Abstract
To assess the comparative public health and budget impact over 5 years of several pneumococcal vaccination strategies (23-valent pneumococcal polysaccharide vaccine [PPV23] and/or 13-valent pneumococcal conjugate vaccine [PCV13]) in Germany, within the context of changing invasive pneumococcal disease (IPD) incidence over time. A multi-cohort, population-based Markov model was developed. Uncertainty around vaccine effectiveness, costs and IPD incidence change was handled through scenario analyses. Between 2012 and 2016, the introduction of PCV13 in adults, compared with the use of PPV23, would be associated with a net estimated budget increase of €59.7 million (+6.7%) to €151.6 million (+13.7%). Impact on IPD incidence ranged from -113 cases (-0.8%) to +298 cases (+2.8%). Introducing PCV13 in adults is expected to significantly affect healthcare budgets. Adult vaccination with PPV23 remains the optimal vaccination strategy from public health and budget perspectives.
PCV13: 13-valent pneumococcal conjugate vaccine; PPV23: 23-valent pneumococcal polysaccharide vaccine.
IPD: Invasive pneumococcal disease; PCV7: Seven-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine; PPV23: 23-valent pneumococcal polysaccharide vaccine.Data taken from Citation[13,31].
![Figure 3. Observed and modeled sero-epidemiological changes of the incidence of pneumococcal serotypes causing invasive pneumococcal disease over time from 2005 to 2021.IPD: Invasive pneumococcal disease; PCV7: Seven-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine; PPV23: 23-valent pneumococcal polysaccharide vaccine.Data taken from Citation[13,31].](/cms/asset/956d1a6b-80c0-4a20-926e-ea4f93a390cf/ierp_a_11215579_f0003_b.jpg)
Scenario A assumed comparable vaccine effectiveness between 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. Scenario D assumed very high vaccine effectiveness for 13-valent pneumococcal conjugate vaccine and very low vaccine effectiveness for 23-valent pneumococcal polysaccharide vaccine. Negative numbers indicate net decrease in the number of cases while positive numbers indicate net increase.IPD: Invasive pneumococcal disease.
Background & objectives
Pneumococcal diseases
Infection with Streptococcus pneumoniae may cause noninvasive and invasive pneumococcal diseases (meningitis, bacteremic pneumonia and bacteremia) Citation[1,2]. Globally, pneumococcal disease is estimated to cause 1.6 million deaths annually. The most vulnerable population consists of children, adults aged 60 years and over, individuals with chronic diseases such as chronic cardiovascular, pulmonary, renal and hepatic diseases and those who are immunocompromised Citation[1–4].
In Germany, the incidence of invasive pneumococcal disease (IPD) was estimated at 3.1 cases per 100,000 patient-years between 2001 and 2003, and at 4.6 cases per 100,000 patient-years in individuals aged 65 years or older Citation[5]. It is assumed that IPD alone accounts for approximately 1200 deaths annually in Germany Citation[6,101].
Vaccination
The only public-health strategy that reduces the burden of pneumococcal diseases is vaccination Citation[2] and pneumococcal vaccination policies or programs targeting at-risk groups and/or the elderly have been in place for many years in almost all European countries Citation[7,8].
Currently, there are two types of vaccines available in Germany. The 23-valent pneumococcal polysaccharide vaccine (PPV23) was introduced in 1982. It has been recommended for at-risk adults (including those with chronic cardiovascular disease, respiratory disease, diabetes, chronic renal disease or nephrotic syndrome, neurological diseases or cerebrospinal fluid fistula) since its authorization Citation[9] and in 1998 the recommendation was extended to all adults aged 60 years and older (hereafter referred to as the elderly) Citation[10]. The vaccine covers 80–90% of serotypes causing IPD in adults in Europe Citation[11–13]. In Germany, it is estimated that 82.3% of IPD cases and 86.5% of bacteremic pneumonia cases that occurred in adults between 2009 and 2010 related to serotypes covered by the vaccine Citation[14]. The efficacy and effectiveness of PPV23 against IPD have been established in immunocompetent adults Citation[4,15] and have also been demonstrated in some studies in immunosuppressed adults (e.g., patients infected with HIV) Citation[16,17]. The vaccination is well tolerated Citation[4,7,18,19]. Recent economic evaluations showed that vaccinating at-risk adults and the elderly with PPV23 was a cost-effective strategy when compared with no vaccination, even when accounting for the decline in incidence of IPD in adults induced by universal childhood vaccination against pneumococcal diseases Citation[7,20–29].
The second vaccine type is the pneumococcal conjugate vaccine (PCV). The seven-valent vaccine (PCV7) was authorized against pneumococcal diseases in children in Europe in 2001, and was replaced by the 13-valent vaccine (PCV13) in 2009 Citation[102,103]. Another 10-valent vaccine (PCV10) was introduced in 2009 for children aged between 6 weeks and 5 years Citation[104]. In Germany, vaccination with PCV has been recommended for children under the age of 24 months since 2006 Citation[30]. The serotypes included in PCV7 covered 57.7% of the serotypes that were causing IPD in children prior to the introduction of the vaccine (which occurred between 2001 and 2002), while the additional six serotypes in PCV13 covered an additional 24.9% of serotypes Citation[31]. After the introduction of PCV vaccines, the serotypes included in PCV7 covered only 16.5% of IPD-causing serotypes in children, while the additional serotypes in PCV13 covered an additional 54.7% (both estimated for 2009/2010) Citation[31]. The efficacy and safety of PCV7 have been confirmed in clinical trials in children Citation[32–36]. For PCV10 and PCV13, evidence is only available in terms of immunogenicity and tolerability in children Citation[36,105]. The efficacy of PCV has not yet been demonstrated in adults.
