Abstract
Zoledronic acid is the only bisphosphonate approved for the prevention or delay of skeletal-related events in patients with bone metastases secondary to prostate cancer. Recently, the US FDA and the EMA approved denosumab (a fully human monoclonal antibody) to treat skeletal-related events in bone-metastatic prostate cancer. This article summarizes the cost–effectiveness literature pertaining to these two agents when used in the prevention of skeletal-related events secondary to malignancy. Zoledronic acid (and denosumab in comparison with zoledronic acid) have been found to be cost effective and cost ineffective depending on the analytical perspective and model parameters.
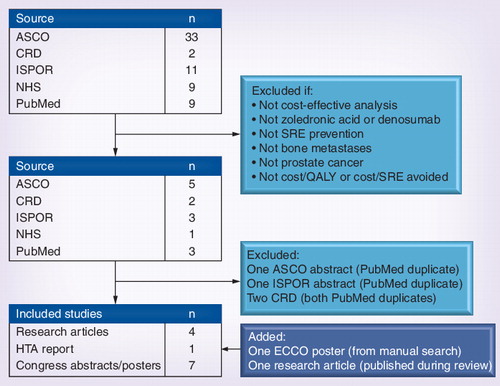
ASCO: American Society of Clinical Oncology; CRD: Center for Reviews and Dissemination; ECCO: The European Cancer Organisation; HTA: Health and Technology Assessment; ISPOR: International Society for Pharmacoeconomics and Outcomes Research;
NHS: National Health Service; QALY: Quality-adjusted life year; SRE: Skeletal-related event;
An estimated 217,730 men in the USA were newly diagnosed with prostate cancer in 2010 Citation[1]. Approximately 65–75% of those patients with advanced prostate cancer will develop bone metastases Citation[2]. Without bisphosphonate treatment, approximately 50% of patients with prostate cancer metastatic to the bone will develop ≥1 skeletal-related event (SRE; e.g., radiotherapy or surgery to bone, pathological fracture or spinal cord compression) within 2 years Citation[3]. These SREs are associated with increased treatment costs Citation[4], decreased survival Citation[5] and impaired quality of life Citation[6,7]. Thus, the prevention or reduction of SREs in bone-metastatic prostate cancer is an important treatment goal.
Patients with metastatic prostate cancer at risk of experiencing SREs often receive zoledronic acid, a third-generation intravenous bisphosphonate. Zoledronic acid is the only member of its class approved in this indication across the USA and Europe Citation[8–10]. A Phase III clinical trial has shown that zoledronic acid (4 mg every 3–4 weeks) was superior to placebo in reducing SREs in hormone-refractory prostate cancer patients with bone metastases Citation[11,12]. Significantly fewer zoledronic acid-treated patients experienced ≥1 SRE at 15 and 24 months (p < 0.05) amounting to a 53% overall reduction in SREs (RR: 0.47; p < 0.05) Citation[11–13]. At 15 months, the median time to the first SRE was 321 days in the placebo group, but had not yet been reached in the zoledronic acid group. After the 24-month extension study, the median time to first SRE was approximately 6 months longer for zoledronic acid-treated versus placebo-treated patients (p = 0.011) Citation[12]. In an exploratory analysis, zoledronic acid was associated with a significant reduction of SREs (-11%; p = 0.019) when excluding asymptomatic fractures Citation[12].
Denosumab (a fully human monoclonal antibody administered via subcutaneous injection) was recently approved by the US FDA Citation[14] and the EMA Citation[15] for SRE prevention in patients with prostate cancer and bone metastases on the basis of a Phase III trial comparing it with zoledronic acid Citation[16]. In the trial, patients with bone-metastatic prostate cancer were randomized to receive monthly denosumab (120 mg subcutaneously) or zoledronic acid (4 mg intravenously) until the first on-study SRE or death Citation[16]. Denosumab demonstrated a primary end point of noninferiority to zoledronic acid for time to first SRE (HR: 0.82; 95% CI: 0.71–0.95; p = 0.0002; p = 0.008 for secondary end point, superiority). No significant differences in overall survival, disease progression and adverse event rates were observed.
While denosumab is a step forward for SRE-preventative therapy in terms of clinical effectiveness, a number of oncologists have raised concerns that denosumab’s clinical advantages and ease of administration may not justify its high incremental cost (e.g., approximately twice that of zoledronic acid in the USA) Citation[17–19]. Regardless, denosumab and zoledronic acid are widely used therapies that have been recommended for SRE-prevention in bone-metastatic prostate cancer at the highest recommendation level by the European Association of Urology Citation[20] and the National Comprehensive Cancer Network Citation[21].
The purpose of the present article is therefore to assess and summarize the available evidence regarding the cost–effectiveness of zoledronic acid and denosumab in bone-metastatic prostate cancer with the goal of informing clinicians and policy makers who have to make formulary and reimbursement decisions regarding these agents.
Systematic literature review
A systematic literature review was conducted to identify all relevant research articles and abstracts that assessed the cost–effectiveness of zoledronic acid and/or denosumab for SRE-prevention in patients with bone metastases secondary to prostate cancer in which the outcome was the incremental cost per quality-adjusted life year (QALY) gained or incremental cost per SRE avoided. A primary search was conducted in PubMed/MEDLINE (years: 2000–20 January 2012) using the following search terms (cost–effectiveness OR cost utility) AND (zoledronic OR denosumab) AND prostate. Expansion of terms traditionally associated with economic analyses (i.e., the inclusion of ‘cost benefit’, ‘economic evaluation’ and ‘value for money’ in the search strategy) did not result in additional analyses.
This was supplemented with a secondary search (using keywords from the primary search) of relevant congresses (International Society for Pharmacoeconomics and Outcomes Research [ISPOR] Citation[101] and the American Society of Clinical Oncology [ASCO] Citation[102]; years: all). Additionally, the Database of Abstracts of Reviews of Effects, National Institute for Health Economic Evaluation Database, and the Health and Technology Assessment Database were searched via the Center for Reviews and Dissemination (years: 2000 to 20 January 2012) Citation[103]. The authors’ personal files and references were also searched. Letters, editorials, correspondences and comments were excluded. All searches were performed without regard to language. Studies were systematically assessed by two reviewers and retained if they satisfied the above inclusion criteria.
The literature search initially identified 64 studies . After inspection, 14 studies remained. Four studies reported in an abstract format corresponded to published, full analyses found in the PubMed primary search. These four studies were deemed duplicate analyses and removed. Following manual review of references and personal files, one study was added. During peer review, a cost–effectiveness analysis of denosumab versus zoledronic acid from a USA managed care perspective was published, and was added to our review as well. Thus, 12 studies were retained in the final review . Outcomes were extracted systematically and presented in the currency and year in which they were originally reported .
