficits at an early stage Citation[207], although this needs replication.
In parallel with RCTs, experimental studies (cell culture and animal models) must be prioritized to identify the mechanistic pathways that could explain some or all of the reported benefits of n-3 PUFAs on mood, cognition and behavior. It is likely that the benefits of n-3 PUFAs for physical health might be mediated mostly by their anti-inflammatory effects, whereas mental health benefits might result from additional mechanisms, such as enhancing BDNF Citation[28] or regulating key signal transduction pathways (the ‘arachidonic acid cascade’ Citation[127]). Future research should also monitor specific biomarkers to examine the putative effects of n-3 PUFAs on the pathophysiological processes of BD, such as decreased neurogenesis and increased apoptosis, oxidative stress, excitotoxicity or neuroinflammation Citation[180]. The interaction of n-3 PUFA status and life-course events, such as chronic stress, may affect the vulnerability to CNS abnormalities and therefore to psychiatric disorders.
Potential benefits of n-3 PUFAs have been described for CVD, MetS and related conditions, autoimmune and inflammatory conditions and cancer Citation[208–211], all of which are frequently associated with BD. It has been hypothesized that n-3 may represent a biological link between affective disorders and CVDs Citation[212]. These comorbidities might be even explained by a common impairment in fatty acid and phospholipid metabolism, which would be corrected by PUFA Citation[201]. Predictably, supplementation with n-3 PUFAs will improve BD patients’ physical health and decrease allostatic load (see later), and this clearly represents an emerging area of research. Here we propose that future studies examine the effects of PUFA supplementation above and beyond measures of clinical outcomes, by also using intermediate variables. The inclusion of biomarkers of allostatic load (e.g., interleukins, TNF-α, C-reactive protein, glucocorticoids, oxidative stress, Systemic Toxicity Index Citation[213]) and neurogenesis/neuroprotection (the BDNF–TrkB signaling pathway and other neurotrophins) represents a significant opportunity for future studies and merits further development. To our knowledge, these issues have not been explored previously.
Similarly, family studies may help to reveal whether the putative n-3 PUFA deficiency and/or abnormal metabolism show a familial association or even fulfill endophenotype criteria Citation[214]. If that is the case, this endophenotype might be used for early detection and treatment of at-risk individuals, genetic studies or the development of animal models of BD. Potential benefits of n-3 PUFAs have been recently demonstrated among subjects at ultra-high-risk for psychosis Citation[215].
Finally, we suggest undertaking multimodal, structured intervention programs in BD. n-3 PUFAs may have a synergistic effect not only with standard medications used to treat BD, but probably also with other lifestyle interventions that enhance neurogenesis or the BDNF–TrkB signaling pathway, such as physical exercise. There is preliminary, preclinical evidence supporting this Citation[216].
Conclusion
Epidemiological, biochemical, experimental and intervention evidence is still limited, but support the hypotheses that low PUFA status is involved in the pathogenesis of BD and that n-3 supplementation is useful for BD, especially to treat depressive symptoms. Longer-term, well-controlled RCTs are justified to confirm this efficacy and establish the minimum dose and length of supplementation required to significantly improve intermediate and clinical outcomes in BD. It is proposed that the n-3–BDNF connection is involved in the pathophysiology of BD and represents a promising target for developing a novel class of rationally devised therapies.
Bipolar disorder is a severe disorder, which is frequently associated with chronic conditions, such as CVD and MetS. Benefits of n-3 fatty acids have been shown for these disorders Citation[217]. n-3 PUFAs are safe and well-tolerated nutrients Citation[218] and only mild, transient adverse events, such as nausea, are likely to occur Citation[116,183]. Moreover, they represent an appealing option for patients, their relatives and clinicians because they are relatively cheap and perceived as a ‘natural remedy’. Altogether, it is predicted that supplementation with n-3 PUFAs will benefit the physical health of BD patients. To our knowledge, this hypothesis has not been tested to date.
Key issues
• The omega-3 (n-3) and omega-6 long-chain, polyunsaturated fatty acids (LC-PUFAs or PUFAs) are important for many functions in the organism, including the structures of cellular membranes, metabolic processes, inflammation and brain function.
• PUFA incorporation into neuron cell membranes increases its fluidity, thereby enhancing neurotransmission and facilitating signal transduction pathways.
• Evidence from animal models of dietary n-3 deficiency suggest that these fatty acids play important roles modulating neurochemical pathways controlling behavioral aspects such as locomotor activity, depressive-like states and responses to reward, domains classically linked to BD models.