In November 2011, based on immunological data, the European indication of PCV13 was extended to adults aged 50 years and older Citation[106]. A Phase IV, randomized, placebo-controlled trial of PCV13 designed to assess efficacy in terms of prevention of IPD and community-acquired pneumonia (CAP) in adults aged 65 years and older is currently ongoing. The efficacy may not be as good as in children owing to pre-existing immunity, lower carriage, immunosenescence and underlying conditions in the older population Citation[37].
With the extension of the indication of PCV13 to include preventing IPD in adults aged 50 years and older, and the change in IPD incidence induced by the vaccination of children, it is critical for decision-makers to understand the respective value of both PPV23 and PCV13 in the current context of limited economic resources. By understanding the implications of different vaccination strategies on costs and health outcomes, an informed decision could then be made regarding the optimal pneumococcal vaccination strategy.
The impact of PCV vaccination of children on IPD epidemiology
Universal PCV7 vaccination in children led to a dramatic change in the epidemiology of IPD. A decrease has been observed in the number of IPD cases associated with vaccine-type pneumococcal serotypes in vaccinated children in Europe and the USA Citation[38,107]. Moreover, unvaccinated adults also benefited from this vaccination through herd protection, as witnessed in the USA and in Spain Citation[7,39,40]. Since then, the burden of IPD has mainly been borne by the elderly and at-risk adults Citation[38].
Following a reduction in the incidence of IPD associated with serotypes covered by PCV7, an increase in the incidence of IPD associated with serotypes not covered by the conjugate vaccines has been reported Citation[41–44]. It is argued that this serotype replacement could offset part of the benefits from herd protection Citation[45]. With the recent introduction of PCV13 vaccination in children, the question of a potential additional effect on serotype replacement can be raised.
Objectives
This study aimed to assess the public health and budget impact of different adult vaccination strategies using both PPV23 and PCV13 or PCV13 alone, compared with the current vaccination strategy (i.e., PPV23 alone) in the German setting.
Modeling approach
Target population & model structure
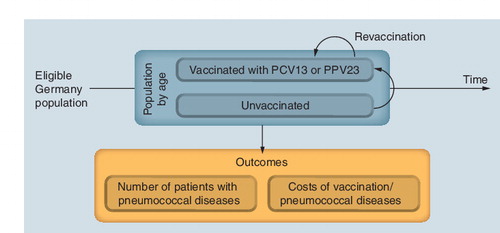
As the analysis aimed to quantify the impact of vaccination at the national level, a population-based, multi-cohort, state-transition Markov model was built following recent recommendations on the approach to assessing vaccines Citation[46]. The model was developed in compliance with the guidelines published by the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG; Cologne, Germany) Citation[108] and the principles of good practice for modeling from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR; NJ, USA) Citation[47].
The model tracked the German population aged 18 years and older who are eligible for vaccination against pneumococcal diseases. Following the most recent German guidelines on economic evaluation Citation[48], two subgroups were considered in the model: individuals younger than 60 years who are at increased risk of developing pneumococcal diseases (‘at-risk adults’); and individuals aged 60 years or older (‘the elderly’). In the ‘at-risk adults’ population, the model distinguished between the immunocompetent (defined as those with chronic respiratory or cardiovascular conditions and/or diabetes), and the immunosuppressed (defined as those infected with HIV or those who had received immunosuppressants such as chemotherapy or high-dose corticosteroids, or who had received a transplantation).
For each calendar year of the study period, the incident cohort (i.e., new patients eligible for vaccination, regardless of whether they received vaccination or not) was identified. Each incident cohort was then run through the model simulating the progression of disease assuming no vaccination, vaccination with PPV23 and vaccination with PCV13 over a period of 5 years unless they reached 100 years of age, in which case they left the model. The corresponding events (IPD, nonbacteremic pneumococcal pneumonia [NBPP], death) and health state of each incident cohort were reported by calendar year. The different strategies (use of PPV23 and/or PCV13) were assessed by considering the mixed population of individuals not vaccinated and vaccinated with each vaccine .
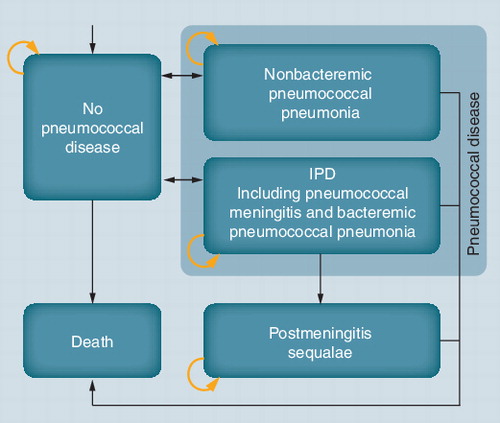
The progression of disease was modeled through a Markov structure, which consisted of an annual cycle length and five health states: no pneumococcal disease, NBPP, IPD (including pneumococcal meningitis and bacteremic pneumococcal pneumonia), post-meningitis sequelae and death (see ). Individuals entered the model in the no pneumococcal disease state and faced a risk of developing IPD or NBPP. Patients with IPD or NBPP had a higher mortality risk than individuals without pneumococcal disease. Following recovery from IPD and NBPP, individuals could return to the no pneumococcal disease state, and the fraction of patients who had developed pneumococcal meningitis could develop post-meningitis sequelae (auditory or neurologic). It was assumed that patients with post-meningitis sequelae could not develop a second episode of IPD or NBPP, as this was regarded as rare (less than one in a million) Citation[5].
The model started in 2012 and results were reported for the period between 2012 and 2016. In order to validate the model using historical data, it was decided to start running the model from 2005. Model predictions for the years 2005–2009 were then compared with observed data.
Most of the assumptions applied to the model parameters were described in a previous paper on a cost–effectiveness analysis of PPV23 [29].