Of these 12 studies, five were fully published reports Citation[22–26] and seven were congress presentations (four from ASCO Citation[27–30]; two from ISPOR Citation[31,32]; one from the European Cancer Organization Citation[33]). Of the seven congress presentations, posters were available for four Citation[29,31–33]. The remaining three Citation[27,28,30] were presented as abstracts only. With the exception of studies of the cost–effectiveness of denosumab versus zoledronic acid in The Netherlands Citation[32], USA Citation[25] and the UK Citation[23], all studies included in this review received funding support from Novartis Pharmaceuticals; the maker of zoledronic acid. The Dutch analysis by Lothgren et al. (2011) Citation[32], the USA analysis by Stopeck et al. (2012) Citation[25] and the Amgen UK analysis presented by Ford et al. (2011; who also performed an independent [i.e., nonpharmaceutical industry funded] analysis based on the Amgen analysis presented in the same report) Citation[23] were funded by Amgen Incorporated; the maker of denosumab. The UK analysis by Ford et al. (2011) Citation[23] was commissioned by the UK’s health technology assessment program, and is thus the only analysis not to have been funded by a pharmaceutical company. Only one study (by Reed et al. 2004 Citation[24]) was prospectively designed and performed alongside a clinical trial. Reed et al. was also the only reviewed analysis to have been performed from a societal (i.e., nonpayer) perspective.
Cost–effectiveness of zoledronic acid & denosumab in metastatic prostate cancer
The cost–effectiveness of zoledronic acid versus placebo (i.e., no therapy) or pamidronate was assessed in six of the 12 studies retained for review and the cost–effectiveness of denosumab versus zoledronic acid was also assessed in six of the 12 studies .
Zoledronic acid versus no therapy
The seminal analysis (published in full) by Reed et al. Citation[24] was a prospectively-designed evaluation from an international, societal perspective (but presented in US$) performed in parallel to the 15-month Phase III clinical trial of zoledronic acid versus placebo Citation[11]. Resource utilization was collected alongside the trial and country-specific costs were drawn from local health economists in non-USA countries. USA costs were based on Medicare reimbursement rates and published average wholesale drug prices. Patients completed the EuroQol Group’s EQ5-D (a descriptive system using five domains each with three levels that defines 245 mutually exclusive health states) to assess the effect of SREs on quality of life every 3 months. EQ-5D data were used to estimate the difference in quality of life between patients with and without SREs, and ultimately, to calculate the QALY difference between treatment groups. Reed et al. reported both the incremental cost per QALY gained and the incremental cost per SRE avoided as measures of cost–effectiveness .
In their analysis, Reed et al. found that the total medical cost (excluding study drug cost) was US$5689 per patient on placebo versus $5365 per patient on zoledronic acid (difference -$324; 95% CI: -1781 to 1140; p = 0.6687). Including drug and administration costs of zoledronic acid, total cost increased to $11,042 per patient on zoledronic acid, resulting in a net increase of $5353 per patient relative to placebo-treated patients. The incremental costs per QALY gained and SRE-avoided were $159,200 (95% CI: 88,500–786,600) and $12,300 (95% CI: 6900–48,700), respectively Citation[24]. Univariate sensitivity analysis indicated that measures of cost–effectiveness were most sensitive to drug acquisition cost (i.e., an increase in zoledronic acid cost to its average wholesale price resulted in $217,900/QALY and $16,900/SRE). The authors concluded that the cost–effectiveness estimates of zoledronic acid versus placebo exceeded typical thresholds for conferring cost–effectiveness Citation[24].
In their fully published cost–effectiveness analysis of zoledronic acid versus no therapy, Carter et al. (2011) Citation[22] assessed the incremental costs per QALY gained from payers’ perspectives in France, Germany, Portugal and The Netherlands over 15 months, corresponding to the timeframe of the clinical trial reported by Saad et al. (2002) Citation[11]. The authors found that zoledronic acid patients experienced an estimated 0.759 fewer SREs (0.831 and 1.590 SREs in zoledronic acid and no-therapy cohorts, respectively) and gained an estimated 0.03566 QALYs versus placebo patients. The QALY gain was estimated directly from the analysis by Reed et al. and SRE costs (which ranged from €144 for vertebral fracture in Germany to €16,829 for bone surgery in The Netherlands) were based on literature estimates and country-specific reimbursement tariffs. Zoledronic acid was associated with reduced SRE-related costs [net costs] (-€2396 [€1,284] in France, -€2606 [€841] in Germany, -€3326 [€309] in Portugal and -€3617 [€87] in The Netherlands). The incremental costs per QALY gained ranged from €2430 (The Netherlands) to €36,007 (France) Citation[22]. The authors concluded that the the cost–effectiveness estimates fell below the standard €50,000/QALY threshold in non-UK European countries, and therefore, that zoledronic acid was a cost-effective alternative to no treatment.
Univariate sensitivity analysis indicated that the cost per infusion of zoledronic acid was the most important determinant of the results in France, Germany and Portugal. That is, variability in the cost per infusion (± 10% as per Reed et al. Citation[24]) was associated with the greatest subsequent variability in costs per QALY gained; with the cost–effectiveness estimates having been as low as -€1537 in Portugal and as high as €46,327 in France as a result of varying the cost per infusion of zoledronic acid. In The Netherlands, nonvertebral fracture cost was the strongest cost-driver (i.e., a difference of €21,812 between the minimum [-€8476] and maximum [€13,336] values). The cost per infusion of zoledronic acid ranked second.
The analysis by Botteman et al. (2009) assessed the cost–effectiveness of zoledronic acid versus no therapy from a French payer perspective over 15 months and was presented as an abstract only Citation[28]. Therefore details were limited. Botteman et al. (2009) employed the same global 15-month analysis as Carter et al. (2011) Citation[22,31] but with French-specific drug, administration and SRE costs. The 15-month cumulative number of SREs was 0.83 for zoledronic acid patients and 1.66 for no therapy patients. Costs related to SRE management were €2659 lower in the zoledronic acid group than no therapy. The subsequent incremental cost associated with zoledronic acid treatment was €266, and incremental QALYs were 0.03566 (estimated from Reed et al. Citation[24]). The incremental cost per QALY gained with zoledronic versus no therapy was €7447, and it remained below €50,000 in various sensitivity analyses for which details were not provided.
El Ouagari et al. (2003) Citation[33] estimated the cost–effectiveness of zoledronic versus no therapy from a Canadian payer perspective, but used the 15-month clinical trial report originally reported by Saad et al. (2002) Citation[11] and not the 24-month extension study Citation[12]. This is despite zoledronic acid not being indicated in Canada for bone-metastatic prostate cancer; although it may be routinely used. The analysis was presented as a poster at the 2003 European Cancer Organization meeting. SRE costs were estimated based on consultation with a Novartis Oncology Advisory Board, which provided a clinically informed assessment of local treatment patterns. Canadian specialists provided information with which resource use patterns were adjusted and validated for Canadians Citation[33]. However, SREs were assumed to incur cost only if they had required treatment and occurred no fewer than 6 days from the previous event in the clinical trial. Drug acquisition and administration costs were also Canadian-specific. The relevant outcome measure of cost–effectiveness was the cost per SRE avoided.
The authors found that patients treated with zoledronic acid incurred greater overall treatment costs (i.e., $6910 vs $0) but the average cost of treating SREs per patient were lower in the zoledronic acid group ($567 vs $999). Given that zoledronic acid reduced the total number of SREs by 43, the cost per SRE avoided was $32,378. Interestingly, El Ouagari also presented the cost per patient avoiding an SRE ($58,890 given an 11% reduction in patients experiencing an SRE) Citation[33]. Scenario analysis indicated that cost–effectiveness estimates were robust to varying SRE-related resource consumption costs ($53,963/QALY), excluding costs associated with bone surgery ($60,390/QALY), and taking healthcare support costs into consideration ($54,309/QALY) Citation[33]. Given that SREs may cost in excess of $40,000, the authors concluded that zoledronic acid offers a reasonable value for money versus no therapy from a Canadian payer perspective Citation[33].
