Abstract
Pulmonary veno-occlusive disease (PVOD) is a rare disorder that can be misdiagnosed as idiopathic pulmonary arterial hypertension (PAH) and accounts for 5–10% of cases initially considered as idiopathic PAH. PVOD and idiopathic PAH share a similar clinical presentation, genetic background and hemodynamic profile. A definite diagnosis of PVOD necessitates a surgical biopsy, but since it represents a high-risk procedure in these patients, it is contraindicated. Therefore, a noninvasive diagnostic approach using chest high-resolution computed tomography, arterial blood gas analysis, pulmonary function tests and bronchoalveolar lavage is helpful to detect PVOD. PVOD is characterized by a poor prognosis and the possibility of developing severe pulmonary edema with specific PAH therapy. Lung transplantation remains the treatment of choice.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.org/journal/expertrespiratory (4) view/print certificate.
Release date: 21 April 2011; Expiration date: 21 April 2012
Learning objectives
Upon completion of this activity, participants should be able to:
• Distinguish the epidemiology of PVOD
• Compare findings in PVOD and idiopathic PAH
• Evaluate diagnostic tools for PVOD
• Analyze treatment options for PVOD
Financial & competing interests disclosure
EDITOR
Elisa ManzottiEditorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles P. Vega, MD,Associate Professor; Residency Director, Department of Family Medicine, University of California, Irvine
Disclosure: Charles P. Vega, MD,has disclosed no relevant financial relationships.
AUTHORS
Alice Huertas,Université Paris-Sud, Faculté de Médecine, France; Centre de Référence de l’Hypertension Pulmonaire Sévère, Hôpital A. Béclère, France; INSERM U999, France
Disclosure:Alice Huertas has disclosed no relevant financial relationships.
Barbara Girerd,Université Paris-Sud, Faculté de Médecine, France; Centre de Référence de l’Hypertension Pulmonaire Sévère, Hôpital A. Béclère, France; INSERM U999, France
Disclosure:Barbara Girerd has disclosed no relevant financial relationships.
Peter Dorfmuller,Université Paris-Sud, Faculté de Médecine, France; Centre de Référence de l’Hypertension Pulmonaire Sévère, Hôpital A. Béclère, France; INSERM U999, France
Disclosure:Peter Dorfmuller has disclosed no relevant financial relationships.
Dermot O’Callaghan,Université Paris-Sud, Faculté de Médecine, France; Centre de Référence de l’Hypertension Pulmonaire Sévère, Hôpital A. Béclère, France; INSERM U999, France
Disclosure:Dermot O’Callaghan has disclosed no relevant financial relationships.
Marc Humbert,Université Paris-Sud, Faculté de Médecine, France; Centre de Référence de l’Hypertension Pulmonaire Sévère, Hôpital A. Béclère, France; INSERM U999, France
Disclosure:Marc Humbert has relationships with drug companies including AB Science, Actelion, BayerSchering, GSK, Novartis, Pfizer and United Therapeutics. In addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards.
David Montani,Université Paris-Sud, Faculté de Médecine, France; Centre de Référence de l’Hypertension Pulmonaire Sévère, Hôpital A. Béclère, France; INSERM U999, France
Disclosure:David Montani has relationships with drug companies including AB Science, Actelion, BayerSchering, GSK, Novartis, Pfizer and United Therapeutics. In addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards.
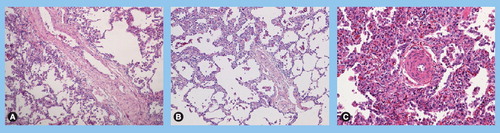
(A) Vascular remodeling in a patient with pulmonary veno-occlusive disease (PVOD): septal vein displaying loose fibrosis with cushion-like narrowing of the native lumen. (B) Vascular remodeling in a patient with PVOD: preseptal venules with occlusive intimal fibrosis; note the partial thickening of alveolar septa due to focal multiplication of alveolar capillaries. (C) Vascular remodeling in a patient with PVOD: associated precapillary remodeling in the same patient with typical hyperplasia of small arterioles; note numerous intra-alveolar macrophages and capillary filling/multiplication as indirect signs of congestion due to post-capillary obstruction. (A–C) Hematoxylin-eosin stained, magnification ×100.
This diagram explains why pulmonary capillary wedge pressure (PCWP) is usually normal in pulmonary veno-occlusive disease (PVOD). PVOD mostly affects small pulmonary veins, leading to an elevation of pressure in this region (Pv), as well as to an elevation in true pulmonary capillary pressure (Pc) and precapillary pulmonary arterial pressure (Pa). Larger pulmonary veins are usually not affected by PVOD, and it is in fact the pressure here that is reflected by PCWP: the static column of blood (hatched) occluded by pulmonary arterial catheter wedging or balloon inflation of a pulmonary arterial branch (balloon 1) reflects the pressure in a vein of similar diameter (balloon 2), usually of a larger size than those vessels affected by PVOD. Therefore, this measurement technique does not reflect the important elevation of pressure in the smaller diameter vessels associated with PVOD.
Reprinted with permission from Citation[5].
![Figure 2. Measurement of pulmonary capillary wedge pressure in pulmonary veno-occlusive diseaseThis diagram explains why pulmonary capillary wedge pressure (PCWP) is usually normal in pulmonary veno-occlusive disease (PVOD). PVOD mostly affects small pulmonary veins, leading to an elevation of pressure in this region (Pv), as well as to an elevation in true pulmonary capillary pressure (Pc) and precapillary pulmonary arterial pressure (Pa). Larger pulmonary veins are usually not affected by PVOD, and it is in fact the pressure here that is reflected by PCWP: the static column of blood (hatched) occluded by pulmonary arterial catheter wedging or balloon inflation of a pulmonary arterial branch (balloon 1) reflects the pressure in a vein of similar diameter (balloon 2), usually of a larger size than those vessels affected by PVOD. Therefore, this measurement technique does not reflect the important elevation of pressure in the smaller diameter vessels associated with PVOD.Reprinted with permission from Citation[5].](/cms/asset/a719f678-5ad3-4795-9cbd-f7451610e1c6/ierx_a_11219173_f0002_b.jpg)
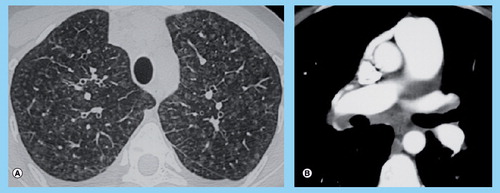
(A) High-resolution computed tomography of the chest showing marked ground-glass opacities with centrilobular pattern, ground-glass opacities with poorly defined nodular opacities, septal lines. (B) High-resolution computed tomography of the chest showing mediastinal lymph node enlargement.