Public health & budget impact analysis: definition
The public-health outcomes of the model included the number of IPD, NBPP and post-meningitis sequelae cases in the target population over time. Budget impact outcomes consisted of total costs for each strategy per year, as well as costs by category (vaccine, management of IPD, NBPP and post-meningitis sequelae). The net budget impact was defined as the difference between the assessed strategy (i.e., PPV23 and PCV13 or PCV13 alone) and the current strategy (i.e., vaccination with PPV23).
Comparative analyses of vaccination strategies for the 2012–2016 period
The comparator used was the current recommended strategy with PPV23, that is, at-risk adults either immunocompetent or immunosuppressed and the elderly aged 60 years and over. Despite the indication of PCV13 being for those aged 50 years and older only, it was assumed that at-risk individuals aged between 18 and 60 years and the elderly aged 60 years and older would receive PCV13, as at the time of the analysis the exact indication was not available. It also ensured that evaluation of the different strategies related to the same target population.
Vaccination recommendations are likely to be reviewed, owing to the launch of PCV13 for the prevention of IPD in adults. Given the context of the analysis, two different strategies were assessed for the comparative analysis:
• A preferential recommendation (full switch) where PCV13 would be the only vaccine available for at-risk adults and the elderly (all adults receive PCV13);
• A nonpreferential recommendation where it was assumed that half of the individuals undertaking vaccination would receive PCV13 and half would receive PPV23.
Vaccine effectiveness
Four sets of vaccine effectiveness parameters were assumed for each scenario analysis (see Tables 1 & 2). In scenario A, vaccine effectiveness of PPV23 against IPD (in all patient subgroups) and against NBPP (in at-risk immunocompetent adults and the elderly) came from clinical trials Citation[15,16,49]. No protection against NBPP was assumed for immunosuppressed subjects. As no information was available on the effectiveness of PCV13 in adults, it was assumed to be the same as PPV23 for serotypes included in the vaccine (referred to as vaccine serotype hereafter). In the other scenarios, the vaccine effectiveness of PCV13 was assumed to be higher than PPV23 for the vaccine serotypes. Scenario D was developed to account for the maximum incremental benefits that PCV13 could add and considered the minimum values described in the literature for PPV23 and the maximum estimates reported for PCV13 (on vaccine serotypes). Owing to a scarcity of data, it was assumed that the vaccines would reduce the incidence of IPD and NBPP but would not have an effect on the severity of disease or on the risk of post-meningitis sequelae.
The vaccines’ effectiveness was assumed to wane, reaching no protection after 8 years and the model applied the waning function based on previously published population-based studies Citation[50,51].
The model considered the possibility for revaccination after 5 years in at-risk adults Citation[48]. Revaccination was assumed to provide the same protection as the initial vaccination Citation[18,52,53]. Three percent of vaccinated at-risk adults were expected to receive revaccination with the same vaccine, in both populations who had previously received PPV23 or PCV13 Citation[31].
Healthcare resources used, unit costs & prices
Costs (in euros) were estimated from the perspective of the German sickness fund (i.e., third-party payer) for the year 2010. No discounting was applied since the aim of the study was to understand the actual impact of the introduction of PCV13 on the sickness fund’s budget Citation[47]. To account for uncertainty around cost of illnesses, scenario analyses explored the impact of increasing or decreasing by 20% the costs of managing all diseases considered (IPD, NBPP and post-meningitis sequelae).
Unit prices of the vaccines took into account the mandatory rebate on ex-factory prices to the third-party payer by manufacturers (16% of the ex-factory price) and by pharmacies (€2.05 per pack). The price of PPV23 (Pneumovax® II, single-dose pack; Sanofi Pasteur MSD) was €30.25 and the current unit price of PCV13 (Prevenar 13®, single-dose pack; Pfizer, Inc.) was assumed to remain stable following the launch of the vaccine in adults (€65.02) Citation[109]. Costs of administration and treatment of pneumococcal disease were retrieved from German sources Citation[110,111] or updated from previous German studies Citation[54,55] and are detailed in .
Invasive pneumococcal disease
The model took into account the epidemiological changes in adults induced by the introduction of universal mass vaccination of children and the model started in 2005. IPD incidence data among adults were obtained from a prospective surveillance study conducted in North-Rhine Westphalia, Germany between 2001 and 2003 Citation[5]. A stable incidence of IPD was assumed between 2003 and 2005 owing to a lack of data. The incidence of IPD from 2005 onwards was estimated through separate calculation as described below.
Although two studies have been identified that document the incidence of IPD by serotype in Germany Citation[13,31], no local time series were available to model the trends over time. Therefore, the trend in IPD incidence (i.e., change in IPD incidence among adults due to pediatric vaccination) was estimated from data observed in the USA, where the incidence of IPD associated with PCV7 serotypes decreased Citation[36] as a function of cumulative vaccine coverage in children, and the incidence of IPD associated with the serotypes included in PPV23 and not in PCV7 (hereafter referred to as ‘PPV23 not PCV7’) increased in adults Citation[39,112]. A cumulative gamma distribution was fitted between the cumulative vaccine uptake in children and the epidemiological changes (by group of serotypes, i.e., PCV7 serotypes and ‘PPV23 not PCV7’ serotypes) in adults because of its goodness of fit Citation[31]. Then, the change in IPD incidence was estimated for Germany based on local PCV7 uptake data observed in children.
As no data are available on the changes in IPD incidence induced by the six additional serotypes included in PCV13 (‘PCV13 not PCV7’), some additional analyses were run . When an additional analysis accounted for a change induced, it was assumed that the trend of IPD incidence associated with the six additional serotypes included in PCV13 (‘PCV13 not PCV7’) would follow the same rate of change as the PCV7 serotypes.