Zoledronic acid versus pamidronate
In some countries, nonapproved bisphosphonates (e.g., pamidronate) are used off-label in place of more effective but more expensive SRE-limiting agents, such as zoledronic acid. Thus, a number of analyses in this review estimated the cost–effectiveness of zoledronic acid versus no therapy and/or pamidronate.
In an analysis presented as a poster at ISPOR, Carter et al. (2011) Citation[31] adapted the same decision-analytic model described previously Citation[22] to calculate the incremental cost per QALY gained of zoledronic acid versus no therapy from a UK payer’s perspective using UK-specific drug and administration costs and SRE-related costs. One important addition in this analysis was the inclusion of a cohort of patients treated with pamidronate (i.e., in addition to no therapy). The rationale for including pamidronate is its off-label use in 17% of patients in the UK despite its lack of efficacy relative to placebo Citation[34]. Hence, in this analysis, it was assumed 17% of patients in the comparator group were treated with pamidronate (and incurred costs associated with pamidronate acquisition and administration) but without any reduction in SREs.
Over 15 months (coinciding with the duration of the clinical trial) Carter et al. estimated zoledronic acid patients to have experienced fewer SREs (-0.831), more QALYs (+0.03566/patient, estimated from Reed et al. Citation[23]) and fewer SRE-related costs (GBP£1639; £2004 vs £3643), but greater treatment and administration costs (+£2419/patient; £2789 vs £370) and greater overall costs (+£780/patient) Citation[31]. Thus, the incremental cost per QALY gained was £21,874 Citation[31]. Because £30,000 per QALY gained is a standard willingness-to-pay threshold in the UK, the authors concluded that zoledronic acid is a cost-effective treatment alternative to pamidronate or no therapy. In univariate sensitivity analysis, key variables (number of infusions, SRE cost, proportion on no therapy and QALY gained) were varied ±10%. In no scenario did the cost–effectiveness exceed £30,000 per QALY gained. Results were most sensitive to the number of (or cost per) infusions of zoledronic acid (i.e., the incremental costs per QALY gained varied by £13,568 from £15,090 to £28,658). Because the singular comparator cohort was comprised of patients receiving pamidronate or no therapy, the results apply to a comparison of zoledronic acid versus a treatment strategy involving a certain proportion receiving pamidronate or no therapy. Therefore, the analysis by Carter et al. did not establish, from a UK payer perspective, whether zoledronic is cost effective versus no therapy or pamidronate, individually Citation[31].
An analysis by Botteman et al. (2006) Citation[27] is the only analysis reviewed herein to have estimated the cost–effectiveness of zoledronic acid versus no therapy and versus pamidronate using the 24-month extension study Citation[12] of the 15-month Phase III trial Citation[11]. As was done in the 2011 ISPOR analysis by Carter et al. Citation[31], Botteman et al. (2006) incorporated pamidronate as a third treatment option (i.e., used costs associated with pamidronate acquisition/administration and efficacy from the placebo cohort to represent patients treated with pamidronate). However, unlike Carter et al. Citation[31], Botteman et al. compared zoledronic acid to no therapy and to pamidronate separately, and did not create one comparator cohort comprised of both. It should also be noted that the QALY gain estimated by Botteman et al. was greater than that used by Reed et al. (and studies that incorporated Reed et al.’s QALY gain estimate) because the time horizon in Botteman et al. was longer (i.e., 24 months as opposed to 15 months). The analysis by Botteman et al. was performed from a Canadian payer perspective. Again, zoledronic acid is not approved in Canada for SRE-prevention in bone-metastatic prostate cancer.
Botteman et al. (2006) estimated the cumulative number of SREs to be 2.69 for pamidronate and no therapy patients and 1.76 for zoledronic acid-treated patients. Total costs were $11,918, $17,593 and $19,312 (+$7394 vs no therapy; +$1719 vs pamidronate) for no therapy, pamidronate and zoledronic acid patients, respectively. Zoledronic acid-treated patients gained 0.094 QALYs compared with pamidronate or no therapy. Compared with no therapy, zoledronic acid resulted in an incremental cost per QALY gained of $78,366. Versus pamidronate, zoledronic acid resulted in $18,343 per QALY gained. The authors concluded that zoledronic acid was cost effective according to Canadian standards versus no therapy and pamidronate Citation[27]. Specific details regarding the results of sensitivity and scenario analyses were not provided.
Denosumab versus zoledronic acid
The cost–effectiveness of denosumab versus zoledronic acid was assessed in six analyses Citation[23,25,26,29,30,32]. Each employed a Markov decision model based on a Phase III trial of denosumab versus zoledronic acid in patients with bone-metastatic prostate cancer described in the introduction Citation[16]. Of these analyses, three were published in full Citation[23,25,26], and the other three were presented at 2011 ASCO Citation[29,30] or 2011 ISPOR Citation[32] meetings.
In the analysis reported by Xie et al. (2011) Citation[26], a nine-state Markov decision-analytic model assessed the pharmacoeconomic value of denosumab versus zoledronic acid on the basis of cost per SRE avoided over 1- and 3-year time horizons from a US payer perspective. Xie et al. used the cost per SRE avoided as the measure of cost–effectiveness. Only literature-derived direct medical costs (i.e., wholesale drug acquisition, creatinine monitoring, labor, SREs, adverse events, disease progression and terminal care) were considered. Health states were defined by SRE occurrence, SRE history, disease progression and death, and patients transited among the nine health states based on transition probabilities derived from the clinical trial Citation[16] and supplemented with the literature estimates. Treatment-related adverse event resource utilization was estimated based on expert opinion. One-way and probabilistic sensitivity analyses were performed to test robustness of model parameters.
Patients treated with denosumab experienced and estimated -0.11 (0.49 vs 0.60) fewer SREs over the 1-year base-case scenario and -0.27 (1.18 vs 1.46) over 3 years; consistent with the Phase III trial Citation[16] in which denosumab was superior to zoledronic acid. Within the 1-year base-case time horizon, the estimated total costs per denosumab-treated patient were $35,341 ($19,230 and $16,111 for drug and nondrug costs, respectively) and $27,528 ($10,960 drug costs and $16,569 nondrug costs) for zoledronic acid. The incremental total direct cost of denosumab was $7813 and $13,856 over 1 and 3 years, respectively Citation[26]. The estimated incremental total direct costs per SRE avoided with the use of denosumab instead of zoledronic acid were $71,027 for 1 year and $51,319 for 3 years. In descending order of robustness, the following were the five most sensitive model parameters: median time to first on-study SRE for denosumab, median time to first on-study SRE for zoledronic acid, denosumab acquisition cost, zoledronic acid acquisition cost and relative risk of having an SRE associated with disease progression. Within the 1- and 3-year model horizons examined, costs per SRE avoided ranged from $27,318 to $161,680 and from $18,870 to $83,767, respectively. The probabilistic sensitivity analysis indicated that based on cost–effectiveness thresholds of $70,000, $50,000 and $30,000 per SRE avoided, denosumab was cost effective in 49.5, 17.5 and 0.3% of the cases at 1 year, respectively. The proportions were 79.0, 49.8 and 4.1% in the 3-year scenario. The authors concluded that denosumab was not a cost-effective treatment alternative to zoledronic acid Citation[26].