• Blood n-3 PUFA content is positively correlated with n-3 PUFA intake and may be a suitable index of PUFA composition in brain cell membranes. Lower levels of n-3 PUFAs have been found in blood and post-mortem brain tissues of BD patients.
• n-3 PUFAs seem to be an effective adjunctive treatment for unipolar and bipolar depression, but further large-scale, well-controlled trials are needed to examine its utility in BD.
• BDNF, a protein involved in neurogenesis and neuroplasticity, has been consistently associated with the pathophysiology of BD. Changes in neurotrophins in BD and the effects of antibipolar medications on neurotrophin levels are well-documented.
• BDNF levels could be a marker of clinical response to treatment in BD and emphasizes the potential therapeutic use of BDNF-enhancing drugs in their treatments.
• n-3 PUFAs have been shown to induce BDNF expression, which may be responsible for their neuroprotective effects.
• The BDNF–TrkB signaling pathway is one of the neurobiological mechanisms of action that have been proposed to explain the mood-regulating effects of n-3 PUFAs in BD. Moreover, the potential antiapoptotic effects of n-3 PUFAs deserve more attention.
Acknowledgements
The authors acknowledge the contribution of Camila Lersch (Bipolar Disorder Program and Laboratory of Molecular Psychiatry, INCT Translational Medicine) in the discussion and preparation of the early draft of this article.
References
- Bowden CL. Bipolar pathophysiology and development of improved treatments. Brain Res.1235, 92–97 (2008).
- Soreca I, Frank E, Kupfer DJ. The phenomenology of bipolar disorder: what drives the high rate of medical burden and determines long-term prognosis? Depress. Anxiety26(1), 73–82 (2009).
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet349, 1436–1442 (1997).
- Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. Pharmacoeconomics21(9), 601–622 (2003).
- Kapczinski F, Vieta E, Andreazza AC et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci. Biobehav. Rev.32(4), 675–692 (2008).
- Sanchez-Moreno J, Martinez-Aran A, Tabarés-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and disability in bipolar disorder: an extensive review. Psychother. Psychosom.78(5), 285–297 (2009).
- Tabarés-Seisdedos R, Balanzá-Martínez V, Sánchez-Moreno J et al. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J. Affect. Disord.109, 286–299 (2008).
- Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord.11, 113–125 (2009).
- Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: A population-based controlled study. Psychosom. Med.68, 684–691 (2006).
- McIntyre RS, Soczynska JK, Beyer JL et al. Medical comorbidity in bipolar disorder: re-prioritizing unmet needs. Curr. Opin. Psychiatry20(4), 406–416 (2007).
- Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann. Clin. Psychiatry20(3), 131–137 (2008).
- McIntyre RS, Konarski JZ, Soczynska JK et al. Medical comorbidity in bipolar disorder: implications for functional outcomes and health service utilization. Psychiatr. Serv.57, 1140–1144 (2006).
- Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am. J. Psychiatry160(1), 112–117 (2003).
- Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int. J. Dev. Neurosci.18(4/5), 383–399 (2000).
- Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem.282, 18661–18665 (2007).
- Fedorova I, Salem Jr N. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids75(4–5), 271–289 (2006).
- Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr.28(5), 525–542 (2009).
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr.91(3), 757–770 (2010).
- Rocha Araujo DM, Vilarim MM, Nardi AE. What is the effectiveness of the use of polyunsaturated fatty acid omega-3 in the treatment of depression? Expert Rev. Neurother.10(7), 1117–1129 (2010).
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma21, 1457–1467 (2004).
- Rao JS, Ertley RN, Lee HJ et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol. Psychiatry12, 36–46 (2007).
- Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci. Biobehav. Rev.31(6), 858–873 (2007).
- Kapczinski F, Frey BN, Kauer-Sant’Anna M, Grassi-Oliveira R. Brain-derived neurotrophic factor and neuroplasticity in bipolar disorder. Expert Rev. Neurother.8(7), 1101–1113 (2008).
- Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brainderived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry54, 70–75 (2003).
- Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl.)158(1), 100–106 (2001).
- Li N, He X, Qi X, Zhang Y, He S. The mood stabilizer lamotrigine produces antidepressant behavioral effects in rats: role of brain-derived neurotrophic factor. J. Psychopharmacol.24(12), 1772–1778 (2010).
- de Oliveira GS, Ceresér KM, Fernandes BS, et al. Decreased brain-derived neurotrophic factor in medicated and drug-free bipolar patients. J. Psychiatr. Res.43(14), 1171–1174 (2009).