In scenarios A and C, a decrease in the incidence of IPD associated with the PCV7 serotypes was assumed Citation[36,39,41] and the same trend was applied to the additional six serotypes covered by PCV13. For the serotypes not included in PCV13, an increase in the incidence of IPD associated with these serotypes was assumed. In scenarios B and D, only the incidence associated with the serotypes included in PCV7 was considered .
As US data were only available for a period of 7 years following introduction of the vaccine in children, it was assumed that the incidence of IPD would be stable afterwards (i.e., beyond 2012, 7 years after the recommendation to vaccinate children with PCV7 in Germany).
In adults, the incidence of IPD was estimated by risk group in order to reflect the higher incidence in those at-risk when compared with all individuals Citation[56,57]. The distribution of IPD by serotype was based on German surveillance data Citation[13].
The case-fatality rate from IPD by serotype group (i.e., PCV7, ‘PCV13 not PCV7’, ‘PPV23 not PCV13’, others not included in any vaccine) was estimated based on Dutch and Danish studies, owing to the lack of local data Citation[58,59].
IPD clinical parameters are summarized in .
Demography & vaccine uptake
German population sizes by age and mortality rates were obtained from Statistisches Bundesamt Deutschland (DESTATIS) Citation[113] and the Human Mortality Database Citation[114]. The proportion of at-risk adults was calculated based on data estimates for the influenza vaccination (at-risk immunocompetent [with chronic cardiovascular or respiratory disease or diabetes]: 12.8%; at-risk immunosuppressed [with HIV/AIDS or transplantation]: 0.2%) Citation[57].
The number of individuals vaccinated up to 2010 was estimated from internal sales data for PPV23 Citation[31] in the absence of publicly available German data on vaccination coverage rates. Based on vaccine uptake trends, it was assumed that 3% of the target population would receive the vaccination each year Citation[31].
No vaccine uptake increase was assumed after the introduction of PCV13 in 2012.
Results
Model validation
The modeled epidemiological changes of IPD incidence among adults due to pediatric vaccination were compared with the observed sero-epidemiological changes in Germany Citation[13,31]. The model fitted the total IPD incidence well but slightly underestimated the contribution of PCV13 serotypes to the incidence of IPD between 2006 and 2008. The drop in incidence between 2009 and 2010 was attributed to the observed vaccine uptake of PCV13 in children.
Public health impact
Vaccinated and unvaccinated German adults who were eligible to receive vaccination against pneumococcal diseases were followed; approximately 20.9% of them were at-risk adults and 79.1% were elderly (aged 60 years and older).
It was estimated that between 2012 and 2016, a total of 4.3 million individuals would receive the initial vaccination, and 208,467 individuals would undertake revaccination. In three out of four scenarios, the current PPV23 recommendation provided the greatest public health benefit .
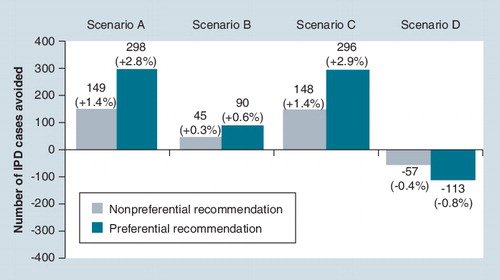
As shown in and Supplementary Table 1, when comparing the strategy with PCV13 to the strategy without PCV13, under a nonpreferential recommendation, scenarios A, B and C predicted an increase in the total number of IPD cases between 2012 and 2016, from 45 (+0.3%) to 149 cases (+1.4%). In scenario D, where a higher effectiveness was assumed for PCV13, the total number of IPD cases was predicted to be reduced by 57 cases (-0.4%). Under a preferential recommendation for PCV13, scenarios A, B and C predicted an increase in the total number of IPD cases by 90 (0.6%) to 298 cases (+2.8%) between 2012 and 2016, whereas the total number of IPD cases was predicted to decrease by 113 cases (-0.8%) in scenario D. In scenarios A, B and C, the preferential recommendations resulted in twice as high an increase in the number of IPD cases compared with the nonpreferential recommendations. In scenario D, the decrease in the number of IPD cases was twice as large with the preferential recommendation compared with the nonpreferential ones, due to the assumption of a better effectiveness profile for PCV13.
The vaccines were assumed to confer the same protection against NBPP in scenarios A, B and C, therefore the difference between vaccination strategies were minimal under these scenarios. Although PCV13 is only indicated against IPD in adults, scenario D assumed a higher effectiveness for PCV13 against NBPP. Introducing PCV13 in adults was then associated with a decrease in the number of NBPP cases ranging from 9168 (-2.3%) to 18,335 cases (-4.5%), under the assumptions of preferential and nonpreferential recommendations, respectively.
The total number of post-meningitis sequelae cases after IPD had little impact on the results. The change ranged between -0.1 and 0.2% across scenarios.
Budget impact
PCV13 implementation resulted in a net increase in healthcare budget expenditure across all scenarios (see & Supplementary Table 2) Under the assumption of a nonpreferential recommendation, vaccinating German at-risk adults and the elderly aged 60 years and older with PCV13 and PPV23 was associated with total costs of €957.3 to €1180.0 million over the 2012–2016 period. The vaccination costs (including administration) accounted for approximately 23% of the total cost. The remaining burden of disease accounted for 77% of the total costs.
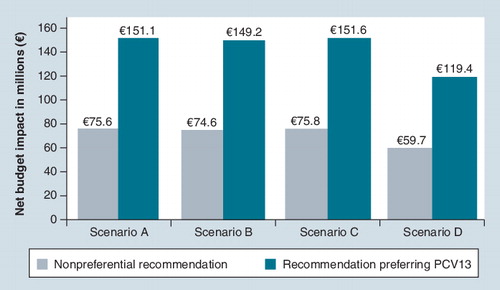
Compared with the current vaccination strategy (with PPV23 only), the net incremental budget impact results ranged from +€59.7 millions (scenario D) to +€75.8 millions (scenario C).