Similar to Xie et al., Yu et al. (2011) Citation[30] assessed the cost–effectiveness of denosumab versus zoledronic acid from the perspective of cost per SRE avoided in the USA. Yu et al. was presented as an abstract only and therefore details beyond the model structure, inputs and base-case results were limited. The authors employed a 1-year Markov model (number of states not defined) populated with transition probabilities drawn from the Phase III trial Citation[16] and costs derived from the literature. Over 1 year, Yu et al. estimated a total incremental cost of $7355 associated with denosumab ($37,854 vs $30,499). The estimated number of SREs during the 1-year period was 0.56 for denosumab and 0.67 for ZA, where the denosumab patients had 0.11 fewer SREs. The incremental cost per SRE avoided with the use of denosumab was $66,864. Sensitivity analysis indicated that the cost per SRE avoided was driven by the differences in the drug costs, risk of progression and risk of first SRE, but outcome values were not provided Citation[30].
The analyses by Snedecor et al. (2011) Citation[29] and Lothgren et al. (2011) Citation[32] were presented at the annual meetings of ASCO and ISPOR, respectively. Both analyses were Markov decision analytic models that assessed the cost–effectiveness of denosumab versus zoledronic acid using the incremental cost per QALY gained and/or incremental cost per SRE avoided and based on the Phase III trial reported by Fizazi et al. (2011) Citation[16]. Snedecor et al. estimated that patients treated with denosumab in the USA experienced fewer SREs (-0.25; 1.04 vs 1.29), more QALYs (+0.00432; 0.93512 vs 0.93080) and fewer SRE-related costs (-$1942; $7604 vs $9528). However, patients treated with denosumab also incurred greater drug, administration and monitoring costs (+$7314; $20,277 vs $12,963), and thus, greater overall costs (+$5390; $27,881 vs $22,491). Consequently, the incremental cost per QALY gained with denosumab was $1,248,051 Citation[29]. This value increased to $1,375,378, $1,674,075 and $1,899,618 in scenario analyses assuming post-SRE discounted therapy, average wholesale drug pricing, and therapy continuation until death, respectively. Univariate sensitivity analysis showed that cost–effectiveness was most influenced by assumptions regarding the effect of radiation to bone on patient utility (range in cost per QALY gained: $666,284 to $4,582,138) and time to first SRE in the denosumab cohort (range in cost–effectiveness per QALY: $318,968 to Dominated). Probabilistic sensitivity analysis indicated a low likelihood that denosumab is cost–effective. That is, 0.36% (18/5000) model replicates had a cost/QALY ≤$100,000 Citation[29]. The authors concluded that denosumab was not a cost-effective treatment alternative to zoledronic acid in this indication from a US payer perspective Citation[29].
The cost–effectiveness analysis by Lothgren et al. (2011) Citation[32] was an assessment of denosumab versus zoledronic acid from a Dutch payer’s perspective. Funded by Amgen, Lothgren et al. had access to patient level data from the Phase III trial reported by Fizazi et al. Citation[16]. As such, Lothgren et al. calculated baseline patient health state utility based on patient reports on the EQ-5D, which was administered every 4 weeks. SRE-related health state utility values were also drawn directly from the trial. This is opposed to the aforementioned analysis by Snedecor et al. (2011) Citation[29] wherein health state utilities were estimated from the literature. In Lothgren et al., denosumab and zoledronic acid treatment-related resource utilization data were also drawn directly from the trial and monetized with local cost data. The specificity of data with which SRE-related treatment costs were calculated was not defined. Costs and outcomes were discounted at 4 and 1.5%, respectively Citation[32].
Over a lifetime model horizon, Lothgren et al. found that patients treated with denosumab experienced fewer SREs (-0.158; 1.550 vs 1.708), more QALYs (+0.013; 1.380 vs 1.368), but greater overall costs (+€542; €11,912 vs €11,370). Thus, the incremental costs per QALY gained and SRE avoided were €42,933 and €3360, respectively. Results of one-way sensitivity analysis indicated that medication administration cost was the strongest driver of modeled outcomes, followed by the impacts of SREs on QALYs and overall treatment costs Citation[32]. Minimal and maximal values were not provided. Given that an incremental €50,000 per QALY gained is the traditional threshold in non-UK European countries, the authors concluded that denosumab is a cost-effective treatment alternative to zoledronic acid in The Netherlands. No such threshold exists for the incremental cost per SRE avoided; however, the authors also state that denosumab represents good value for money from this perspective.
Commissioned by NICE, Ford et al. (2011) Citation[23] presented a partially redacted cost–effectiveness analysis of denosumab versus zoledronic acid supplied to NICE by Amgen (hereto for referred to as Amgen’s analysis). The authors also presented their own analysis based on Amgen’s (hereto for referred to as NICE AG), but with additional or modified assumptions. These analyses were from a UK payer perspective using a lifetime-horizon Markov model wherein costs and QALYs were discounted 3.5% per annum. SRE-related costs were based on an unpublished, prospective, global study commissioned by Amgen (STARS costing study). Quality of life effects of SREs (i.e., disutility) were measured directly from the clinical trial with the EQ-5D. Drug and administration costs were estimated based on an unpublished microcosting study in the UK and using UK-specific costs.
In Amgen’s analysis for bone-metastatic prostate cancer, denosumab was compared with zoledronic acid on the basis of incremental cost per QALY gained in patients with ≥1 SRE prior to initiating therapy (i.e., SRE-experienced). Ford et al. note that Amgen’s reasoning for not including a comparison in SRE-naive patients (who were treated with supportive care in the analysis) was due to how zoledronic acid is indicated in the UK for metastatic prostate cancer patients. In the UK, zoledronic acid is recommended by NICE for reimbursement in bone-metastatic prostate cancer only if there is uncontrolled pain. While there was no assessment of ‘uncontrolled’ pain by Amgen, they noted in their NICE submission that 80% of SRE-experienced bone-metastatic prostate cancer patients (26% of trial participants) had bone pain at trial baseline Citation[23]. Thus, in the Amgen analysis the designation of ‘SRE-experienced’ was used as a proxy for uncontrolled bone pain, and so the comparison of denosumab to zoledronic acid was carried out for SRE-experienced patients only.
The results of the Amgen analysis showed that patients treated with denosumab compared with zoledronic acid experienced fewer SREs (-0.14; 1.98 vs 2.12) and more QALYs (0.006; 1.089 vs 1.083), but greater overall costs (£922). Unfortunately treatment-specific overall costs were redacted from the report. The resulting incremental cost per QALY gained was £157,276; cost ineffective at NICE’s willingness-to-pay threshold of £30,000/QALY. Alternatively, Amgen (and Ford et al.) conducted the analysis under a scenario wherein the price of denosumab is essentially lowered with a NICE-approved patient access scheme (PAS; details were redacted from the report). Used to help patients gain access to high-cost therapies and improve cost–effectiveness, PAS is a predefined strategy of discounts and rebates offered by the drug manufacturer to the UK National Health Service. When including PAS, denosumab saved £281 and zoledronic acid was dominated. In non-PAS scenarios, outcomes were relatively sensitive to excluding the rule that SREs must be ≥21 days apart (resulting in a reduction to £89,000/QALY), basing SRE-related utilities on an alternate source Citation[35] (resulting in an increase to £348,000 per QALY), and zoledronic acid dosing frequency (resulting in a decrease to £125,000 per QALY) Citation[23]. Incidentally, in SRE-naive patients, the Amgen model estimated that denosumab was cost ineffective versus best supportive care with PAS (£71,320) and without PAS (£102,067).