- Logan AC. Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern. Med. Rev.8(4), 410–425 (2003).
- Rao JS, Ertley RN, DeMar JC Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry.12, 151–157 (2007).
- Levant B, Ozias MK, Davis PF et al. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology33, 1279–1292 (2008).
- Stoll AL, Locke CA, Marangell LB, Severus WE. Omega-3 fatty acids and bipolar disorder: a review. Prostaglandins Leukot. Essent. Fatty Acids60, 329–337 (1999).
- Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta1213, 277–288 (1994).
- Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Compartmental modeling to quantify α-linolenic acid conversion after longer term intake of multiple tracer boluses. J. Lipid Res.46, 1474–1483 (2005).
- Bezard J, Blond JP, Bernard A, Clouet P. The metabolism and availability of essential fatty acids in animal abd human tissues. Reprod. Nutr. Dev.34, 539–568 (1994).
- Nakamura MT, Nara TY. Structure, function and dietary regulation of δ-6, δ-5 and δ-9 desaturases. Annu. Rev. Nutr.24(4), 345–376 (2004).
- Smith WL. Prostanoid biosynthesis and mechanism of action. Am. J. Physiol. Renal Physiol.263(2 Pt 2), F181–F191 (1992).
- Simopoulos AP. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev. Int.20(1), 77–90 (2004).
- Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res.242, 69–176 (1985).
- Kim HY, Akbar M, Kim YS. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids82(4–6), 165–172 (2010).
- Ouellet M, Emond V, Chen CT et al. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood–brain barrier: an in situ cerebral perfusion study. Neurochem. Int.55(7), 476–482 (2009).
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev.31, 79–83 (1979).
- Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr.1204(Pt 2), S129–S138 (1992).
- Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proc. Nutr. Soc.651, 42–50 (2006).
- Su H. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J. Nutr. Biochem.21, 364–373 (2010).
- Bourre JM, Dumont O, Durand G. Brain phospholipids as dietary source of (n-3) polyunsaturated fatty acids for nervous tissue in the rat. J. Neurochem.60, 2018–2028 (1993).
- Cunnane SC, Francescutti V, Brenna JT, Crawford MA. Breast-fed infants achieve a higher rate of brain and whole body docosahexaenoate accumulation than formula-fed infants not consuming dietary docosahexaenoate. Lipids35(1), 105–111 (2000).
- Aid S, Vancassel S, Poumes-Ballihaut C, Chalon S, Guesnet P, Lavialle M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J. Lipid Res.44(8), 1545–1551 (2003).
- Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fatty Acids75(4–5), 259–269 (2006).
- Alessandri JM, Guesnet P, Vancassel S et al. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev.44(6), 509–538 (2004).
- Hichami A, Datiche F, Ullah S et al. Olfactory discrimination ability and brain expression of c-fos, Gir and Glut1mRNA are altered in n-3 fatty acid-depleted rats. Behav. Brain Res.184(1), 1–10 (2007).
- Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res.1237, 35–43 (2008).
- Chung WL, Chen JJ, Su HM. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr.1386, 1165–1171 (2008).
- Lim SY, Hoshiba J, Moriguchi T, Salem N Jr. N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr. Res.584, 741–748 (2005).
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience155(3), 751–759 (2008).
- Mizuno K, Giese KP. Hippocampus-dependent memory formation: do memory type-specific mechanisms exist? J. Pharmacol. Sci.983, 191–197 (2005).
- Bas O, Songur A, Sahin O et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem. Int.503, 548–554 (2007).
- Umezawa M, Kogishi K, Tojo H et al. High-linoleate and high-α-linolenate diets affect learning ability and natural behavior in samr1 mice. J. Nutr.129(2), 431– 437 (1999).
- Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav. Brain Res.152(1), 49–57 (2004).
- Vancassel S, Aid S, Pifferi F et al. Cerebral asymmetry and behavioral lateralization in rats chronically lacking n-3 polyunsaturated fatty acids. Biol. Psychiatry58(10), 805–811 (2005).
- Lavialle M, Champeil-Potokar G, Alessandri JM, et al. An (n-3) polyunsaturated fatty acid-deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J. Nutr.138(9), 1719–1724 (2008).
- McNamara RK, Sullivan J, Richtand NM et al. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: relationship with ventral striatum dopamine concentrations. Synapse62(10), 725–735 (2008).
- DeMar JC Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res.47, 172–180 (2006).