Pulmonary veno-occlusive disease (PVOD) is a rare disorder classified as a subgroup of pulmonary arterial hypertension (PAH) Citation[1]. PAH is a heterogeneous group of diseases, defined as an increase in resting mean pulmonary arterial pressure (mPAP) ≥25 mmHg and a pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, that can lead to right heart failure and death Citation[1,2]. PVOD shares several characteristics with idiopathic PAH, from risk factors to clinical presentation, which can easily lead to misdiagnosis between these two conditions. PVOD and PAH are both severe and have clinical and hemodynamic similarities . These two conditions can only be distinguished by the PVOD histopathological hallmark, which is represented by a widespread fibrous intimal proliferation that predominantly involves the pulmonary venules and small veins Citation[3,4], while idiopathic PAH is characterized by a major remodeling of small pre-capillary pulmonary arteries with frequent plexiform and possible thrombotic lesions. Histological proof is required for a definitive diagnosis of PVOD. Since surgical lung biopsy is a high-risk procedure in these patients and it is therefore contraindicated, the differential diagnosis between PVOD and PAH is often challenging. The importance of establishing a correct and early diagnosis is justified by the worse prognosis in PVOD patients compared with idiopathic PAH patients and by the risk of developing severe pulmonary edema with specific PAH therapy by PVOD patients Citation[5]. Another important clinical hallmark of PVOD is that patients exposed to novel PAH-specific treatments may experience an abrupt and potentially life-threatening deterioration Citation[6]. Clinical worsening as a result of disease progression is almost universal and few patients are alive more than 2 years after diagnosis. It has been hypothesized that idiopathic PAH and PVOD may represent two parts of the same disease spectrum, with lesions preferentially affecting pre-capillary vessels in PAH and post-capillary vessels in PVOD. Owing to the differences in pathological assessment, in response to specific PAH therapy and in prognosis, PVOD is now more clearly identified as a unique subgroup of PAH (revised classification of the 4th World Symposium on pulmonary hypertension Citation[1]). PVOD remains a challenging entity from both a diagnostic and therapeutic point of view. This article aims to provide current knowledge of PVOD.
Epidemiology & risk factors
As PVOD is a difficult-to-diagnose subgroup of a rare disease, the prevalence and incidence are difficult to evaluate. PVOD, without any associated conditions, is an infrequent subgroup of a rare disease and is usually considered to represent approximately 5–10% of histological cases where patients were initially diagnosed as ‘idiopathic’ PAH Citation[7]. Based on data from the French National PAH Registry, we estimate that the annual incidence of PVOD is approximately 0.1–0.2 cases per million in the general population: these data are probably underestimated owing to the diagnostic difficulties Citation[8,9].
As observed in idiopathic PAH, PVOD has been diagnosed throughout a very wide age range, from the first weeks of life to the seventh decade Citation[7]. With regard to sex distribution, PVOD occurs equally in men and women whereas idiopathic PAH has a clear female predominance Citation[2,8–10].
As previously described in idiopathic PAH, we have shown that even in the absence of a diagnosed autoimmune disorder, PVOD shares a similar autoimmune background with idiopathic PAH: auto-antibodies (antinuclear, antiphospholipid or thyroid antibodies) were found in 30 and 33% in PVOD and idiopathic PAH, respectively Citation[8]. PVOD can also occur in conditions usually associated with pulmonary hypertension including connective tissue diseases Citation[11–13], sarcoidosis Citation[14], pulmonary Langerhans cell histiocytosis Citation[7,15,16] and, more rarely, HIV infection Citation[17–19].
There is a clear and significant risk of developing PAH after anorexigen (fenfluramine derivatives) exposure and it has been clearly demonstrated that PAH associated with anorexigens shares a similar presentation and evolution to idiopathic PAH Citation[20–24]. We have recently reported a case of PVOD in a patient with a history of fenfluramine exposure, therefore suggesting a possible association between anorexigen exposure and PVOD Citation[8].
PVOD has been reported in association with various chemotherapy regimens including bleomycin, bis-chloronitrosourea and mitomycin Citation[25–28] and after bone marrow transplantation Citation[29–36]. Chemical exposure has been suggested to influence the development of PVOD while it is not considered as a risk factor of PAH. This association has been reported in isolated case reports; however, the two largest series of PVOD found no significant association with chemical exposure, but did not include specific exposure history elicited by questionnaire Citation[8,10]. In a recent series, we reported a higher tobacco exposure and an increased proportion of smokers in PVOD as compared with idiopathic PAH Citation[8]. Even if it has been previously demonstrated that tobacco exposure may contribute to pulmonary vascular injury Citation[37,38], it is not clear why tobacco exposure would be a specific risk factor for PVOD and not for PAH Citation[21].
Genetic analysis performed in PAH patients revealed the presence of a germline mutations in the bone morphogenetic protein receptor 2 (BMPR2) gene in approsimately 75% of familial cases and in 10–40% of apparently idiopathic PAH Citation[39–41]. Because BMPR2 mutations have been detected in apparently idiopathic cases with no family history, the distinction between idiopathic and familial BMPR2 mutation carriers may be artificial. All patients with BMPR2 mutations have heritable disease, whether the patient is the first identified case or other family members were previously diagnosed with PAH. Therefore, recent expert discussion pleads in favor of the term ‘heritable’ PAH to describe these genetic forms of the disease Citation[1]. Interestingly, PVOD can appear in a familial context and some patients with a definite diagnosis of PVOD have been reported to carry a BMPR2 mutation. These observations suggest the existence of a genetic risk factor in the development of PVOD Citation[8,42–46]. The involvement of a genetic pattern in the development of PVOD emphasizes once more the similarities between PVOD and PAH. As in heritable PAH, ‘heritable’ PVOD may occur in the absence of BMPR2 mutation suggesting that other genetic risk factors may also be contributing to the disease process Citation[42]. In the French Referral Center for Pulmonary Hypertension, we propose systematic screening for a possible familial history of pulmonary vascular disease and similar genetic counseling in both PAH and PVOD patients. In our center, after obtaining written informed consent, exonic and intronic point mutations and large rearrangements of the BMPR2 gene were searched as previously described Citation[47].
Clinical presentation
Idiopathic PAH and PVOD have very similar clinical presentations, therefore the differentiation between these two entities is difficult and the clinical exam is often unhelpful. As in PAH, progressive dyspnea on exertion is the main symptom in PVOD, although often neglected by patients, leading to the frequent diagnostic delay. In PVOD, most of the patients have severe exertional dyspnea with a New York Heart Association (NYHA) functional class III or IV at the time of the diagnosis Citation[8,10]. We found no difference in NYHA functional class at diagnosis between patients displaying PVOD as compared with idiopathic PAH. Right-sided cardiac dysfunction and right ventricular failure, due to the sustained pressure overload, often complicate the more advanced stages of the disease. Signs of right heart failure are frequently elicited on clinical examination. Cardiac auscultation reveals a prominent pulmonic component of the second heart sound and a systolic murmur of tricuspid regurgitation Citation[10]. Respiratory auscultatory crackles may occur in PVOD patients with predominant pulmonary infiltrates but the precise prevalence is unknown Citation[7,10]. Pleural effusions have been suggested to be more frequent in PVOD, however, the studies focused on radiography with high-resolution computed tomography (HRCT) of the chest show a similar proportion of pleural effusions in both diseases Citation[8,10,48,49]. Hemoptysis has been described in PVOD; however, in our recent study, hemoptysis was reported infrequently and equally in both idiopathic PAH and PVOD, confirming that alveolar hemorrhage in PVOD is generally occult Citation[8,50]. Clubbing and Raynauld’s phenomenon have been reported as clinical signs in PVOD but this was not reproduced in our recent series, as these signs remain rare and can also occur in idiopathic PAH Citation[8].