Under the assumption of a preferential recommendation for PCV13, vaccinating at-risk adult patients and the elderly was associated with total costs of €1017.0 to €1255.8 million over the study period. The costs of vaccine and administration contributed to approximately 29% of the incremental costs. The remaining burden of disease accounted for 71% of the total costs.
Compared with the use of PPV23 only, PCV13 was expected to add a net incremental budget impact ranging from +€119.4 million (scenario D) to +€151.6 million (scenario C) over the study period. The estimated incremental budget impact associated with PCV13 introduction would be comparable with the total health expenditure associated with HIV (ICD-10: B20-B24), €144 million, or 1.5–2-times of that associated with influenza (ICD-10: J10-J11), €71, both estimated in 2008 in Germany Citation[115].
Discussion
The model estimated the public health and budget impact of introducing PCV13 vaccination in German adults in 2012 compared with the use of PPV23, the current vaccination program. Two alternative situations were assumed to explore the impact of a change in the recommendation for adult immunization against pneumococcal diseases, that is, either preferential (use of PCV13 only) or nonpreferential for a specific vaccine (i.e., use of PCV13 with PPV23). While the preferential recommendation was developed to account for the maximum impact both in terms of public health and budget impact, the nonpreferential recommendation was simulated to capture a more realistic picture of what could occur. These hypotheses are arbitrary and disconnected from any policy advice. To allow comparisons, the PCV13 indication was assumed to be identical to the PPV23 indication (not known at the time of the analysis).
To account for uncertainties regarding vaccine effectiveness, costs of disease and changes in IPD incidence induced by pediatric vaccination, scenario analyses were developed. Vaccine effectiveness was assumed to be either low or high, and in most scenarios higher for PCV13. The analysis assumed that the current price of PCV13 (for the vaccination of children) would be maintained.
While three out of four scenarios were in favor of the PPV23 vaccination strategy in terms of public health, scenario analyses were always in favor of PPV23 compared with PCV13 when focusing on the budget impact. Results were driven by the difference between vaccine costs and an uncertain impact in terms of IPD cases avoided with the introduction of PCV13, due to a change in IPD incidence among adults over time and a broader serotype coverage of PPV23.
In most scenarios, the introduction of PCV13 was associated with an increase in the number of IPD cases, due to the lower serotype coverage of the vaccine compared with PPV23. The number of NBPP cases was stable. When PCV13 was assumed to have higher effectiveness against IPD and NBPP (scenario D), the number of IPD and NBPP cases decreased slightly with the introduction of PCV13. The number of post-meningitis sequelae cases remained unchanged. It must be noted that the efficacy of PCV13 against pneumococcal diseases has not been confirmed and it does not have an indication against NBPP.
Even with the most favorable clinical assumptions versus PPV23 (scenario D), PCV13 was associated with a marked increase in the healthcare budget (+6.7 to +13.3%), because not enough cases of pneumococcal disease were avoided to offset the costs associated with the vaccine, when compared with vaccinating with PPV23 alone. Using PCV13 alone was associated with a net budget impact of +€119.4 million, and assuming that half of the vaccinated population would receive PCV13 resulted in a net budget impact of +€59.7 million. In other scenarios less favorable to PCV13, the net budget impact ranged from +€74.6 million (+8.3%; scenario B, nonpreferential recommendation) to +€151.6 million (+13.7%; scenario C, recommendation preferring PCV13).
Strengths
The model has several strengths. First, it takes into account the changing epidemiology of pneumococcal diseases. As observed in the USA and some European countries, the introduction of routine childhood PCV vaccination affected the incidence of IPD and serotypes distribution in adults Citation[7,39,40]. It is therefore important to understand the effect of introducing PCV13 in adults in this environment. Moreover, to account for uncertainty around the impact of PCV vaccination of children on IPD epidemiology, scenario analyses ranging from no impact to a maximum impact on all serotypes included in PCV13 were run.
Secondly, the model was based on local burden of disease and epidemiological data. The vaccine uptake of PCV7/13 in children and PPV23 in adults was based on German sales data Citation[31]. The modeled results of sero-epidemiological changes were also comparable with the national data observed in Germany Citation[13,31].
Owing to the lack of data on the effectiveness of PCV13 against IPD and NBPP, different scenarios were run in order to understand the implication of different vaccine profiles, from pessimistic to optimistic. For PPV23, all scenarios were based on published literature reporting results from efficacy and effectiveness studies. This is the first study that compared PCV13 and PPV23 in a health economic framework with several sets of assumptions.
Limitations
There are several weaknesses to the current study. First of all, in spite of employing the German incidence of IPD for the period before mass PCV vaccination of children, the epidemiological trends were based on data from the USA, another industrialized country. This approach has been validated by comparing the observed trend and that predicted by the model , although differences exist in the exact percentage changes. The analysis will need to be updated when more data become available.
Secondly, owing to a lack of data on the level of potential herd immunity associated with adult vaccination with PCV13 and PPV23, a static model was developed. Further data on the indirect immunity conferred by PCV vaccines in adults would be required to model disease transmission under the different vaccination strategies.
The incidence of NBPP has been identified as an important driver of the budget. Further data would be required to obtain a reliable estimate of the incidence of NBPP.