- Hamazaki T, Sawazaki S, Itomura M et al. The effect of docosahexaenoic acid on aggression in young adults. A placebo-controlled double-blind study. J. Clin. Invest.97(4), 1129–1133 (1996).
- Takeuchi T, Iwanaga M, Harada E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res.964(1), 136–143 (2003).
- Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. αlinolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J. Neurochem.66, 1582–1591 (1996).
- Zimmer L, Hembert S, Durand G, et al. Chronic n-3 polyunsaturated fatty acid diet-deficiency acts on dopamine metabolism in the rat frontal cortex: a microdialysis study. Neurosci. Lett.240, 177–181 (1998).
- Zimmer L, Breton P, Durand G, Guilloteau D, Besnard JC, Chalon S. Prominent role of n-3 polyunsaturated fatty acids in cortical dopamine metabolism. Nutr. Neurosci.2, 257–265 (1999).
- Zimmer L, Delion-Vancassel S, Durand G et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J. Lipid Res.41, 32–40 (2000).
- Zimmer L, Vancassel S, Cantagrel S et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am. J. Clin. Nutr.75(4), 662–667 (2002).
- Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J. Nutr.137(1), 130–134 (2007).
- Hamilton L, Greiner R, Salem N Jr, Kim HY. n-3 fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids35(8), 863–869 (2000).
- Garcia MC, Ward G, Ma YC, Salem N Jr, Kim HY. Effect of docosahexaenoic acid on the synthesis of phosphatidylserine in rat brain in microsomes and C6 glioma cells. J. Neurochem.70(1), 24–30 (1998).
- Tam O, Innis SM. Dietary polyunsaturated fatty acids in gestation alter fetal cortical phospholipids, fatty acids and phosphatidylserine synthesis. Dev. Neurosci.28(3), 222–229 (2006).
- Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl Acad. Sci. USA102(31), 10858–10863 (2005).
- Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. J. Neurochem.81, 1328–1337 (2002).
- Pifferi F, Roux F, Langelier B et al. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J. Nutr.135, 2241–2246 (2005).
- Kuperstein F, Yakubov E, Dinerman P et al. Overexpression of dopamine receptor genes and their products in the postnatal rat brain following maternal n-3 fatty acid dietary deficiency. J. Neurochem.95(6), 1550–1562 (2005).
- Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J. Neurochem.106, 662–671 (2008).
- Ahmad SO, Park JH, Radel JD, Levant B. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci. Lett.438(3), 303–307 (2008).
- Weisinger RS, Armitage JA, Chen N et al. Sodium appetite in adult rats following omega-3 polyunsaturated fatty acid deficiency in early development. Appetite55(3), 393–397 (2010).
- Begg DP, Sinclair AJ, Stahl LA et al. Hypertension induced by ω-3 polyunsaturated fatty acid deficiency is alleviated by α-linolenic acid regardless of dietary source. Hypertens. Res.33(8), 808–813 (2010).
- Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nature Med.7(3), 258–259 (2001).
- Begg DP, Sinclair AJ, Stahl LA, Garg ML, Jois M, Weisinger RS. Dietary protein level interacts with omega-3 polyunsaturated fatty acid deficiency to induce hypertension. Am. J. Hypertens.23(2), 125–128 (2010).
- Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes40(2), 280–289 (1991).
- Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr.84(2), 175–184 (2000).
- Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in Type 2 diabetes: a review. J. Am. Diet. Assoc.105(3), 428–440 (2005).
- Abeywardena MY, Head RJ. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc. Res.52(3), 361–371 (2001).
- Das UN. Long-chain polyunsaturated fatty acids interact with nitric oxide superoxide anion, and transforming growth factor-β to prevent human essential hypertension. Eur. J. Clin. Nutr.58, 195–203 (2004).
- Hassanali Z, Ametaj BN, Field CJ, Proctor SD, Vine DF. Dietary supplementation of n-3 PUFA reduces weight gain and improves postprandial lipaemia and the associated inflammatory response in the obese JCR:LA-cp rat. Diabetes Obes. Metab.12(2), 139–147 (2010).
- Matthews K, Wilkinson LS, Robbins TW. Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol. Behav.59(1), 99–107 (1996).
- Mathieu G, Denis S, Lavialle M, Vancassel S. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot. Essent. Fatty Acids78(6), 391–401 (2008).
- Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J. Lipid Res.43(8), 1209–1219 (2002).
- Lin P-Y, Su K-P. A meta-analytic review of doubleblind, placebo-controlled trials of anti-depressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry68, 1056–1061 (2007).
- Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis.6, 21 (2007).
- Liperoti R, Landi F, Fusco O, Bernabei R, Onder G. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence. Curr. Pharm. Des.15(36), 41654172 (2009).
- McNamara RK. Evaluation of docosahexaenoic acid deficiency as a preventable risk factor for recurrent affective disorders: current status, future directions, and dietary recommendations. Prostaglandins Leukot. Essent. Fatty Acids81(2–3), 223–231 (2009).
- Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am. J. Psychiatry160, 2222–2227 (2003).
- Hibbeln JR. Fish consumption and major depression. Lancet351(9110), 1213 (1998).
- Hibbeln JR. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J. Affect. Disord.69, 15–29 (2002).
- Tanskanen A, Hibbeln JR, Tuomilehto J et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr. Serv.52(4), 529–531 (2001).
- Sanchez-Villegas A, Henríquez P, Figueiras A, Ortuño F, Lahortiga F, Martínez-González MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur. J. Nutr.46(6), 337–346 (2007).
- Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am. J. Psychiatry161(3), 567–569 (2004).
- Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr.86(6), 1621–1625 (2007).
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr.60(2), 189–194 (1994).
- Chiu CC, Huang SY, Su KP et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur. Neuropsychopharmacol.13, 99–103 (2003).
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J. Affect. Disord.126(1–2), 303–311 (2010).
- Ranjekar PK, Hinge A, Hegde MV et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res.121, 109–122 (2003).
- Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord.9, 759–765 (2007).
- Sobczak S, Honig A, Christophe A et al. Lower high-density lipoprotein cholesterol and increased omega-6 polyunsaturated fatty acids in first-degree relatives of bipolar patients. Psychol. Med.34(1), 103–112 (2004).
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids43(11), 1031–1038 (2008).
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry68(2), 140–147 (2010).
- Appleton KM, Rogers PJ, Ness AR. Is there a role for n-3 long-chain polyunsaturated fatty acids in the regulation of mood and behaviour? A review of the evidence to date from epidemiological studies, clinical studies and intervention trials. Nutr. Res. Rev.21(1), 13–41 (2008).
- McNamara RK, Jandacek R, Rider T et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res.160(3), 285–299 (2008).
- Schwarz E, Prabakaran S, Whitfield P et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J. Proteome Res.7(10), 4266–4277 (2008).
- Young G, Conquer J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod. Nutr. Dev.45(1), 1–28 (2005).
- Freeman MP, Hibblen JR, Wisner KL et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry67, 1954–1967 (2006).
- Crawford MA, Bazinet RP, Sinclair AJ. Fat intake and CNS functioning: ageing and disease. Ann. Nutr. Metab.55(1–3), 202–228 (2009).
- Stoll AL, Severus WE, Freeman MP et al. Omega-3 fatty acids in bipolar disorder a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry.56, 407–412 (1999).
- Frangou S, Lewis M, McCrone P. Efficacy of ethyleicosapentaenoic acid in bipolar depression: randomized double-blind placebo-controlled study. Br. J. Psychiatry188, 46–50 (2006).
- Keck PE Jr, Mintz J, McElroy SL et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol. Psychiatry60, 1020–1022 (2006).
- Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord.12(2), 142–154 (2010).
- Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J. Clin. Psychiatry66(6), 726–729 (2005).
- Sagduyu K, Dokucu ME, Eddy BA, Craigen G, Baldassano CF, Yildiz A. Omega-3 fatty acids decreased irritability of patients with bipolar disorder in an add-on, open label study. Nutr. J.4, 1–6 (2005).
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur. J. Clin. Nutr.63(8), 1037–1040 (2009).
- Wozniak J, Biederman J, Mick E et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur. Neuropsychopharmacol.17(6–7), 440–447 (2007).
- Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac. J. Clin. Nutr.16(Suppl. 1), 391–397 (2007).
- Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol. Psychiatry13(6), 585–596 (2008).
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol.3, 279–312 (2008).
- Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res.50(Suppl.), S400–S405 (2009).
- Kitajka K, Sinclair AJ, Weisinger RS et al. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc. Natl Acad. Sci. USA101, 10931–10936 (2004).
- Katsumata T, Katayama Y, Obo R, Muramatsu H, Ohtori T, Terashi A. Delayed administration of ethyl eicosapentate improves local cerebral blood flow and metabolism without affecting infarct volumes in the rat focal ischemic model. Eur. J. Pharmacol.372(2), 167–174 (1999).