Diagnosis
Histopathological assessment
Pulmonary veno-occlusive disease is a rare pulmonary vascular disease causing pulmonary hypertension and has been considered together with pulmonary capillary hemangiomatosis (PCH), a subgroup of PAH, until recently, but is now separated according to the latest revised classification of the 4th World Symposium on pulmonary hypertension Citation[1]. In PVOD, as in PCH, vascular lesions predominate on the post-capillary level of pulmonary vasculature. However, lesions frequently concern both veins and arteries in lungs of patients with PVOD. The observed post-capillary lesions concern septal veins and pre-septal venules and frequently consist of loose, fibrous remodeling of the intima, that may totally occlude the lumen . The involvement of pre-septal venules is considered as important, if not necessary, for the histological diagnosis of PVOD, as fibrous occlusion of large septal veins can be encountered in many forms of pulmonary venous hypertension, including a frequently reported obstruction of large pulmonary veins following catheter ablation for cardiac atrial fibrillation Citation[51]. While septal veins usually display a paucicellular, cushion-like fibrous obstruction, intimal thickening of pre-septal venules can present with a dense pattern and increased cellularity, including smooth muscle cells. Pleural and pulmonary lymphatic vessels are usually dilated. The presence of calcium-encrusting elastic fibers in the vessel wall or the perivascular space, and consecutive inflammatory activation through a foreign body giant-cell response is considered as an argument in favor of PVOD as compared to secondary venous hypertension Citation[3]. Moreover, post-capillary obstruction may frequently lead to capillary angiectasia and even capillary angioproliferation; in PVOD cases, doubling and trebling of the alveolar septal capillary layers may be focally present.
Lately, this histological peculiarity has raised questions concerning a possible overlap between PVOD and cases of PCH, a disease classically characterized by an aggressive patch-like angioproliferation of capillaries; indeed, Lantuejoul et al. have recently reported 35 cases of PVOD and PCH with a more or less similar pattern and evoke the possiblity of the same disease Citation[4]. Our experiences in the French Referral Centre for Pulmonary Hypertension, which includes a large paraffin and frozen tissue collection confirm this view: most, if not all, PVOD cases display patchy capillary hemangiomatosis, which is virtually indistinguishable from the very rare cases of PCH. This latter pseudoentity always includes fibrous lesions of veins and venules.
Occult pulmonary hemorrhage regularly occurs in patients displaying PVOD contrarily to idiopathic PAH where alveolar hemorrhage is classically absent Citation[50]. This is possibly due to the post-capillary bloc, and is of particular diagnostic interest, as bronchoalveolar lavage (BAL) can reveal the presence of occult hemorrhage. The degree of hemorrhage is evaluated semiquantitatively and qualitatively using the Golde Score, a siderophagic scoring system firstly described in pulmonary hemorrhage of patients suffering from leukemia Citation[52]. This assessment tool for the cytologist/pathologist takes the number of intra-alveolar siderin-laden macrophages but also the degree of their staining (Perls-Prussian blue or iron staining) into consideration. A coefficient from 1 to 4 is accorded to different staining intensities, allowing a qualitative assessment during the cytological cell count: a Golde Score above 100 is considered as occult alveolar hemorrhage and may suggest PVOD in the setting of pulmonary hypertension Citation[50]. Interestingly, venous lesions and post-capillary obstruction in PVOD are commonly associated with capillary remodeling, including angiectasia and even capillary angioproliferation . A doubling or tripling of the alveolar septal capillary layers may focally be observed. Despite the constant occurrence of arterial lesions in the lungs of PVOD patients, complex lesions, as defined here above, do not usually occur in the context of PVOD Citation[3].
Hemodynamic evaluation
Pulmonary capillary wedge pressure
As for all patients with suspected PAH, hemodynamic evaluation is required in PVOD to confirm the diagnosis. Both idiopathic PAH and PVOD patients have evidence of severe pre-capillary PAH with an elevated resting mPAP ≥25 mmHg with a PCWP ≤15 mmHg Citation[1,5,53–55]. When biopsy-proven idiopathic PAH patients were compared with a group with biopsy-proven PVOD in a recent large series, similar hemodynamic characteristics were observed with the exception of right atrial pressure, which was lower in PVOD Citation[8].
Case reports Citation[56] and case series Citation[8,10,50] demonstrated that PCWP is usually normal (<15 mmHg) in PVOD patients. The difference between PVOD and idiopathic PAH is at the level of obstruction to blood flow, noted in pre-capillary vessels in PAH and in capillary as well as post-capillary vessels in PVOD. By definition, true capillary pressure (Pc) and measured PCWP are normal (<15 mmHg) in idiopathic PAH. Since Pc measurement is not suitable for clinical application, PCWP is currently the most frequently used method for estimating Pc in a broad range of clinical and/or experimental conditions Citation[57,58]. By contrast, in PVOD experimental conditions, the post-capillary obstruction leads to an elevated Pc without any increase in PCPW Citation[59,60]. During hemodynamic evaluation, the measured PCWP is reflective of the pressure in the column of blood distal to the inflated balloon . Then, PCWP reflects the pressure in a pulmonary vein of similar diameter to the occluded pulmonary arterial branch, which is larger than the small veins affected by PVOD, explaining the normal PCWP usually obtained in PVOD patients Citation[3,5,8]. In conclusion, because PCWP does not reflect true capillary pressure but reflects the pressure in large veins, PCWP is not helpful to discriminate idiopathic PAH and PVOD. Measurement of the Pc may theoretically be useful in PVOD; the principle of Pc pressure measurement is extrapolated from a canine model whereby the pressure decay following balloon occlusion is mathematically analyzed to represent the emptying of the capillary compartment Citation[61]. Interestingly, Fesler et al. showed showed that this method helps to locate the site of predominantly increased pulmonary vascular resistance in severe PAH and that patients displaying PVOD may have higher Pc than PAH patients using this method Citation[57,58].
Acute vasodilator testing
In idiopathic PAH, acute vasoreactivity testing is predictive of long-term responsiveness to calcium channel blockers Citation[55,62]. There are clear differences in the long-term prognosis in the group of idiopathic PAH patients who have a positive response to this challenge when compared with nonresponders Citation[55,62]. There is a recent report of one patient with PVOD who responded to nitric oxide (NO); however, within 48 h after initiation of calcium channel blocker therapy, severe pulmonary edema developed Citation[8]. This suggests that an acute vasodilator response in PVOD is not predictive of a better prognosis and that calcium channel blockers should be avoided in PVOD, even in the context of a positive acute test. The main concern in PVOD is the risk of pulmonary edema with all specific PAH therapies, in particular with continuous intravenous epoprostenol Citation[6,8,9]. We showed that acute pulmonary edema occurs in 40% of the reported cases treated with specific PAH therapies Citation[8]. In our experience, less than 10% of all suspected untreated PVOD patients develop an acute pulmonary edema during the time course of the disease, whereas all PVOD patients treated with vasodilators develop an acute pulmonary edema in the following 72 h that could lead to death Citation[63]. However, in chronic therapy, we did not observe a response with calcium channel blockers in PVOD patients Citation[63]. Continuous intravenous epoprostenol can be initiated in the most severe patients with a slowly increasing dose and high-dose diuretics under close medical monitoring. Since 2003, the French Referral Center has proposed this approach as a bridge therapy to lung transplantation in several severe highly probable PVOD patients (later confirmed by histology after lung transplantation) Citation[5]. In these patients, intravenous epoprostenol may improve hemodynamics without major adverse complications Citation[64].
Pulmonary edema has been described following vasodilator testing Citation[6,10]. However, in a recent series of 24 histologically confirmed PVOD patients, inhalation of 10 ppm NO for a short period (5–10 min) was used to test acute vasoreactivity. In this study, none of the patients developed pulmonary edema acutely Citation[8]. This regimen of NO administration seems to be safe in patients with suspected PVOD. However, in this series, acute vasoreactivity testing was unable to predict those patients who later developed pulmonary edema following initiation of a PAH-specific therapy. It appears that acute vasoreactivity testing is probably not helpful in the management of PVOD patients, as calcium channel blocker responders have not been described Citation[63]. Furthermore, acute vasoreactivity testing is not predictive of those patients at risk of developing pulmonary edema with specific PAH therapy. Given these important observations, there may be little merit of systemically performing vasodilator studies in those patients with a strong clinical suspicion of PVOD, since the hemodynamic findings are unlikely to impact on therapeutic decisions. Noninvasive tools (HRCT and pulmonary function testing, in particular diffusing lung capacity of carbon monoxide [DLCO]) may help to discriminate PVOD from PAH patients.