Ford et al. noted that, with regard to SREs, Amgen applied to their analysis of denosumab versus zoledronic acid in SRE-experienced patients trial-based hazard ratios and relative risks pooled across SRE-experienced and SRE-naive patients. Ford et al. stated that the SRE-experienced subgroup-specific central estimates suggest a smaller treatment effect of denosumab compared with pooled estimates, and that this effect was particularly robust in the prostate cancer analysis Citation[23].
Ford et al. also presented their own model based on the model submitted by Amgen, but which made the comparison across and within SRE-experienced and SRE-naive patients. When using pooled SRE-related hazard ratios and relative risks (as was done in Amgen’s model), the authors found that, compared with zoledronic acid, denosumab’s cost–effectiveness was £46,976, £35,732 and £167,503/QALY in all patients, SRE-naive and SRE-experienced, respectively . Including PAS, denosumab was dominant in each patient group. Ford et al. conducted the same analysis using SRE-naive and SRE-experienced-specific SRE effects. In this scenario, denosumab was cost ineffective at £113,237, £93,575 and £249,575/QALY in all patients, SRE-naive and SRE-experienced, respectively. Again, when including PAS, denosumab was cost-saving and dominated zoledronic acid. The costs per QALY were most sensitive to SRE-naive and SRE-experienced specific hazard ratios and relative risks Citation[23].
None of the scenarios presented by Ford and colleagues took into account the effect of generic zoledronic acid in 2013 on modeled outcomes. Ford et al. indicated that an increase in the incremental cost of denosumab due to generic zoledronic acid will likely increase the increase the incremental cost per QALY gained with denosumab and that such considerations are imperative to health-economic comparisons of these agents. However, consistently across these analyses, denosumab was cost ineffective at its current price but dominant when including PAS (except when versus best supportive care in SRE-naive patients).
Stopeck et al. (2012) Citation[25] reported the only fully published analysis of denosumab versus zoledronic acid from a US payer perspective. The authors used a 28-day cycle, lifetime (i.e., 15-year) Markov model (based on the clinical trial report by Fizazi et al. Citation[16]) wherein QALY decrements were estimated from nontrial based time trade-off studies and SRE costs were estimated from a US commercial claims database. Outcomes were discounted 3% per year. Interestingly, this was also the only analysis reviewed that incorporated real-world retrospective claims data Citation[36] in order to adjust the trial-based cumulative number of SREs modeled over patients’ lifetimes. The cumulative number of SREs estimated to have been experienced by zoledronic acid-treated patients was based on a 2.01 upward adjustment of the trial-based SRE rate, from which the total number of SREs experienced in the denosumab cohort was calculated using the relative treatment effects reported in the clinical trial Citation[25]. The authors justified this assumption with the explanation that SREs have been under-reported in clinical trials, and therefore the adjustment ensured that the model better reflected a US managed care perspective Citation[25].
It was estimated that, on average, denosumab-treated patients experienced 0.81 (3.23 vs 4.04) fewer SREs, gained 0.14 more QALYs (0.97 vs 0.83), and incurred $6910 greater total lifetime costs ($76,486 vs $69,577). The subsequent incremental costs per QALY gained and SRE avoided were $49,405 and $8567, respectively. One-way sensitivity analyses indicated that the most influential variables were denosumab drug cost (with outcomes ranging from approximately -$100,000/QALY to $200,000/QALY), zoledronic acid drug cost (with outcomes ranging from approximately -$40,000/QALY to $125,000/QALY), and the adjustment factor by which the total number of SREs was upwardly adjusted (with outcomes ranging from approximately $25,000/QALY to $120,000/QALY) Citation[25]. The probabilistic sensitivity analysis estimated that the probabilities of denosumab being cost effective at $100,000, $150,000 and $200,000 per QALY thresholds were 0.83, 0.94 and 0.98, respectively Citation[25]. The authors concluded that denosumab is a cost-effective treatment alternative to zoledronic acid from a US managed care perspective.
Expert commentary
Zoledronic acid and denosumab are SRE-limiting agents proven effective in patients with bone-metastatic prostate cancer. As was presented in this article, denosumab has been compared with zoledronic acid from USA, UK and Dutch payer perspectives, and zoledronic acid has been compared with pamidronate and no therapy across Europe and North America. Zoledronic acid was found to be cost effective across Europe and Canada but not in the USA, while denosumab was found to be cost effective in The Netherlands, the UK and the USA in certain analyses, but cost ineffective in other analyses in the UK and the USA. Across the reviewed analyses, by far the most robust driver of modeled outcomes was drug acquisition cost.
It was also noteworthy that analyses based on the same clinical trial data estimated SRE-limiting agents to be cost effective in some settings but not in others despite having also employed very similar model structures. For example, while both were based on the Phase III clinical trial reported by Saad et al. (2002) Citation[11], Carter et al. (2011) Citation[22] found zoledronic acid to be a cost-effective treatment alternative to placebo from a European payer perspective whereas Reed et al. Citation[23] found it to be cost-ineffective from a US societal perspective. Likewise, Snedecor et al. Citation[29] found denosumab to be cost ineffective versus zoledronic acid from a US payer perspective, while Lothgren et al. found denosumab to be cost effective from a Dutch payer perspective. Both analyses were populated with clinical data from the Phase III clinical trial reported by Fizazi et al. Citation[16]. These seemingly discrepant findings among analyses cannot be ascribed to a single cause. Rather, differences in outcomes were likely due to factors such as but not limited to: the use of country-specific drug acquisition and SRE-treatment costs, the assumed distribution of subsequent SRE-types, and study-specific assumptions related to the magnitude of effect of SREs on quality of life. These are inputs not typically presented in published clinical trial reports and so will vary among cost–effectiveness analyses. Therefore, when assessing multiple cost–effectiveness analyses that compare the same agents, one should pay particular attention to the inputs and assumptions specific to each analysis.
Similarly, in reviewing the cost–effectiveness of SRE-limiting agents in bone-metastatic solid tumors, it is important to emphasize that the choice of economic end points (i.e., incremental costs per QALY gained or SRE avoided) is integral to how one might interpret the analyses and findings. Rader et al. Citation[37] criticized Xie et al.’s Citation[26] choice of incremental cost per SRE avoided as the end point on the basis that it did not consider the effect of SREs on patients’ quality of life within the context of decreasing time remaining in patients’ lives. Cost per SRE avoided measures incremental effectiveness in only two dimensions (i.e., considering the total costs in the numerator and the number of SREs avoided in the denominator), and thus fails to consider both the timing of SREs and duration of their effect. Conversely, the incremental cost per QALY gained provides a better indication of therapeutic value by considering costs and benefits across at least two additional dimensions: time and severity. Specifically, QALYs integrate SRE-related effects on quality of life and duration of life and the number and timing of SREs in relation to both treatment initiation and time of death. Because of these differences, two treatments with the same cost per SRE avoided could be seen as either cost effective or cost ineffective from an incremental cost per QALY gained perspective.