Comparison with previous studies
The only study reporting the public health and budget impact associated with the introduction of PCV13 in adults that is publicly available is a presentation given at the Advisory Committee on Immunization Practices of the US Centers for Disease Control and Prevention Citation[116]. A model similar to the current study was developed to compare a cohort of US individuals aged 50 years and older receiving PCV13 or PPV23 vaccination. Only one scenario was considered: vaccine effectiveness was assumed to be similar to that in scenario D in the current study. The analysis concluded that the introduction of PCV13 would reduce IPD, inpatient pneumonia and outpatient pneumonia by 19,000, 840,000 and 650,000 cases, respectively, over a lifetime. Moreover, PCV13 vaccination was also expected to save $6.4 billion from the third-party payer’s perspective. As noted in the study presented at ACIP, the results were very sensitive to assumptions related to NBPP, which is in line with this study. Owing to the lack of information on assumptions made for the US study, differences between the two analyses cannot be identified. However, based on available information, one important difference lies in the assumptions on resource utilization associated with NBPP. The German source applied in the current study Citation[54] reported a relatively low rate, whereas the rate used in the study presented at ACIP appeared to be much higher, as seen in the number of inpatient/outpatient CAP cases avoided and the corresponding assumptions on vaccine effectiveness. Data sources were not detailed. Furthermore, since the US study did not explore other possible scenarios with regard to vaccine effectiveness against IPD and NBPP, the results are difficult to interpret, especially given that evidence on the effectiveness of PCV13 in adults is largely missing and that PCV13 is not currently indicated for NBPP in Europe.
Conclusion
Within the context of the changing epidemiology of IPD among adults and the elderly, due to pediatric vaccination, the present analysis showed that, in at-risk adults and the elderly, PPV23 remains the optimal vaccination strategy from public health and economic perspectives. The introduction of PCV13 is expected to impose a significant impact on the German healthcare budget when compared with the current vaccination strategy with PPV23 only. Further research is needed to understand changes in the epidemiology of IPD following routine childhood vaccination.
Expert commentary
The present study shows that if PCV13 does not demonstrate a significant clinical benefit versus PPV23, adult vaccination with PPV23 remains the optimal vaccination strategy from public health and economic perspectives. The introduction of PCV13 in adults is likely to impose a significant impact on the German healthcare budget, in an environment where the epidemiology of IPD is changing. When focusing on preventing severe forms of pneumococcal disease (i.e., IPD), the analysis concluded that PCV13 would bring no, or very limited, additional benefits.
As seen from the cost–effectiveness analysis mentioned earlier, which compared PPV vaccination with no vaccination [29], the results were largely driven by assumptions about NBPP, for which more real-life data are required in order to refine the model estimates. Indeed, data should be collected to understand whether the cost difference associated with a decreased number of NBPP cases can leverage the additional costs. Additional epidemiological studies are required to understand the indirect effects of vaccinating children and adults in Germany. The model should be updated once further data related to the effectiveness of PCV13 become available.
Although a clinical trial is currently ongoing (the CAPITA trial), it will not provide effectiveness data versus PPV23. Future studies should be planned to understand the relative value of PCV13 compared with PPV23.
Five-year view
More data need to be collected on the effectiveness, efficacy and safety of PCV13 in adults, as well as on herd protection induced by the six additional serotypes included in PCV13 from the universal vaccination of children. Even if higher levels of effectiveness were reported for PCV13, it is expected that PPV23 will still be a good option owing to broad serotype coverage, its good cost-effectiveness profile and the changing IPD epidemiology.
The question of a life-course vaccination with a single vaccine that induces herd protection from children to adults including the elderly, and the resulting epidemiological changes, will need to be raised.
Future effectiveness studies should be planned to understand the relative value of PCV13 compared with PPV23.
Table 1. Scenario analysis (accounting for the uncertainties of parameters).
Table 2. Model parameters: vaccine effectiveness.
Table 3. Model parameters: costs.
Table 4. Model parameters: clinical parameters.
Key issues
• In Germany, the incidence of invasive pneumococcal disease (IPD) was estimated at 4.6 cases per 100,000 patient-years in the elderly between 2001 and 2003.
• In the USA, routine childhood pneumococcal conjugate vaccines (PCV) led to a change in the epidemiology of IPD in vaccinated children, as well as in unvaccinated adults.
• The model showed that owing to serotype replacement, 13-valent pneumococcal conjugate vaccine introduction is likely to be associated with an increase in the number of IPD and post-meningitis sequelae cases compared with the current strategy where only 23-valent pneumococcal polysaccharide vaccine (PPV23) is used, and it is expected to impose a significant impact on the German healthcare budget.
• Over a 5-year period, the model predicted that adult vaccination with PPV23 remains the optimal vaccination strategy from public health and economic perspectives, owing to its broader serotype coverage.
• To enhance the analysis, studies should be conducted to understand the comparative effectiveness of 13-valent pneumococcal conjugate vaccine and PPV23.
Acknowledgements
The authors thank F Baron-Papillon (Sanofi Pasteur MSD) for her assistance in reviewing the manuscript.
Disclaimer
The authors take sole responsibility for the content of this article.
Financial & competing interests disclosure
This study was conducted by Amaris and funded by Sanofi Pasteur MSD. Y Jiang and A Gauthier are employees of Amaris. L Annemans received a consultancy fee from Sanofi Pasteur MSD for his role as an advisor in the design of the model. M van der Linden has received research grants from GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, is a member of advisory boards for GlaxoSmithKline, and has received speakers’ honoraria from GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD. L Nicolas-Spony and X Bresse are employees of Sanofi Pasteur MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Lynch JP 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin. Respir. Crit. Care Med. 30(2), 189–209 (2009).
- Prato R, Tafuri S, Fortunato F, Martinelli D. Why it is still important that countries know the burden of pneumococcal disease. Hum. Vaccin. 6(11), 918–921 (2010).
- Cartwright K. Pneumococcal disease in Western Europe: burden of disease, antibiotic resistance and management. Eur. J. Pediatr. 161(4), 188–195 (2002).