Noninvasive tools
The gold standard for a definite diagnosis of PVOD requires histological analysis of a lung sample. As this patient population often presents with more advanced disease, surgical lung biopsy is invasive, highly risky and thus contraindicated. Therefore, there is a need to use less invasive tools to aid in the diagnosis of suspected PVOD. Recent data have shown that HRCT of the chest, arterial blood gases, pulmonary function tests and BAL could be helpful to define a subgroup of PAH with a high probability of PVOD.
Transthoracic echocardiography
Transthoracic echocardiography is an important initial non-invasive diagnostic tool in the evaluation of all patients in whom pulmonary hypertension is suspected Citation[65]. A systolic pulmonary artery pressure cutoff greater than 40 mmHg, as estimated by the velocity of the regurgitating flow through the tricuspid valve, is a sensitive but not a specific diagnostic threshold. False positives using this value are common and formal diagnosis of pulmonary hypertension requires confirmation by right heart catheterization. Furthermore, there are no distinct echocardiographic features that help distinguish PVOD from other forms of pulmonary hypertension. Nonetheless, echocardiography is useful to exclude the presence of associated left ventricular, valvular or pericardial abnormalities Citation[66].
High-resolution computed tomography of the chest
In idiopathic PAH, the radiographic findings are generally limited to enlargement of the right and left pulmonary arteries without evidence of pulmonary parenchymal abnormalities. In contrast, the chest radiograph in PVOD may show significant abnormalities with Kerley B lines or signs of pulmonary edema, most frequently occurring after initiation of pulmonary vasodilator therapy Citation[6,8,10,48,67]. HRCT of the chest may also help to discriminate PVOD and PAH in less acute situations Citation[8,10,48,49]. It has been clearly demonstrated that HRCT in PVOD was characterized by a higher frequency of centrilobular ground-glass opacities, septal lines and mediastinal lymph node enlargement as compared with idiopathic PAH Citation[49]. Pleural effusion and other abnormal parenchymal findings were not significantly associated with PVOD Citation[49]. We have reported that the presence of two or three radiological abnormalities (including lymph node enlargement, septal lines and centrilobuar ground-glass opacities) were present in 75% of HRCT from patients displaying histologically proven PVOD Citation[8]. However, this means that one-quarter of histologically proven PVOD patients had only one or no radiological abnormalities on HRCT; inversely, 15% of patients with confirmed idiopathic PAH had two or three abnormalities Citation[8]. These findings suggest that HRCT was a useful tool to discriminate PVOD, but normal HCRT could not rule out the diagnosis of PVOD, and should be included in a multifactorial noninvasive approach.
Pulmonary function testing & oxygen parameters
Results of previous reports have suggested that PVOD patients may have mild obstructive or restrictive patterns noted on pulmonary function testing Citation[10]. However, a large series of ‘idiopathic’ PVOD patients showed that these patients had normal mean values of forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity ratio and total lung capacity. Indeed, no difference was observed in pulmonary function tests analyzed during spirometry and plethysmography as compared with idiopathic PAH Citation[8]. A decrease in DLCO has been well established in patients displaying idiopathic PAH and PVOD Citation[9,68]. However, we have compared DLCO and the DLCO/alveolar volume between PVOD and PAH patients, and the results indicated that both these parameters were more significantly reduced in PVOD as compared with idiopathic PAH Citation[8]. PVOD patients usually had a very low DLCO and in this series, half of the histologically confirmed PVOD patients had a mean DLCO of 52 ± 19% as compared with 71 ± 15% in idiopathic PAH patients Citation[8]. In PVOD patients, alveolar hemorrhages occur quite often Citation[50] so the DLCO should be increased in these patients. Therefore, it is reasonable to suggest that the DLCO measurement in PVOD is probably overestimated and that the real DLCO is even lower than the observed one.
Hypoxemia has been reported in patients displaying PVOD or idiopathic PAH Citation[10,55]. Our recent report showed that the baseline partial pressure of arterial oxygen at rest is significantly lower in PVOD patients than in idiopathic PAH patients (61 ± 17 and 75 ± 14, respectively) Citation[8]. This study also evaluated the partial pressure of arterial carbon dioxide which was decreased in a similar pattern in both PVOD and PAH patients. Many factors likely contribute to the pathophysiological mechanisms of exaggerated hypoxemia in PVOD patients in comparison to patients with idiopathic PAH. These include a probable combination of more extensive obliteration of the pulmonary vascular bed, a diffusion limitation, possible alveolar hemorrhage and pulmonary edema in the most severe cases.
The 6-min walk distance (6MWD) has been found to correlate with functional status and survival in idiopathic PAH. Additionally, it is used as a measure of baseline severity and a surrogate marker of response to treatment in idiopathic PAH. Only a few studies have evaluated this parameter in PVOD. A series comparing idiopathic PAH and PVOD patients demonstrated a significantly lower 6MWD in the PVOD group Citation[50]. Our recent case series showed that PVOD patients had a similar 6MWD compared with the PAH patients; however, the PVOD patients had lower nadir pulse oxygen saturation during the test Citation[8].
Bronchoalveolar lavage
In patients undergoing an evaluation for PAH, bronchoscopy is rarely performed as a routine investigation. However, in the context of possible PVOD, a bronchoscopic evaluation with BAL may be helpful Citation[50]. In PVOD, it has been suggested that hyperemia of the lobar and segmental bronchi may be observed on bronchoscopic airway inspection as it was described in mitral stenosis, where chronic pulmonary venous hypertension leads to engorgement and dilatation of the bronchial venous plexus and veins Citation[69,70]. Of note, bronchial biopsies are not informative of PVOD and transbronchial biopsies are contraindicated in patients with pulmonary hypertension due to the risk of life-threatening hemoptysis Citation[71]. When comparing the BAL results of eight PVOD patients and 11 idiopathic PAH patients, Rabiller et al. showed a significantly elevated percentage of hemosiderin-laden macrophages and a higher Golde Score, demonstrating that PVOD may be associated with occult alveolar hemorrhage Citation[50]. The absence of alveolar hemorrhage could not exclude the diagnosis of PVOD, however, confirmation of alveolar hemorrhage is highly suggestive of PVOD. As histological diagnosis is difficult to obtain in this patient population, the role of bronchoscopy to detect occult alveolar hemorrhage as part of the diagnostic work-up for suspected PVOD is somewhat attractive.
Prognosis
Historically, the prognosis has been poor in idiopathic PAH and unfortunately, the survival in PVOD seems to be even more dismal than for idiopathic PAH. Data suggest that the 1-year mortality rate may be as high as 72% in PVOD Citation[10], and the most recent series of a group of 24 histologically confirmed severe PVOD patients found a mean time from first symptoms (or diagnosis confirmed by right heart catheterization) to death or lung transplantation of 24.4 ± 22.2 or 11.8 ± 16.4 months, respectively, which is significantly lower in PVOD when compared with 57.9 ± 38.2 or 42.3 ± 29.9 months, respectively, for patients with idiopathic PAH Citation[8]. In this series, even if baseline parameters including NYHA functional class, 6MWD and baseline hemodynamics are similar between the idiopathic PAH and the PVOD groups, PVOD patients seem to have a poorer prognosis, suggesting that these parameters might be less accurate to predict the evolution of PVOD than they are in idiopathic PAH. Treatment options are unfortunately very limited in PVOD. Therefore, early diagnosis and referral for lung transplantation in appropriate patients is critical in PVOD.