The following example illustrates this point. Assume a US-based pharmacoeconomic comparison of hypothetical SRE-limiting Therapy A (which costs $20,000/year) versus B (which costs $10,000/year), within a time-horizon of 2 years, and with a given average SRE cost of $12,000. Over 2 years, patients treated with Therapy A experienced 0.15 fewer SREs and 0.10 more QALYs than patients treated with Therapy B. However, assume that Therapy A did not prevent SREs until the end of the time-horizon (i.e., the incremental benefit of Therapy A over B was not apparent until after nearly 2 years of treatment). In this scenario, the incremental costs per SRE avoided and QALY gained with Therapy A are $121,333 and $182,000, respectively. One would conclude that Therapy A is cost ineffective using both cost–effectiveness end points.
Now assume a similar scenario in which Therapy A prevented SREs earlier in the time-horizon (i.e., its incremental benefit over Therapy B was evidenced at the beginning of treatment) such that patients treated with Therapy A still experienced 0.15 fewer SREs, but 0.20 more QALYs (rather than 0.10 in the aforementioned scenario) than Therapy B. For the purpose of this example, it is assumed that the QALY increased from 0.10 to 0.20 due to an increase in remaining lifetime and due to the value of preventing SREs in patients who are not as close to death (i.e., model termination). In this scenario, the incremental cost per SRE avoided with Therapy A is unchanged ($121,333) but the cost per QALY decreases to $91,000. Here, one would conclude that Therapy A is cost effective from the perspective of incremental cost per QALY gained. Thus, despite identical results in terms of incremental cost per SRE avoided, one reaches different conclusions based on cost per QALY gained.
These examples illustrate that different conclusions regarding the economic value of a therapy can be reached when one considers the incremental cost per QALY gained versus the incremental cost per SRE avoided metric. It also shows that the direction of the disagreement may not be predictable and depends on many factors. Since the cost per QALY metric is a more sensitive and more meaningful measure, in addition to being a universal measure of cost–effectiveness that can be compared across therapies and indications, it should be the preferred outcome in health-economic evaluations of SRE-limiting agents.
The analyses reviewed herein were populated with clinical data drawn from either a 15-month Phase III trial of zoledronic acid versus placebo (or its 24-month extension study) Citation[11,12] or from the 27-month trial of denosumab versus zoledronic acid Citation[16]. That these models, which sought to determine the cost–effectiveness of two treatments routinely used in real-world practice, were populated with clinical trial data, which may not reflect real-world practice, is worth exploration. In both clinical trials, patients underwent skeletal surveys and radiographic assessments approximately every 12 weeks Citation[11,16]. Frequent clinical trial skeletal surveillance (especially with regard to asymptomatic vertebral fractures) may lead to overestimation of the number of SREs relative to what would be seen in a nonexperimental, real-world treatment setting and thus an overestimation of the effects of denosumab and zoledronic acid. In some of these analyses SREs that would not normally be brought to clinical attention, that require conservative therapy and are only identified by routine skeletal surveillance in clinical trials are accorded the same weight (via modeled economic and quality of life impacts) as those that require costly treatment and that have a marked impact on patients’ quality of life. Furthermore, the rates of asymptomatic skeletal events identified in clinical trials, with the exception of Saad et al. (2004) Citation[12] where overall rates of symptomatic SRE rates were reported but not stratified by type, have not been reported. Therefore, there is the risk of overestimation of SRE burden and thus an overestimation of the value of SRE-limiting therapies in the existing cost–effectiveness literature.
Such concerns have been voiced previously Citation[38]. In a research highlight of the Phase III denosumab trial reported by Fizazi et al. (2011), Sartor et al. postulated that possible over-identification of asymptomatic vertebral fractures in the Fizazi et al. and Saad et al. trials would overestimate the value of SRE-limiting agents that largely reduce asymptomatic events in patients with advanced disease Citation[38]. By extension, it is possible that because these trials may have overestimated the real-world incidence of SREs and because all the reviewed cost–effectiveness analyses were based on these trials, the estimated cost–effectiveness values of SRE-limiting agents may be optimistically low. Interestingly, the Saad et al. (2004) 24-month extension study of the original 15-month zoledronic acid trial reported the overall SRE rates in each treatment cohort exclusive of asymptomatic SREs, but no cost–effectiveness analysis reviewed herein incorporated this into the base-case or scenario analyses. However, three analyses in this review Citation[22,23,29] adjusted pathologic fracture cost for severity based on previous research, indicating that only a proportion of pathologic fractures are symptomatic Citation[39,40].
Conversely, it has been argued that clinical trials of SRE-limiting agents under-report the true rate of SREs due to having enrolled patients who are healthier (i.e., less progressed) than those treated in real-world practice Citation[37]. In a response to the cost–effectiveness analysis of denosumab versus zoledronic acid reported by Xie et al. (2011) Citation[26], Rader et al. (2011) Citation[37] indicated that it would be appropriate to upwardly adjust the SRE rates reported in the trial Citation[16] to reflect real-word incidence rate. In particular, Rader et al. Citation[37] suggested one use the results of a real-world retrospective analysis of SREs in patients treated with zoledronic acid reported by Hatoum et al. (2008) Citation[36]. In Hatoum et al., the SRE rate in zoledronic acid-treated patients adhering to treatment guidelines was 1.92 SREs/year Citation[36]. In the clinical trial of denosumab versus zoledronic acid in bone-metastatic prostate cancer, the rates for patients treated with denosumab or zoledronic acid were lower; 0.49 SREs/year and 0.60 SREs/year, respectively Citation[16]. However, Hatoum and colleagues reported that the zoledronic acid cohort included approximately 75% of patients who had already experienced ≥1 SRE before initiating therapy. In comparison, the proportion was 24% in the clinical trial Citation[16]. Therefore, while it is likely that SRE rates reported in clinical trials do not reflect precisely the rates in real-world practice, an upward adjustment of clinical trial-based SRE rates with data from Hatoum et al. would produce a modeled cohort that is almost entirely comprised of patients with advanced disease and at an increased risk of experiencing an SRE compared with patients from the clinical trial.
In light of this, Stopeck and colleagues Citation[25], chose to upwardly adjust the SRE rate in the zoledronic acid-treated cohort by 2.01 based on the analysis by Hatoum et al. and then extrapolate the total number of SREs in the denosumab-treated cohort using trial-based efficacy data. This assumption is potentially problematic for the very same reasons described above. In addition, Stopeck et al. provided no indication as to whether survival data and baseline quality of life data in their model (which were derived directly from the clinical trial wherein patients had far less advanced disease than in Hatoum et al.) were adjusted to correspond to a patient cohort with more advanced disease created by upwardly adjusting the SRE rate. Therefore, given that the Stopeck et al. analysis pertains primarily to patients who had already experienced ≥1 SRE at baseline, one might compare their results to the results of Amgen’s cost–effectiveness submission to NICE as reported by Ford et al. who also presented their own analysis in the same report (, NICE AG) Citation[23]. In its analysis, Amgen reported that over a lifetime model horizon, SRE-experienced patients with bone-metastatic prostate cancer treated with denosumab versus zoledronic acid experienced 0.14 fewer SREs (1.98 vs 2.12) and 0.006 more QALYs (1.089 vs 1.083). Conversely, in Stopeck et al., wherein an artificially constructed cohort composed primarily of SRE-experienced patients by virtue of having incorporated data from Hatoum et al., denosumab-treated patients experienced 0.81 fewer SREs and 0.14 more QALYs over a lifetime analysis. Thus, in comparing these analyses, one finds that denosumab’s treatment benefits in terms of SREs avoided and QALYs gained increased by 579% (i.e., 0.81/0.14 × 100) and 2333% (i.e., 0.14/0.006 × 100), respectively in the Stopeck et al. analysis. For the sake of argument, if one combines the incremental costs associated with denosumab in Stopeck et al. with the QALYs gained from Amgen’s analysis, the incremental cost per QALY gained would be $1,151,667 as opposed to the original value of $49,405. The higher of the two cost–effectiveness values closely approximates the results of Snedecor et al. Citation[29] who reported a cost per QALY of $1,248,051. Therefore, because of methodological differences it is likely that the incremental cost per QALY gained reported by Stopeck et al. was underestimated to a meaningful degree.