- No authors listed. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly. Epidemiol. Rec. 83(42), 373–384 (2008).
- Reinert RR, Haupts S, van der Linden M et al. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clin. Microbiol. Infect. 11(12), 985–991 (2005).
- Hülsse C, Littmann M, Fiedler K, Kaltofen U, Hundt C. Epidemiologic and serologic studies of pneumococcal infections with reference to the new STIKO recommendations. Gesundheitswesen 61(8-9), 393–397 (1999).
- Fedson DS, Nicolas-Spony L, Klemets P et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev. Vaccines 10(8), 1143–1167 (2011).
- Pebody RG, Leino T, Nohynek H, Hellenbrand W, Salmaso S, Ruutu P. Pneumococcal vaccination policy in Europe. Euro Surveill. 10(9), 174–178 (2005).
- No authors listed. Bekanntmachungen des Bundesgesundheitsamtes. Bundesgesundhbl. 25(5), 170–171 (1982).
- No authors listed. Impfempfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut/Stand: März 1998. Epid. Bull. 15, 101–114 (1998).
- Varon E. Epidemiology of acute bacterial meningitis in adult patients in France. Med. Mal. Infect. 39(7-8), 432–444 (2009).
- Trotter CL, Waight P, Andrews NJ et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: England and Wales, 1996–2006. J. Infect. 60(3), 200–208 (2010).
- Imöhl M, Reinert RR, van der Linden M. Temporal variations among invasive pneumococcal disease serotypes in children and adults in Germany (1992–2008). Int. J. Microbiol. 2010, 874189 (2010).
- van der Linden M, Imohl M. R2762 serotype distribution among bacteraemic pneumococcal pneumonia in adults in Germany. Clin. Microbiol. Infect. 17(S4), S832 (2011).
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 1, CD000422 (2008).
- Rodriguez-Barradas MC, Goulet J, Brown S et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin. Infect. Dis. 46(7), 1093–1100 (2008).
- Peñaranda M, Falco V, Payeras A et al. Effectiveness of polysaccharide pneumococcal vaccine in HIV-infected patients: a case–control study. Clin. Infect. Dis. 45(7), e82–e87 (2007).
- Musher DM, Manof SB, Liss C et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J. Infect. Dis. 201(4), 516–524 (2010).
- Hammitt LL, Bulkow LR, Singleton RJ et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55–74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine 29(12), 2287–2295 (2011).
- Smith KJ, Zimmerman RK, Lin CJ et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost–effectiveness analysis. Vaccine 26(11), 1420–1431 (2008).
- Evers SM, Ament AJ, Colombo GL et al. Cost–effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur. J. Clin. Microbiol. Infect. Dis. 26(8), 531–540 (2007).
- Ament A, Baltussen R, Duru G et al. Cost–effectiveness of pneumococcal vaccination of older people: a study in 5 western European countries. Clin. Infect. Dis. 31(2), 444–450 (2000).
- Smith KJ, Lee BY, Nowalk MP, Raymund M, Zimmerman RK. Cost–effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine 28(48), 7620–7625 (2010).
- Kawakami K, Ohkusa Y, Kuroki R et al. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine 28(43), 7063–7069 (2010).
- Ogilvie I, Khoury AE, Cui Y, Dasbach E, Grabenstein JD, Goetghebeur M. Cost–effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine 27(36), 4891–4904 (2009).
- Akin L, Kaya M, Altinel S, Durand L. Cost of pneumococcal infections and cost–effectiveness analysis of pneumococcal vaccination at risk adults and elderly in Turkey. Hum. Vaccin. 7(4), 441–450 (2011).
- Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost–effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann. Intern. Med. 138(12), 960–968 (2003).
- Ament A, Fedson DS, Christie P. Pneumococcal vaccination and pneumonia: even a low level of clinical effectiveness is highly cost–effective. Clin. Infect. Dis. 33(12), 2078–2079 (2001).
- Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. Cost–effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Rev. Pharmacoecon. Outcomes Res. doi:10.1586/ERP.12.54 (2012) (Epub ahead of print).
- No authors listed. Mitteilung der Ständigen Impfkommission am Robert Koch-Institut: Neuerungen in den aktuellen Empfehlungen der Ständigen Impfkommission (STIKO) am RKI vom Juli 2006. Epid. Bull. 32, 271–276 (2006).
- Sanofi Pasteur MSD data on file (2011).
- No authors listed. Pneumococcal conjugate vaccine for childhood immunization – WHO position paper. Wkly. Epidemiol. Rec. 82(12) 93–104 (2007).
- No authors listed. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children – Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 59(9), 258–261 (2010).
- Weinberger DM, Dagan R, Givon-Lavi N, Regev-Yochay G, Malley R, Lipsitch M. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J. Infect. Dis. 197(11), 1511–1518 (2008).
- Black S, Shinefield H, Fireman B et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19(3), 187–195 (2000).
- Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children – use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recomm. Rep. 59(RR-11), 1–18 (2010).
- O’Brien KL. Pneumococcal conjugate vaccine, polysaccharide vaccine, or both for adults? We’re not there yet. Clin. Infect. Dis. 49(9), 1326–1328 (2009).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report on Communicable Diseases in Europe 2010. ECDC, Stockholm, Sweden (2010).
- Pilishvili T, Lexau C, Farley MM et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201(1), 32–41 (2010).
- Ardanuy C, Domenech A, Rolo D et al. Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993–2008). J. Antimicrob. Chemother. 65(4), 634–643 (2010).
- No authors listed. Changing epidemiology of pneumococcal serotypes after introduction of conjugate vaccine: July 2010 report. Wkly. Epidemiol. Rec. 85(43), 434–436 (2010).