Therapy
Conventional therapy
A significant aggravating factor in PAH is hypoxic vasoconstriction. Therefore, oxygen therapy should be considered and initiated in hypoxemic patients with both idiopathic PAH and PVOD Citation[55]. It has been noted that PVOD patients have a lower partial pressure of arterial oxygen at rest than idiopathic PAH patients, suggesting that PVOD patients may require oxygen therapy earlier than other PAH patients Citation[8]. In appropriate patients with resting or exercise-induced hypoxemia, oxygen therapy may provide symptomatic benefit as well as helping to avoid PAH deterioration Citation[55].
Patients with idiopathic PAH have improved outcomes with the initiation of warfarin therapy. Although there are no specific data pertaining to PVOD, the rationale is similar and related to reducing organized thrombi Citation[72] and subsequent in situ thrombosis, which manifest in both conditions Citation[73]. The current recommendations suggest warfarin therapy with an international normalized ratio to be maintained between 1.5 and 2.5 in idiopathic PAH Citation[55]. This recommendation has been generalized to all PAH patients without contraindications, despite the fact that the evidence was obtained only in the idiopathic PAH group. In PVOD, one could suggest that caution should be taken in the application of anticoagulation, because of the frequent association with occult alveolar hemorrhage. However, we have reported the same proportion of hemoptysis in idiopathic PAH and PVOD, even with the use of anticoagulation. As in idiopathic PAH, no data support the role of immunosuppressive therapy in idiopathic PVOD, but it may be of interest in PAH or PVOD associated with other inflammatory conditions, including sarcoidosis, mixed connective tissue disease and systemic lupus erythematosus, but not in scleroderma-associated PAH Citation[14,74,75].
A number of additional measures are routinely advised for PVOD patients, although these are mostly based on expert consensus, often with extrapolation from historical studies in other diseases. Empiric evidence from well-designed prospective clinical trials to support the use of most of these ‘conventional’ therapies is lacking as published studies are mostly of a retrospective or uncontrolled retrospective nature. Indeed, some of these interventions likely offer only symptomatic benefits without necessarily impacting on prognosis. Nevertheless, their application is biologically plausible and thus broadly advocated Citation[76].
General recommendations include limiting of physical activity to tolerance and avoidance of concomitant medications that can potentially aggravate pulmonary hypertension (such as β-adrenergic receptor blockers) or interfere with the metabolism of vitamin K antagonist anticoagulation therapy. Vaccinations to prevent pneumococcal pneumonia and influenza are advised. Given a possible etiological link between tobacco exposure and PVOD, smoking cessation advice and appropriate pharmacotherapy is particularly important and should routinely be offered to cigarette smokers. Diuretics offer symptomatic benefit in those with right ventricular volume overload that is not controlled by dietary measures alone. The effect of diuretics on mortality in PVOD has not been systemically examined.
The additional hemodynamic stresses of pregnancy and delivery are poorly tolerated in PVOD patients, being associated with increased rate of deterioration and very high mortality rates. Moreover, commonly prescribed treatments (e.g., warfarin and bosentan) are known teratogens. Female patients of child-bearing age should therefore be counseled on effective contraception measures.
Specific PAH therapies
Presently, the evidence for specific vasodilator therapies is strong in idiopathic PAH Citation[54–56,77–79] but is modest and conflicting in PVOD, and the primary concern remains the risk of pulmonary edema. Recent reports have illustrated the occurrence of pulmonary edema with different specific PAH therapies (prostacyclins, endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, calcium channel blockers), highlighting that pulmonary edema is not limited to a single therapeutic class and can occur with all specific PAH therapies Citation[5,8,64]. One of the suspected mechanisms is a relative vasodilatation of the pre-capillary resistance vessels greater than the pulmonary capillaries and veins, associated with increased blood flow that leads to an increase of transcapillary hydrostatic pressure and transudation of fluid into the pulmonary interstitium and alveoli. Furthermore, lymphatic involvement is frequently observed in PVOD and may participate in the mechanism of pulmonary edema by a decrease in fluid reabsorption. In our recent cohort of histologically confirmed PVOD patients, seven of 16 patients who received specific PAH therapy developed pulmonary edema. However, none of these patients developed pulmonary edema during acute testing with NO, nor were any of the clinical, functional or hemodynamic characteristics predictive of the onset of this complication after initiation of specific PAH therapy Citation[8]. In selected cases, there have been reported mild clinical improvement or stabilization with continuous intravenous epoprostenol Citation[10,80–83], iloprost therapy Citation[84,85] and oral sildenafil Citation[86,87]. In contrast with idiopathic PAH, the benefits of specific PAH treatments in patients with PVOD remain unclear. Continuous intravenous epoprostenol can be initiated in the most severe patients with a slowly increasing dose and high-dose diuretics under close medical monitoring. Since 2003, the French referral center has proposed this approach as a bridge therapy to lung transplantation Citation[5]. In these patients, intravenous epoprostenol may improve hemodynamics without major adverse complications Citation[64].
Immunomodulatory agents
The basis for the use of immunosuppressive agents in some selected PAH patients was initially anecdotal with rare reports of clinical and hemodynamic improvements in PAH and PVOD patients, suggesting the use of corticosteroids, cyclophosphamide and azathioprine Citation[17,29,74,75,88,89]. PVOD is also thought to be present in some cases of pulmonary hypertension patients displaying sarcoidosis. However, in these patients, the pulmonary vascular component may be less responsive to treatment with glucocorticoids than the parenchymal lung disease Citation[14]. There has been mounting evidence that PAH has a significant inflammatory component Citation[90], and that PAH seen in some connective tissue diseases responds well to immunosuppressive therapy Citation[75,80,89]. Specific patients who may improve with immunosuppression include those with mixed connective tissue disease and systemic lupus erythematosus, but not in scleroderma-associated PAH Citation[75,89]. A full histopathological assessment has shown that severe connective tissue disease-associated PAH has a major venous pathological component in 75% of cases Citation[11]. In cases where such venous occlusion was observed, the post-capillary arterial involvement was important, and there was also an association with local inflammatory infiltrates Citation[11]. The reason why this subset of connective tissue disease-associated PAH patients do not improve with pulmonary vasodilator therapy might be due at least in part to venous involvement. More studies are needed to understand whether PVOD, either idiopathic or associated with other conditions such as connective tissue diseases, are responsive to immunosuppressive therapies. Currently, corticosteroids or immunosuppressive therapy should only be considered in the context of sarcoidosis or connective tissue disease (except scleroderma).
Lung transplantation
Lung transplantation is the only curative intervention of PAH and historically it was the only form of treatment for severe PAH. However, with the advent of specific PAH therapy, transplantation is now considered in idiopathic PAH when the disease progresses despite optimal medical management Citation[53–55,91,92]. As the prognosis in PVOD patients is worse than in idiopathic PAH, and due to limited medical therapy, lung transplantation should be considered and discussed early in the course of PVOD or suspected PVOD (HRCT findings, worsening symptoms on treatment or refractoriness to medical therapy, development of worsening hypoxemia or chronic heart failure). In these patients, PAH-specific therapies may serve as a bridge to lung transplantation Citation[5,83]. The survival has been suggested to be broadly similar to that in idiopathic PAH. Both single and bilateral procedures have been performed, apparently with similar survival. However, any complication occurring in the allograft following single lung transplantation is associated with severe hypoxemia Citation[54]. Currently, the vast majority of patients worldwide receive bilateral lungs.