The analysis by Ford et al. (2011; i.e., the analysis conducted by NICE AG ) Citation[23] was the only one reviewed that analyzed patients with advanced disease (i.e., SRE-experienced) separately from those with less advanced disease (i.e., SRE-naive). Contrary to Rader et al.’s possible implication that denosumab may be more valuable in patients with more advanced disease (i.e., that one should upwardly adjust the total number of SREs based on an analysis where nearly 75% of patients were SRE-experienced), Ford et al. found that the incremental cost per QALY gained with denosumab versus zoledronic acid was approximately 260% higher in these patients compared with those with less advanced disease Citation[23]. The results of Ford et al. are particularly pertinent to understanding the trade-off between treating patients at lower risk of SREs (i.e., SRE-naive and less progressed) versus treating patients at a higher risk (i.e., SRE-experienced and more progressed). At the initially proposed price in the UK (‘no-PAS’ in ) and using hazard/risk ratios specific to SRE-experienced and SRE-naive subgroups, denosumab was cost ineffective in SRE-naive (£93,575/QALY) and SRE-experienced patients (£249,575/QALY). It was also cost ineffective across all patients (£113,237/QALY). The percent reductions in the incremental number of SREs and QALYs associated with denosumab from naive to experienced patients were 39 and 63%, respectively; and thus, the increase in cost per QALY from naive to experienced patients was probably due mainly to the reduction in QALYs gained.
Essentially, SRE-experienced patients may have reduced survival and more SREs (due to advanced disease) and therefore fewer life-years remaining and lower quality of life than SRE-naive patients. This amounts to a marked decrease in QALYs gained, even with a treatment that significantly reduces the number of SREs and increases the time to first SRE. Hence, future studies should seek to determine either the cost–effectiveness of SRE-limiting agents in SRE-experienced and SRE-naive subgroups independently, or calibrate the models based on the real-world proportion of SRE naive and experienced patients so as to have external validity for the entire indicated cohort (bone-metastatic prostate cancer patients with or without prior SRE). It should be noted again that the Ford et al. analysis showed that denosumab was cost effective (in fact dominant) versus zoledronic acid, when a PAS was used.
Although this review illustrates a number of important and useful characteristics of the cost–effectiveness landscape involving zoledronic acid and denosumab, it was limited by a restricted number of fully published, peer-reviewed studies. That is, many of the analyses reviewed were presented at congress as posters (n = 4) or abstracts only (n = 3), and thus, did not contain sufficient detail regarding inputs, methodology or outcomes with which to make more meaningful comparisons. Additionally, only three of the analyses presented a lifetime model horizon, while the rest did not extrapolate beyond the data available from 15–27 months of follow-up. It is likely that the proportion of patients who survive beyond the clinical trials (i.e., beyond 15–27 months) accrue more benefits from SRE-limiting therapies to some extent than are reflected in these analyses. However, survival in bone metastatic prostate cancer is often short; especially with the experience of ≥1 SRE(s). A recent retrospective (1999–2007) population-based cohort analysis of survival in Danish prostate cancer patients found that the proportions of bone-metastatic prostate cancer patients surviving 5 years were 3 versus <1%, respectively for patients with bone metastases only versus bone metastases with SRE(s) Citation[41]. Thus, while it is recommended that future analyses seek to establish the cost–effectiveness of these agents within an analytical horizon that reflects real-world survival, the difference between real-world survival and clinical trial time horizon is likely less pronounced in metastatic prostate cancer than other solid tumors, such as those of the breast.
Five-year view
Given the recent FDA and EMA approvals of denosumab for SRE prevention in patients with bone-metastatic prostate cancer and the anticipated introduction of generic forms of zoledronic acid in 2013, the cost–effectiveness of SRE-limiting agents will be driven almost exclusively by drug acquisition price. With its increased efficacy and ease of administration, denosumab will likely be the new standard of care against which SRE-limiting therapies (primarily zoledronic acid) are measured, clinically.
However, denosumab does not improve overall survival, and zoledronic acid will have a substantially discounted generic price and thus, the pharmacoeconomic value of denosumab may be difficult to justify on the basis of costs per QALY gained or SRE avoided unless denosumab is found to be cost effective in specific subgroups (e.g., those with high baseline N-telopeptide of type I collagen or are otherwise at increased risk of SREs). Therefore, it is likely that most pharmacoeconomic comparisons of SRE-limiting agents in the near future will have to assess the relative merits of denosumab versus zoledronic acid among patient subgroups. Models populated with clinical data derived from retrospective claims analyses, as opposed to modeled outcomes based on clinical trial reports, are also expected.
Table 1. Cost–effectiveness analyses of zoledronic acid versus no therapy or pamidronate in prostate cancer patients with bone metastases.
Table 2. Description of cost–effectiveness analyses of zoledronic acid and denosumab in prostate cancer patients with bone metastases.
Key issues
• Denosumab and zoledronic acid are clinically efficacious agents for the prevention or reduction of skeletal-related events (SREs) in patients with prostate cancer metastatic to the bone.
• Denosumab has been found to be cost effective in The Netherlands from the perspective of cost/quality-adjusted life year, but cost ineffective in the USA from the perspectives of cost/quality-adjusted life year and cost/SRE-avoided. In the UK, it was found to be cost ineffective unless used under a patient access scheme.
• Zoledronic acid has been found to be cost effective across Europe and in Canada, but cost ineffective in the USA from the perspective of cost per quality-adjusted life year gained.
• A synthesis of sensitivity analyses indicated that health–economic end points were most sensitive to drug acquisition costs and assumptions regarding patient survival and quality of life.
• Most of the reviewed analyses were conducted and reported by a narrow group of researchers who employed similar models with funding secured almost exclusively from the pharmaceutical companies that produce denosumab (Amgen) and zoledronic acid (Novartis).
• Additional cost–effectiveness analyses based on head-to-head comparisons of denosumab and zoledronic acid are encouraged. Models based on real-world data (as opposed to clinical trials) would improve the external validity and usefulness of these analyses.
• Particular attention should be paid to the effect of generic zoledronic acid pricing and to identify subgroups that may derive the greatest economic benefit from SRE-limiting therapy.
Financial & competing interests disclosure
JA Carter and MF Botteman received a consulting fee related to the development of this manuscript from Novartis Pharmaceuticals Corporation, which is the manufacturer of zoledronic acid. Novartis Pharmaceuticals Corporation was not involved in the planning or execution of this review. Pharmerit, the research and consulting group for which JA Carter is a research analyst and MF Botteman is managing partner, has received consulting fees from Amgen (maker of denosumab), but has received no consulting fees associated with denosumab-related projects or projects related to the subject matter herein per Expert Review guidelines regarding drug profiles, a Novartis employee checked the completed manuscript for scientific and medical accuracy. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomarkers Prev. 19(8), 1893–1907 (2010).