- Hanage WP, Finkelstein JA, Huang SS et al. Evidence that pneumococcal serotype replacement in Massachusetts following conjugate vaccination is now complete. Epidemics 2(2), 80–84 (2010).
- Rose M, Zielen S. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev. Vaccines 8(10), 1351–1364 (2009).
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect. Dis. 11(10), 760–768 (2011).
- Hanage WP. Serotype-specific problems associated with pneumococcal conjugate vaccination. Future Microbiol. 3(1), 23–30 (2008).
- Annemans L. Progress in vaccines, progress in health economics. Vaccine 28(Suppl. 6), G1–G2 (2010).
- Weinstein MC, O’Brien B, Hornberger J et al. ISPOR Task Force on Good Research Practices–Modeling Studies. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health 6(1), 9–17 (2003).
- No authors listed. Mitteilung der Ständigen Impfkommission am Robert Koch-Institut. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut/Stand: Juli 2011. Epid. Bull. 30, 275–294 (2011).
- Vila-Córcoles A, Ochoa-Gondar O, Hospital I et al. EVAN Study Group. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin. Infect. Dis. 43(7), 860–868 (2006).
- Middleton DB, Lin CJ, Smith KJ et al. Economic evaluation of standing order programs for pneumococcal vaccination of hospitalized elderly patients. Infect. Control Hosp. Epidemiol. 29(5), 385–394 (2008).
- Shapiro ED, Berg AT, Austrian R et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325(21), 1453–1460 (1991).
- Singleton RJ, Hennessy TW, Bulkow LR et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297(16), 1784–1792 (2007).
- Manoff SB, Liss C, Caulfield MJ et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. J. Infect. Dis. 201(4), 525–533 (2010).
- Claes C, Reinert RR, von der Schulenburg JM. Cost–effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur. J. Health Econ. 10(1), 25–38 (2009).
- Schulze-Gattermann H, Illg A, Schoenermark M, Lenarz T, Lesinski-Schiedat A. Cost–benefit analysis of pediatric cochlear implantation: German experience. Otol. Neurotol. 23(5), 674–681 (2002).
- Kyaw MH, Rose CE Jr, Fry AM et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J. Infect. Dis. 192(3), 377–386 (2005).
- Ryan J, Zoellner Y, Gradl B, Palache B, Medema J. Establishing the health and economic impact of influenza vaccination within the European Union 25 countries. Vaccine 24(47-48), 6812–6822 (2006).
- Jansen AG, Rodenburg GD, de Greeff SC et al. Invasive pneumococcal disease in the Netherlands: syndromes, outcome and potential vaccine benefits. Vaccine 27(17), 2394–2401 (2009).
- Harboe ZB, Thomsen RW, Riis A et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 6(5), e1000081 (2009).
- Ewig S, Birkner N, Strauss R et al. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 64(12), 1062–1069 (2009).
- Welte T, Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ Network. Semin. Respir. Crit. Care Med. 30(2), 127–135 (2009).
- des Portes V. Long-term follow-up of bacterial meningitis - sequels in children and adults: incidence, type, and assessment issues. Med. Mal. Infect. 39(7-8), 572–580 (2009).
Websites
- Reinert RR, Bauer T, Bogner JR et al. Positionspapier. Für höhere Impfraten gegen Pneumokokken-Erkrankungen. (2004). www.pneumologie.de/fileadmin/pneumologie/downloads/PP_Pneumokokkenimpfung.pdf?cntmark. (Accessed 27 March 2012)
- European Medicines Agency. EPAR Prevenar. Click here for link (Accessed 22 March 2012)
- European Medicines Agency. EPAR Prevenar 13. Click here for link (Accessed 22 March 2012)
- European Medicines Agency. EPAR Synflorix. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000973/WC500054347.pdf(Accessed 22 March 2012)
- European Medicines Agency. Assessment report for Synflorix.www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000973/WC500054349.pdf(Accessed 22 March 2012)
- Pfizer. Gebrauchsinformation Prevenar 13 Injektionssuspension (2011). Click here for link
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report: Emerging Infections Program Network, Streptococcus pneumoniae, 2009 (2010). www.cdc.gov/abcs/reports-findings/survreports/spneu09.pdf(Accessed 27 March 2012)
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG). General methods for the assessment of the relation of benefits to costs. www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf(Accessed 22 March 2012)
- WEBAPO® LAUER-Taxe. Pricing database.www.lauer-taxe-online.de(Accessed 22 March 2012)
- Eurostat. Harmonized Indices of Consumer Prices (HCIP). ttp://epp.eurostat.ec.europa.eu/portal/page/portal/hicp/introduction(Accessed 27 March 2012)
- Vereinbarung gemäß § 10 Abs. 9 KHEntgG für den Vereinbarungszeitraum 2010. www.gkv-spitzenverband.de/upload/BBFW_2010_unterschrb_9744.pdf(Accessed 27 March 2012)
- ACIP Pneumococcal Vaccines Workgroup. Use of pneumococcal polysaccharide vaccine (PPV23) in adults aged >50 years. (2008). www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun08/03-1-pneu.pdf (Accessed 22 March 2012)
- Statistisches Bundesamt Deutschland. Bevölkerungsentwicklung in Deutschland bis 2060. Click here for link (Accessed 22 March 2012)
- The Human Mortality Database.www.mortality.org/cgi-bin/hmd/country.php?cntr=DEU&level=2 (Accessed 22 March 2012)
- Statistisches Bundesamt Deutschland. Total cost of illness in millions of Euro. Click here for link (Accessed 11 July 2012)
- ACIP. June 2011 ACIP minutes. www.cdc.gov/vaccines/recs/acip/downloads/min-jun11.pdf (Accessed 27 March 2012)