Conclusion
Pulmonary veno-occlusive disease is a rare subgroup of PAH characterized by specific pathological changes of post-capillary venous pulmonary vessels. PVOD shares a broadly similar clinical presentation with idiopathic PAH , making this diagnosis much more challenging to the clinician. In contrast with idiopathic PAH, patients with PVOD have a poorer prognosis and are at risk of developing pulmonary edema with specific PAH therapy. Hemodynamic parameters do not help discriminate between these two diseases and even if PVOD is defined by an involvement of post-capillary and capillary vessels, PCWP is normal (<15 mmHg) in both groups. However, there are some features that may help to discriminate between the two entities in order to prevent risky histological confirmation. These include and may identify a higher probability of PVOD: history of tobacco exposure, oxygen parameters (low partial pressure of arterial oxygen at rest, low pulsoximetry saturation of O2 during 6-min walking test), pulmonary functional tests (low DLCO), HRCT of the chest (centrilobular ground-glass opacities, septal lines, lymph node enlargement) and occult alveolar hemorrhage on BAL when it is feasible. In PVOD, cautious initiation of specific PAH therapy may be considered but should not delay consideration and referral for lung transplantation, which remains the best treatment. Further studies are needed to understand the pathophysiology of this subgroup of PAH, the role of specific PAH therapies and whether new therapeutic approaches with antiproliferative therapies might be helpful.
Expert commentary
There is now a wealth of accumulated clinical experience with strategies employing combinations of PAH-specific drugs in order to target the multifarious aberrant molecular pathways characteristic of the disorder and thereby theoretically to enhance therapeutic gain. Indeed, this approach is routinely pursued in many centers of expertise, despite a lack of robust evidence demonstrating additional clinical benefit. Theoretically, the risk of pulmonary edema developing in the context of PVOD might be further increased by using a regimen involving more than one pulmonary vasodilator. However, in patients with severe disease and poor expected survival time, cautious introduction of additional treatments has been attempted in the hope of slowing clinical deterioration. Nonetheless, experience is extremely limited to date and because of the risk of severe pulmonary edema, this approach is not recommended outside of expert centers.
Five-year view
In recent years, remarkable advances in the pharmacologic options available to clinicians treating PAH patients have been made. Regulatory approval has now been granted for a number of drug therapies that have pulmonary-specific vasodilatory and antiproliferative effects, resulting in meaningful and sustained improvements in relevant clinical parameters. As our understanding of the pathophysiology of PVOD and PAH increases, so does the identification of possible therapeutic targets and potential novel treatments. In 5 years’ time, further studies are needed in order to refine the understanding of the molecular characteristics of PVOD and PAH. The discovery of agents that modify these aberrant pathways will determine whether new therapeutic approaches with antiproliferative therapies might have any role in PVOD.
Table 1. Selected characteristics of idiopathic pulmonary arterial hypertension and idiopathic pulmonary veno-occlusive disease.
Key issues
• Pulmonary veno-occlusive disease (PVOD) is a rare form of pulmonary arterial hypertension (PAH) with several similarities but with worse prognosis.
• PVOD has been described as idiopathic, heritable or complicating other conditions, such as connective tissue diseases, HIV infection, chronic respiratory disease, malignancy or bone marrow transplantation, among others.
• Compared to PAH, PVOD is characterized by a higher male:female ratio, higher tobacco exposure, lower arterial oxygen tension at rest, lower diffusing capacity of the lung for carbon monoxide, and lower oxygen saturation nadir during the 6-min walk test.
• PVOD histopathological hallmark is represented by a widespread fibrous intimal proliferation that predominantly affects the post-capillary venous pulmonary vessels, venules and small veins.
• A definite diagnosis of PVOD necessitates a surgical biopsy, but since it represents a high-risk procedure in these patients, it is contraindicated.
• A noninvasive approach using high-resolution computed tomography of the chest, arterial blood gas analyses, pulmonary function tests and bronchoalveolar lavage could be helpful for the detection of PVOD.
• Treatment of PVOD is challenging as exposure to pulmonary vasodilators and PAH-specific therapies may precipitate acute pulmonary edema.
• Lung transplantation is the treatment of choice.
References
- Simonneau G, Robbins IM, Beghetti M et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol.54(Suppl. 1), S43–S54 (2009).
- Rubin LJ. Primary pulmonary hypertension. N. Engl. J. Med.336(2), 111–117 (1997).
- Pietra GG, Capron F, Stewart S et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J. Am. Coll. Cardiol.43(Suppl. 12), 25S–32S (2004).
- Lantuejoul S, Sheppard MN, Corrin B, Burke MM, Nicholson AG. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: a clinicopathologic study of 35 cases. Am. J. Surg. Pathol.30(7), 850–857 (2006).
- Montani D, Price LC, Dorfmuller P et al. Pulmonary veno-occlusive disease. Eur. Respir. J.33(1), 189–200 (2009).
- Palmer SM, Robinson LJ, Wang A, Gossage JR, Bashore T, Tapson VF. Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest113(1), 237–240 (1998).
- Mandel J, Mark EJ, Hales CA. Pulmonary veno-occlusive disease. Am. J. Respir. Crit. Care Med.162(5), 1964–1973 (2000).
- Montani D, Achouh L, Dorfmuller P et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine (Baltimore)87(4), 220–233 (2008).
- Humbert M, Sitbon O, Chaouat A et al. Pulmonary arterial hypertension in France: results from a national registry. Am. J. Respir. Crit. Care Med.173(9), 1023–1030 (2006).
- Holcomb BW Jr., Loyd JE, Ely EW, Johnson J, Robbins IM. Pulmonary veno-occlusive disease: a case series and new observations. Chest118(6), 1671–1679 (2000).
- Dorfmuller P, Humbert M, Perros F et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum. Pathol.38(6), 893–902 (2007).
- Zhang L, Visscher D, Rihal C, Aubry MC. Pulmonary veno-occlusive disease as a primary cause of pulmonary hypertension in a patient with mixed connective tissue disease. Rheumatol. Int.27(12), 1163–1165 (2007).
- Johnson SR, Patsios D, Hwang DM, Granton JT. Pulmonary veno-occlusive disease and scleroderma associated pulmonary hypertension. J. Rheumatol.33(11), 2347–2350 (2006).
- Nunes H, Humbert M, Capron F et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax61(1), 68–74 (2006).
- Fartoukh M, Humbert M, Capron F et al. Severe pulmonary hypertension in histiocytosis X. Am. J. Respir. Crit. Care Med.161(1), 216–223 (2000).
- Hamada K, Teramoto S, Narita N, Yamada E, Teramoto K, Kobzik L. Pulmonary veno-occlusive disease in pulmonary Langerhans’ cell granulomatosis. Eur. Respir. J.15(2), 421–423 (2000).
- Escamilla R, Hermant C, Berjaud J, Mazerolles C, Daussy X. Pulmonary veno-occlusive disease in a HIV-infected intravenous drug abuser. Eur. Respir. J.8(11), 1982–1984 (1995).
- Hourseau M, Capron F, Nunes H, Godmer P, Martin A, Kambouchner M. Pulmonary veno-occlusive disease in a patient with HIV infection. A case report with autopsy findings. Ann. Pathol.22(6), 472–475 (2002).