- Chalon S, Delion-Vancassel D, Belzung C et al. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J. Nutr.128, 2512–2519 (1998).
- Mitchell DC, Niu SL, Litman BJ. DHA-rich phospholipids optimize G-protein-coupled signaling. J. Pediatr.143(Suppl.), S80–S86 (2003).
- Wood JN. Essential fatty acids and their metabolites in signal transduction. Biochem. Soc. Trans.18, 785–786 (1990).
- du Bois TM, Deng C, Bell W, Huang XF. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience139, 1397–1403 (2006).
- Fenton WS, Hibblen J, Knable M. Essential fatty acids, lipid membrane abnormalities and the diagnosis and treatment of schizophrenia. Biol. Psychiatry47, 8–21 (2000).
- Cole GM, Frautschy SA. DHA may prevent age-related dementia. J. Nutr.140(4), 869–874 (2010).
- Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease – but how and why? Prostaglandins Leukot. Essent. Fatty Acids78(1), 11–19 (2008).
- Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J. Psychopharmacol.21, 435–439 (2007).
- Berger GE, Wood SJ, Wellard RM et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology33(10), 2467–2473 (2008).
- Lee HJ, Rao JS, Rapoport SI, Bazinet RP. Antimanic therapies target brain arachidonic acid signaling: lessons learned about the regulation of brain fatty acid metabolism. Prostaglandins Leukot. Essent. Fatty Acids77(5–6), 239–246 (2007).
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics90(3), 397–406 (2007).
- Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol. Cell. Neurosci.28(3), 556–570 (2005).
- Chalovich EM, Zhu JH, Caltagarone J, Bowser R, Chu CT. Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J. Biol. Chem.281, 17870–17881 (2006).
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron25, 11–14 (2000).
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog. Neurobiol.69, 341–374 (2003).
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J.9(8), 24592464 (1990).
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci.91(4), 267–270 (2003).
- Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol. Neurobiol.22(5–6), 663–674 (2002).
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist14(2), 147–156 (2008).
- Dias VV, Brissos S, Frey BN, Andreazza AC, Cardoso C, Kapczinski F. Cognitive function and serum levels of brain-derived neurotrophic factor in patients with bipolar disorder. Bipolar Disord.11(6), 663–671 (2009).
- Balanzá-Martínez V, Tabarés-Seisdedos R, Selva-Vera G et al. Persistent cognitive dysfunctions in bipolar -I and schizophrenic patients: a 3-year follow-up study. Psychother. Psychosom.74(2), 113–119 (2005).
- Martinez-Aran A, Vieta E, Torrent C et al. Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar Disord.9, 103–113 (2007).
- Egan MF, Kojima M, Callicott JH et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell112(2), 257–269 (2003).
- Rybakowski JK, Borkowska A, Skibinska M, Hauser J. Illness-specific association of val66met BDNF polymorphism with performance on Wisconsin Card Sorting Test in bipolar mood disorder. Mol. Psychiatry11(2), 122–124 (2006).
- McIntosh AM, Moorhead TW, McKirdy J et al. Temporal grey matter reductions in bipolar disorder are associated with the BDNF Val66Met polymorphism. Mol. Psychiatry12(10), 902–903 (2007).
- Cunha AB, Frey BN, Andreazza AC et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci. Lett.398(3), 215–219 (2006).
- Kauer-Sant’Anna M, Kapczinski F, Andreazza AC et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early-vs. late-stage bipolar disorder. Int. J. Neuropsychopharmacol.12(4), 447–458 (2009).
- Tramontina JF, Andreazza AC, Kauer-Sant’anna M et al. Brain-derived neurotrophic factor serum levels before and after treatment for acute mania. Neurosci. Lett.452(2), 111–113 (2009).
- Palomino A, Vallejo-Illarramendi A, González-Pinto A et al. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr. Res.86(1–3), 321–322 (2006).
- Lin P. State-dependent decrease in levels of brain-derived neurotrophic factor in bipolar disorder: a meta-analytic study. Neurosci Lett.466, 139–143 (2009).
- Tsaltas E, Kontis D, Boulougouris V et al. Enhancing effects of chronic lithium on memory in the rat. Behav. Brain Res.177(1), 51–60 (2007).
- Walz JC, Frey BN, Andreazza AC et al. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. J. Psychiatr. Res.42(5), 416–421 (2008).
- Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot. Essent. Fatty Acids79(3–5), 153–156 (2008).