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 27(3), 165–176 (2001).
- Costa L, Lipton A, Coleman RE. Role of bisphosphonates for the management of skeletal complications and bone pain from skeletal metastases. Support Cancer Ther. 3(3), 143–153 (2006).
- Barlev A, Song X, Ivanov B, Setty V, Chung K. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J. Manag. Care Pharm. 16(9), 693–702 (2010).
- Sathiakumar N, Delzell E, Morrisey MA et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic. Dis. 14(2), 177–183 (2011).
- Bouganim N, Clemons MJ. Bone-targeted agents in the treatment of bone metastases: RANK outsider or new kid on the block? Future Oncol. 7(3), 381–383 (2011).
- Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur. Urol. 46(6), 731–739 (2004).
- Coleman RE, Seaman JJ. The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases. Semin. Oncol. 28(2 Suppl. 6), S11–S16 (2001).
- Coleman RE. Bisphosphonates: clinical experience. Oncologist 9(Suppl. 4), S14–S27 (2004).
- Lipton A. Treatment of bone metastases and bone pain with bisphosphonates. Support Cancer Ther. 4(2), 92–100 (2007).
- Saad F, Gleason DM, Murray R et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 94(19), 1458–1468 (2002).
- Saad F, Gleason DM, Murray R et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl Cancer Inst. 96(11), 879–882 (2004).
- Polascik TJ, Given RW, Metzger C et al. Open-label trial evaluating the safety and efficacy of zoledronic acid in preventing bone loss in patients with hormone-sensitive prostate cancer and bone metastases. Urology 66(5), 1054–1059 (2005).
- US Food and Drug Administration. Xgeva BLA 125320/7 Approval Letter. 2010
- European Medicines Agency. Xgeva. In: EPAR Summary for the Public. European Medicines Agency, London, UK (2012).
- Fizazi K, Carducci M, Smith M et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377(9768), 813–822 (2011).
- Aapro MS. Denosumab for bone metastases from breast cancer: a new therapy option? J. Clin. Oncol. 29(14), e419–e420 (2011).
- Aragon-Ching JB. Unravelling the role of denosumab in prostate cancer. Lancet 377(9768), 785–786 (2011).
- West H. Denosumab for prevention of skeletal-related events in patients with bone metastases from solid tumors: incremental benefit, debatable value. J. Clin. Oncol. 29(9), 1095–1098 (2011).
- Mottet N, Bellmunt J, Bolla M et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol. Esp. 35(10), 565–579 (2011).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™ Prostate Cancer, Version 1.2012. In: National Comprehensive Cancer Network; 2012. National Comprehensive Cancer Network, PA, USA, 1–68 (2012).
- Carter JA, Joshi A, Kaura S, Botteman MF. Cost–effectiveness of zoledronic acid in the management of skeletal metastases in hormone-refractory prostate cancer patients in France, Germany, Portugal, and The Netherlands. J. Med. Econ. 14(3), 288–298 (2011).
- Ford J, Cummins E, Sharma P et al. Systematic review of the clinical effectiveness and cost–effectiveness and economic evaluation of denosumab for the treatment of bone metastases from solid tumors. In: Aberdeen HTA Group, Institure of Applied Sciences. University of Aberdeen, UK (2011).
- Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA. Cost–effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J. Urol. 171(4), 1537–1542 (2004).
- Stopeck A, Rader M, Henry D et al. Cost–effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J. Med. Econ. doi:10.3111/13696998.2012.675380 (2012) (Epub ahead of print).
- Xie J, Namjoshi M, Wu EQ et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J. Manag. Care Pharm. 17(8), 621–643 (2011).
- Botteman M, Bargout V, El Ouagari K. Cost–effectiveness of zoledronic acid vs pamidronate in the management of hormone refractory prostate cancer (HRPC) patients with bone metastases [Abstract]. Presented at: American Society of Clinical Oncology Genitourinary Cancer Symposium. Atlanta, GA, USA, 2–6 June 2006.
- Botteman M, Meijboom M, Kaura S, Durand-Zaleski I. Cost–effectiveness of zoledronic acid for the prevention of skeletal-related events in prostate cancer patients with bone metastases in France [Abstract]. Presented at: American Society of Clinical Oncology Genitourinary Cancer Symposium. Orlando, FL, USA, 29 May–2 June 2009.
- Snedecor SJ, Carter JA, Kaura S, Botteman M. Cost–effectiveness of zoledronic acid (ZOL) versus denosumab (Dmab) in prevention of skeletal-related events (SREs) in castration-resistant prostate cancer metastatic to the bone (mCRPC). J. Clin. Oncol. (Suppl.), S29 (2011).
- Yu A, Namjoshi J, Xie K et al. Economic evaluation of denosumab compared with zoledronic acid in patients with hormone-refractory prostate cancer with bone metastases [Abstract]. Presented at: American Society of Clinical Oncology Genitourinary Cancer Symposium. Chicago, IL, USA, 3–7 June 2011.
- Carter J, Bains M, Chandiwana D, Kaura S, Botteman M. Cost–effectiveness of zoledronic acid versus pamidronate or no therapy for the treatment of bone metastases secondary to prostate cancer. Presented at: International Society for Pharmacoeconomics and Outcomes Research 14th Annual European Congress. Madrid, Spain, 5–8 November 2011.
- Lothgren M, Bracco A, Lucius B et al. Cost–effectiveness of denosumab vs zoledronic acid (ZA) for the prevention of skeletal-related events (SRE) in patients with bone metastases from solid tumors in The Netherlands [Abstract]. Presented at: International Society for Pharmacoeconomics and Outcomes Research 14th Annual European Congress. Madrid, Spain, 5–8 November 2011.
- El Ouagari K, Baladi J. Cost–effectiveness of treatment with zoledronic acid (Zometa®) in prostate cancer patients. Eur. J. Cancer 1(5), S256 (2003).
- Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J. Clin. Oncol. 21(23), 4277–4284 (2003).
- Weinfurt KP, Li Y, Castel LD et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 16(4), 579–584 (2005).
- Hatoum HT, Lin SJ, Smith MR, Barghout V, Lipton A. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 113(6), 1438–1445 (2008).
- Rader M, Goessl C, Cong Z. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J. Manag. Care Pharm. 18(1), 74–75 (2012).
- Sartor O. Denosumab in bone-metastatic prostate cancer: known effects on skeletal-related events but unknown effects on quality of life. Asian J. Androl. 13(4), 612–613 (2011).
- McKean H, Miller RC, Jatoi A. Non-traumatic vertebral fractures in patients with locally advanced esophageal cancer: a previously unreported, unrecognized problem. Dis. Esophagus 20(2), 102–106 (2007).
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pain and disability associated with new vertebral fractures and other spinal conditions. J. Clin. Epidemiol. 47(3), 231–239 (1994).
- Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal-related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J. Urol. 184(1), 162–167 (2010).
Websites
- International Society for Pharmacoeconomics and Outcomes Research. www.ispor.org
- American Society of Clinical Oncology. www.asco.org
- Centre for Reviews and Dissemination. www.york.ac.uk/inst/crd