- Ruchelli ED, Nojadera G, Rutstein RM, Rudy B. Pulmonary veno-occlusive disease. Another vascular disorder associated with human immunodeficiency virus infection? Arch. Pathol. Lab. Med.118(6), 664–666 (1994).
- Humbert M, Nunes H, Sitbon O, Parent F, Hervé P, Simonneau G. Risk factors for pulmonary arterial hypertension. Clin. Chest Med.22, 459–475 (2001).
- Abenhaim L, Moride Y, Brenot F et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hpertension Study Group. N. Engl. J. Med.335(9), 609–616 (1996).
- Souza R, Humbert M, Sztrymf B et al. Pulmonary arterial hypertension associated with fenfluramine exposure: report of 109 cases. Eur. Respir. J.31(2), 343–348 (2008).
- Douglas JG, Munro JF, Kitchin AH, Muir AL, Proudfoot AT. Pulmonary hypertension and fenfluramine. Br. Med. J. (Clin. Res. Ed.)283(6296), 881–883 (1981).
- Gurtner HP. Aminorex and pulmonary hypertension. A review. Cor Vasa27(2–3), 160–171 (1985).
- Joselson R, Warnock M. Pulmonary veno-occlusive disease after chemotherapy. Hum. Pathol.14(1), 88–91 (1983).
- Knight BK, Rose AG. Pulmonary veno-occlusive disease after chemotherapy. Thorax40(11), 874–875 (1985).
- Swift GL, Gibbs A, Campbell IA, Wagenvoort CA, Tuthill D. Pulmonary veno-occlusive disease and Hodgkin’s lymphoma. Eur. Respir. J.6(4), 596–598 (1993).
- Waldhorn RE, Tsou E, Smith FP, Kerwin DM. Pulmonary veno-occlusive disease associated with microangiopathic hemolytic anemia and chemotherapy of gastric adenocarcinoma. Med. Pediatr. Oncol.12(6), 394–396 (1984).
- Hackman RC, Madtes DK, Petersen FB, Clark JG. Pulmonary venoocclusive disease following bone marrow transplantation. Transplantation47(6), 989–992 (1989).
- Williams LM, Fussell S, Veith RW, Nelson S, Mason CM. Pulmonary veno-occlusive disease in an adult following bone marrow transplantation. Case report and review of the literature. Chest109(5), 1388–1391 (1996).
- Kuga T, Kohda K, Hirayama Y et al. Pulmonary veno-occlusive disease accompanied by microangiopathic hemolytic anemia 1 year after a second bone marrow transplantation for acute lymphoblastic leukemia. Int. J. Hematol.64(2), 143–150 (1996).
- Troussard X, Bernaudin JF, Cordonnier C et al. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax39(12), 956–957 (1984).
- Salzman D, Adkins DR, Craig F, Freytes C, LeMaistre CF. Malignancy-associated pulmonary veno-occlusive disease: report of a case following autologous bone marrow transplantation and review. Bone Marrow Transplant.18(4), 755–760 (1996).
- Seguchi M, Hirabayashi N, Fujii Y et al. Pulmonary hypertension associated with pulmonary occlusive vasculopathy after allogeneic bone marrow transplantation. Transplantation69(1), 177–179 (2000).
- Trobaugh-Lotrario AD, Greffe B, Deterding R, Deutsch G, Quinones R. Pulmonary veno-occlusive disease after autologous bone marrow transplant in a child with stage IV neuroblastoma: case report and literature review. J. Pediatr. Hematol. Oncol.25(5), 405–409 (2003).
- Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant.41(8), 677–686 (2008).
- Wright JL, Tai H, Churg A. Cigarette smoke induces persisting increases of vasoactive mediators in pulmonary arteries. Am. J. Respir. Cell. Mol. Biol.31(5), 501–509 (2004).
- Wright JL, Tai H, Churg A. Vasoactive mediators and pulmonary hypertension after cigarette smoke exposure in the guinea pig. J. Appl. Physiol.100(2), 672–678 (2006).
- Rosenzweig EB, Morse JH, Knowles JA et al. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J. Heart Lung Transplant.27(6), 668–674 (2008).
- Sztrymf B, Coulet F, Girerd B et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am. J. Respir. Crit. Care Med.177(12), 1377–1383 (2008).
- Humbert M. Update in pulmonary hypertension 2008. Am. J. Respir. Crit. Care Med.179(8), 650–656 (2009).
- Runo JR, Vnencak-Jones CL, Prince M et al. Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am. J. Respir. Crit. Care Med.167(6), 889–894 (2003).
- Machado RD, Aldred MA, James V et al. Mutations of the TGF-β type II receptor BMPR2 in pulmonary arterial hypertension. Hum. Mutat.27(2), 121–132 (2006).
- Aldred MA, Vijayakrishnan J, James V et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum. Mutat.27(2), 212–213 (2006).
- Davies P, Reid L. Pulmonary veno-occlusive disease in siblings: case reports and morphometric study. Hum. Pathol.13(10), 911–915 (1982).
- Voordes CG, Kuipers JR, Elema JD. Familial pulmonary veno-occlusive disease: a case report. Thorax32(6), 763–766 (1977).
- Girerd B, Montani D, Coulet F et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am. J. Respir. Crit. Care Med.181(8), 851–861 (2010).
- Dufour B, Maitre S, Humbert M, Capron F, Simonneau G, Musset D. High-resolution CT of the chest in four patients with pulmonary capillary hemangiomatosis or pulmonary venoocclusive disease. AJR Am. J. Roentgenol.171(5), 1321–1324 (1998).
- Resten A, Maitre S, Humbert M et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am. J. Roentgenol.183(1), 65–70 (2004).
- Rabiller A, Jais X, Hamid A et al. Occult alveolar haemorrhage in pulmonary veno-occlusive disease. Eur. Respir. J.27(1), 108–113 (2006).
- Di Biase L, Fahmy TS, Wazni OM et al. Pulmonary vein total occlusion following catheter ablation for atrial fibrillation: clinical implications after long-term follow-up. J. Am. Coll. Cardiol.48(12), 2493–2499 (2006).
- Golde DW, Drew WL, Klein HZ, Finley TN, Cline MJ. Occult pulmonary haemorrhage in leukaemia. Br. Med. J.2(5964), 166–168 (1975).
- Galie N, Hoeper MM, Humbert M et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J.34(6), 1219–1263 (2009).
- Galie N, Hoeper MM, Humbert M et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J.30(20), 2493–2537 (2009).
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N. Engl. J. Med.351(14), 1425–1436 (2004).
- Rambihar VS, Fallen EL, Cairns JA. Pulmonary veno-occlusive disease: antemortem diagnosis from roentgenographic and hemodynamic findings. Can. Med. Assoc. J.120(12), 1519–1522 (1979).
- Fesler P, Pagnamenta A, Vachiery JL et al. Single arterial occlusion to locate resistance in patients with pulmonary hypertension. Eur. Respir. J.21(1), 31–36 (2003).
- Souza R, Amato MB, Demarzo SE et al. Pulmonary capillary pressure in pulmonary hypertension. Crit. Care9(2), R132–R138 (2005).
- Gaar KA Jr, Taylor AE, Owens LJ, Guyton AC. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am. J. Physiol.213(4), 910–914 (1967).
- Grimbert FA. Effective pulmonary capillary pressure. Eur. Respir. J.1(4), 297–301 (1988).