- Basselin M, Kim HW, Chen M et al. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide model of neuroinflammation. J. Lipid Res.51(5), 1049–1056 (2010).
- Cole GM, Ma Q, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fatty Acids81, 213–221 (2009).
- Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience1393, 991–997 (2006).
- Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci. Lett.415(2), 154–158 (2007).
- Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J. Neurosci. Res.88(10), 2091–2102 (2010).
- Beck RD Jr, Wasserfall C, Ha GK et al. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res.1041(2), 223–230 (2005).
- Grimaldi PA. Fatty acid regulation of gene expression. Curr. Opin. Clin. Nutr. Metab. Care4(5), 433–437 (2001).
- Jump DB. Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr. Opin. Lipidol.13(2), 155–164 (2002).
- Lapillonne A, Clarke SD, Heird WC. Polyunsaturated fatty acids and gene expression. Curr. Opin. Clin. Nutr. Metab. Care7(2), 151–156 (2004).
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience112, 803–814 (2002).
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience123, 429–440 (2004).
- Frey BN, Andreazza AC, Ceresér KM et al. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci.79(3), 281–286 (2006).
- Blondeau N, Nguemeni C, Debruyne DN et al. Subchronic α-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology34(12), 2548–2459 (2009).
- Jiang L, Shi Y, Long Y, Yang Z. The influence of orally administered docosahexaenoic acid on monoamine neurotransmitter, nerve growth factor, and brain-derived neurotrophic factor in aged mice. Pharmaceut. Biol.47(7), 584–591 (2009).
- Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology34(2), 199–211 (2009).
- Berk M, Conus P, Kapczinski F et al. From neuroprogression to neuroprotection: implications for clinical care. Med. J. Aust.193(4 Suppl.), S36–S40 (2010).
- Darmon N, Drewnowski A. Does social class predict diet quality? Am. J. Clin. Nutr.87(5), 1107–1117 (2008).
- Medina JM, Tabernero A. Astrocyte-synthesized oleic acid behaves as a neurotrophic factor for neurons. J. Physiol. Paris96(3–4), 265–271 (2002).
- Montgomery P, Richardson AJ. Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst. Rev.2, CD005169 (2008).
- Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP. Omega-3 fatty acids on the forced-swimming test. J. Psychiatr. Res.42(1), 58–63 (2008).
- Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord.11(8), 787–806 (2009).
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord.10(1), 1–37 (2008).
- Blumberg HP, Leung HC, Skudlarski P et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch. Gen. Psychiatry.60(6), 601–609 (2003).
- Sher L, Carballo JJ, Grunebaum MF et al. A prospective study of the association of cerebrospinal fluid monoamine metabolite levels with lethality of suicide attempts in patients with bipolar disorder. Bipolar Disord.8(5 Pt 2), 543–550 (2006).
- Gerner RH, Fairbanks L, Anderson GM et al. CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am. J. Psychiatry141(12), 1533–1540 (1984).
- Sethom MM, Fares S, Bouaziz N et al. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot. Essent. Fatty Acids83(3), 131–136 (2010).
- Marchioli R, Barzi F, Bomba E et al. GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation105(16), 1897–1903 (2002).
- Jicha GA, Markesbery WR. Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clin. Interv. Aging5, 45–61 (2010).
- Berk M, Hallam KT, McGorry PD. The potential utility of a staging model as a course specifier: a bipolar disorder perspective. J. Affect. Disord.100, 279–281 (2007).
- Kapczinski F, Dias VV, Kauer-Sant’Anna M et al. Clinical implications of a staging model for bipolar disorders. Expert Rev. Neurother.9, 957–966 (2009).
- Su KP, Huang SY, Peng CY et al. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-α-induced depression by regulating polyunsaturated fatty acids levels. Biol. Psychiatry67(6), 550–557 (2010).
- Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr.138(11), 2222–2228 (2008).
- Schaeffer L, Gohlke H, Müller M et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet.15(11), 1745–1756 (2006).
- Fallin MD, Lasseter VK, Wolyniec PS et al. Genomewide linkage scan for bipolar-disorder susceptibility loci among Ashkenazi Jewish families. Am. J. Hum. Genet.75(2), 204–219 (2004).
- Sontrop J, Campbell MK. n-3 Polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev. Med.42, 4–13 (2006).
- Liu Y, McNamara RK. Elevated δ-6 desaturase (FADS2) gene expression in the prefrontal cortex of patients with bipolar disorder. J. Psychiatr. Res.45, 269–272 (2011).