- Cope DK, Allison RC, Parmentier JL, Miller JN, Taylor AE. Measurement of effective pulmonary capillary pressure using the pressure profile after pulmonary artery occlusion. Crit. Care Med.14(1), 16–22 (1986).
- Sitbon O, Humbert M, Jais X et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation111(23), 3105–3111 (2005).
- Montani D, Savale L, Natali D et al. Long-term response to calcium-channel blockers in non-idiopathic pulmonary hypertension. Eur. Heart J.31(15), 1898–1907 (2010).
- Montani D, Jaïs X, Dorfmuller P, Simonneau G, Sitbon O, Humbert M. Goal-oriented therapy in pulmonary veno-occlusive disease: a word of caution. Eur. Respir. J.34(5), 1204–1206 (2009).
- McLaughlin VV, Archer SL, Badesch DB et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Coll. Cardiol.53(17), 1573–1619 (2009).
- Rubin LJ; ACoC Physicians. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest126(Suppl. 1), 7S–10S (2004).
- Swensen SJ, Tashjian JH, Myers JL et al. Pulmonary venoocclusive disease: CT findings in eight patients. AJR Am. J. Roentgenol.167(4), 937–940 (1996).
- Elliott CG, Colby TV, Hill T, Crapo RO. Pulmonary veno-occlusive disease associated with severe reduction of single-breath carbon monoxide diffusing capacity. Respiration53(4), 262–266 (1988).
- Matthews AW, Buchanan R. A case of pulmonary veno-occlusive disease and a new bronchoscopic sign. Respir. Med.84(6), 503–505 (1990).
- Ohmichi M, Tagaki S, Nomura N, Tsunematsu K, Suzuki A. Endobronchial changes in chronic pulmonary venous hypertension. Chest94(6), 1127–1132 (1988).
- Wahidi MM, Rocha AT, Hollingsworth JW, Govert JA, Feller-Kopman D, Ernst A. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. A survey of pulmonologists and review of the literature. Respiration72(3), 285–295 (2005).
- Wagenvoort CA, Wagenvoort N. The pathology of pulmonary veno-occlusive disease. Virchows Arch. A Pathol. Anat. Histol.364(1), 69–79 (1974).
- Chazova I, Robbins I, Loyd J et al. Venous and arterial changes in pulmonary veno-occlusive disease, mitral stenosis and fibrosing mediastinitis. Eur. Respir. J.15(1), 116–122 (2000).
- Jais X, Launay D, Yaici A et al. Management of lupus and mixed connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum.58, 521–531 (2008).
- Sanchez O, Sitbon O, Jais X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest130(1), 182–189 (2006).
- Alam S, Palevsky HI. Standard therapies for pulmonary arterial hypertension. Clin. Chest Med.28(1), 91–115; VIII (2007).
- Barst RJ, Rubin LJ, Long WA et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N. Engl. J. Med.334(5), 296–302 (1996).
- Sitbon O, Humbert M, Nunes H et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J. Am. Coll. Cardiol.40(4), 780–788 (2002).
- Montani D, O’Callaghan D, Jaïs X et al. Implementing the ESC/ERS pulmonary hypertension guidelines: real-life cases from a national referral centre. Eur. Respir. Rev.18(114), 231–249 (2009).
- Okumura H, Nagaya N, Kyotani S et al. Effects of continuous IV prostacyclin in a patient with pulmonary veno-occlusive disease. Chest122(3), 1096–1098 (2002).
- Resten A, Maitre S, Humbert M et al. Pulmonary arterial hypertension: thin-section CT predictors of epoprostenol therapy failure. Radiology222(3), 782–788 (2002).
- Davis LL, deBoisblanc BP, Glynn CE, Ramirez C, Summer WR. Effect of prostacyclin on microvascular pressures in a patient with pulmonary veno-occlusive disease. Chest108(6), 1754–1756 (1995).
- Montani D, Jaïs X, Price LC et al. Cautious epoprostenol therapy is a safe bridge to lung transplantation in pulmonary veno-occlusive disease. Eur. Respir. J.34(6), 1348–1356 (2009).
- Shackelford GD, Sacks EJ, Mullins JD, McAlister WH. Pulmonary venoocclusive disease: case report and review of the literature. AJR Am. J. Roentgenol.128(4), 643–648 (1977).
- Hoeper MM, Eschenbruch C, Zink-Wohlfart C et al. Effects of inhaled nitric oxide and aerosolized iloprost in pulmonary veno-occlusive disease. Respir. Med.93(1), 62–64 (1999).
- Kuroda T, Hirota H, Masaki M et al. Sildenafil as adjunct therapy to high-dose epoprostenol in a patient with pulmonary veno-occlusive disease. Heart Lung Circ.15(2), 139–142 (2006).
- Barreto AC, Franchi SM, Castro CR, Lopes AA. One-year follow-up of the effects of sildenafil on pulmonary arterial hypertension and veno-occlusive disease. Braz. J. Med. Biol. Res.38(2), 185–195 (2005).
- Gilroy RJ Jr, Teague MW, Loyd JE. Pulmonary veno-occlusive disease. Fatal progression of pulmonary hypertension despite steroid-induced remission of interstitial pneumonitis. Am. Rev. Respir. Dis.143(5 Pt 1), 1130–1133 (1991).
- Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur. Respir. J.22(2), 358–363 (2003).
- Sanchez O, Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary hypertension secondary to connective tissue diseases. Thorax54, 273–277 (1999).
- Sitbon O, Humbert M, Simonneau G. Primary pulmonary hypertension: current therapy. Prog. Cardiovasc. Dis.45(2), 115–128 (2002).
- Cassart M, Gevenois PA, Kramer M et al. Pulmonary venoocclusive disease: CT findings before and after single-lung transplantation. AJR Am. J. Roentgenol.160(4), 759–760 (1993).
- Montani D, Kemp K, Dorfmuller P, Sitbon O, Simonneau G, Humbert M. Idiopathic pulmonary arterial hypertension and pulmonary veno-occlusive disease. Semin. Respir. Crit. Care Med.30, 411–420 (2009).
Pulmonary veno-occlusive disease: advances in clinical management and treatments
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/expertrespiratory. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 40-year-old previously healthy man who complains of 4 months of progressive dyspnea. He now has some bilateral lower extremity swelling as well. Basic pulmonary function testing was normal and chest radiography demonstrated enlargement of the pulmonary arteries. You suspect pulmonary arterial hypertension (PAH) or possibly PVOD (pulmonary veno-occlusive disease). Which of the following statements regarding the epidemiology of PVOD is most accurate?
□ A It represents approximately 5%-10% of cases of idiopathic PAH
□ B It is almost always diagnosed among older adults
□ C It is more common among men
□ D It is less associated with tobacco exposure compared with idiopathic PAH
2. Which diagnostic test is least indicated in cases of PVOD?
□ A Transthoracic echocardiography
□ B High-resolution CT
□ C Bronchoalveolar lavage
□ D Acute vasodilatory testing
3. Which of the following findings would be most helpful in differentiating PVOD from PAH in this patient?
□ A Lower New York Heart Association (NYHA) class
□ B Normal diffusing capacity of the lung for carbon monoxide (DLCO)
□ C Centrilobular ground glass opacities and septal lines on high-resolution CT
□ D A pulmonary capillary wedge pressure (Ppcw) over 25 mm Hg
4. The patient is diagnosed with hypoxia due to probable PVOD. Which of the following treatments is most likely to make a significant difference in his disease course?
□ A Prostacyclins
□ B Corticosteroids
□ C Cyclophosphamide
□ D Lung transplant