- Horrobin DF, Bennett CN. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot. Essent. Fatty Acids60, 217– 234 (1999).
- Milte CM, Sinn N, Howe PR. Polyunsaturated fatty acid status in attention deficit hyperactivity disorder, depression, and Alzheimer’s disease: towards an omega-3 index for mental health? Nutr. Rev.67(10), 573–590 (2009).
- Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev. Med.39(1), 212–220 (2004).
- Rizzo AM, Montorfano G, Negroni M et al. A rapid method for determining arachidonic:eicosapentaenoic acid ratios in whole blood lipids: correlation with erythrocyte membrane ratios and validation in a large Italian population of various ages and pathologies. Lipids Health Dis.9, 7 (2010).
- Connor SL, Zhu N, Anderson GJ et al. Cheek cell phospholipids in human infants: a marker of docosahexaenoic and arachidonic acids in the diet, plasma, and red blood cells. Am. J. Clin. Nutr.71(1), 21–27 (2000).
- Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA. The association of fatty acid deficiency symptoms (FADS) with actual essential fatty acid status in cheek cells. Prostaglandins Leukot. Essent. Fatty Acids83(1), 1–8 (2010).
- Slagsvold JE, Thorstensen K, Kvitland M et al. Fatty acid desaturase expression in human leukocytes correlates with plasma phopholipid fatty acid status. Scand. J. Clin. Lab. Invest.69(4), 496–504 (2009).
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the antiinflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev.68(5), 280–289 (2010).
- He K, Liu K, Daviglus ML et al. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol.103(9), 1238–1243 (2009).
- Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann. Nutr. Metab.55(1–3), 123–139 (2009).
- Robinson LE, Buchholz AC, Mazurak VC. Inflammation, obesity, and fatty acid metabolism: influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl. Physiol. Nutr. Metab.32(6), 1008–1024 (2007).
- Chang JPC, Chen YT, Su KP. Hypothesis: omega-3 polyunsaturated fatty acids (n-3 PUFAs) in cardiovascular diseases (CVD) and depression: the missing link? Cardiovasc. Psychiatry Neurol.2009, 725310 (2009).
- Kapczinski F, Dal-Pizzol F, Teixeira AL et al. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol. Psychiatry15(8), 784786 (2010).
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry160(4), 636–645 (2003).
- Amminger GP, Schäfer MR, Papageorgiou K et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry67(2), 146–154 (2010).
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience1553, 751–759 (2008).
- Ruxton CHS, Derbyshire E. Latest evidence on omega-3 fatty acids and health. Nutr. Food Sci.39(4), 423–438 (2009).
- Eritsland J. Safety considerations of polyunsaturated fatty acids. Am. J. Clin. Nutr.71(1 Suppl.), S197–S201 (2000).
Therapeutic use of omega-3 fatty acids in bipolar disorder
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/expertneurothera. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Based on the above review by Dr. Balanzá-Martínez and colleagues, which of the following statements about the role of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in brain development and functioning is most likely correct?
□ A Animal models of n-3 PUFA deficiency suggest that the neurotransmitter most affected is acetylcholine
□ B The site of action of n-3 PUFAs within the neuron is the nucleus
□ C n-3 PUFAs modulate neurochemical pathways controlling locomotor activity, depressive-like states, and responses to reward
□ D n-3 PUFA intake is not associated with blood n-3 PUFA content
2. Your patient is a 34-year-old white female with bipolar disorder. Based on the above review, which of the following statements are you most likely to tell her about the role of n-3 PUFAs in managing her condition?
□ A n-3 PUFAs are most effective to treat manic symptoms
□ B Adverse effects of n-3 PUFAs preclude their routine use
□ C Long-term, well-designed, randomized, controlled trials (RCTs) have proven that a specific dose regimen and duration of n-3 PUFA supplementation is an effective stand-alone treatment for bipolar depression
□ D Preclinical and clinical evidence suggests a role for n-3 PUFAs as adjunctive treatment for bipolar depression
3. Based on the above review, which of the following statements about the role of brain-derived neurotrophic factor (BDNF) in bipolar disorder is most likely correct?
□ A BDNF is a neurotrophic factor involved in neurogenesis and neuroplasticity
□ B Antibipolar medications have not been shown to affect neurotrophin levels
□ C Many studies have shown an association between BDNF and n-3 PUFAs in bipolar patients
□ D Dietary nutrients have not been shown to affect BDNF
