Abstract
The vaccine landscape has changed considerably over the last decade with many new vaccines and technological developments, unprecedented progress in reaching out to children and the development of new financing mechanisms. At the same time, there are more demands and additional expectations of national policy makers, donors and other interested parties for increased protection through immunization. The Global Immunization Vision and Strategy (GIVS), which broadens the previous scope of immunization efforts, sets a number of goals to be met by countries. The WHO has recently reviewed and adjusted both its policy making structure and processes for vaccines and immunization to include an enlarged consultation process to generate evidence-based recommendations, thereby ensuring the transparency of the decision making process and improving communications. This article describes the process of development of immunization policy recommendations at the global level and some of their impacts. It focuses on the roles and modes of operating of the Strategic Advisory Group of Experts on immunization, which is the overarching advisory group involved with the issuance of policy recommendations, monitoring and facilitating the achievement of the GIVS goals. The article also describes the process leading to the publication of WHO vaccine position papers, which provide WHO recommendations on vaccine use. WHO vaccine-related recommendations have become a necessary step in the pathway to the introduction and use of vaccines, especially in developing countries and, consequently, have a clear and significant impact.
WHO mandate & goals
WHO’s role & mandate
The WHO’s Constitution, signed by 193 Member States, recognizes WHO as a UN specialized agency Citation[1] whose objective is the attainment by all peoples of the highest possible level of health Citation[2]. The Constitution mandates WHO to set standards and formulate global health policy recommendations. Article 2 of the Constitution states that the organization shall act as the directing and coordinating authority on international health work and shall establish and maintain effective collaboration with the UN, specialized agencies, governmental health administrations, professional groups and such other organizations as may be deemed appropriate. It specifically stresses the need to:
• Promote cooperation among scientific and professional groups that contribute to the advancement of health;
• Provide information, counsel and assistance in the field of health;
• Assist in developing an informed public opinion among all peoples on matters of health;
• Develop, establish and promote international standards with respect to food, biological, pharmaceutical and similar products.
In May 1974, the World Health Assembly Citation[3] requested that WHO provide technical advice on the use of vaccines and assist countries in developing suitable programs. This led to the Expanded Program on Immunization (EPI), whose aim was to use available immunization tools to produce the maximum impact on avoidable mortality. The initial phase of this program focused on extending immunization to cover a maximum number of infants and pregnant women with the vaccines available at that time. This resulted in a rapid improvement in global immunization coverage Citation[4]. In the 1990s, global immunization coverage in excess of 70% was maintained with basic EPI vaccines (diphtheria, tetanus, polio, pertussis, measles, BCG, yet this success masked large disparities between and within countries with millions of children left exposed to potentially fatal childhood diseases Citation[4].
This led to a new vision, driven by the considerable changes in the field of immunization, including an increasing demand for vaccines, rapid progress in availability of new vaccines and technological developments, continuing health-sector development, increasing awareness of the vulnerability to pandemics and other health emergencies and more potential opportunities for partnerships Citation[5].
In 2005, the 58th World Health Assembly, recognizing the value of immunization and the role that vaccines and immunization can play in reducing mortality in individuals under 5 years of age and the attainment of the Millennium Development Goals, welcomed the Global Immunization Vision and Strategy (GIVS) 2006–2015, which was developed by WHO and UNICEF as a framework for strengthening national immunization programs Citation[6,7]. The goal of GIVS is to protect as many people as possible against a larger number of diseases by expanding the reach of immunization to every eligible person and ensuring that immunization is high on every health agenda. GIVS aims to increase, or at least sustain, very high levels of vaccine coverage for all age groups, to introduce new vaccines and to link immunization with the delivery of other health interventions. GIVS acknowledges that immunization can benefit from, and contribute to, the development of the health sector and help overcome system-wide barriers. The vision was inspired by seven guiding principles, which include exclusive reliance on assured quality and safe products and services, as well as policies and strategies based on evidence and best practices. These principles are reflected in the vision’s global goals (see Box 1).
Goals & nature of WHO recommendations
WHO recommendations for vaccine use are of both a scientific and strategic nature and are intended primarily for Member States, specifically for the government agencies responsible for decision making, implementation of immunization programs, surveillance of vaccine-preventable diseases, vaccine safety and licensing, and National Immunization Technical Advisory Groups (NITAGs). Recommendations are also useful for international professional associations, nonprofit organizations, bilateral and multilateral donor agencies, and international organizations such as UNICEF and the Global Alliance for Vaccines and Immunization (GAVI) Alliance to help adjust country programs and assistance, including vaccine procurement. The recommendations are also of interest to the pharmaceutical industry. A robust and clear policy process would mean that the global priorities for vaccine development are recognized and the investments by donors aligned with these priorities, and that industry innovation and production focuses on needed vaccines presented in a relevant formulation. Any gaps in the process may result in costly mistakes and delays in implementing a public health intervention that could have major benefits.
Global recommendations are particularly needed in the context of global efforts for disease control such as pandemic influenza, or disease eradication as in the case of the global polio eradication initiative. During the A (H1N1) 2009 pandemic, a small number of industrialized countries had access to most of the global vaccine output over the next 12 months as advanced-purchase agreements limited availability for the rest of the world (especially developing countries). The decision by some national regulatory authorities only to license nonadjuvanted higher antigen content pandemic vaccine rather than antigen-sparing products further limited the global production capabilities Citation[8].
Polio eradication requires that countries achieve a high level of population immunity through routine and supplementary immunization activities. Low immunization rates and resulting outbreaks at country level pose a serious threat to nonimmune children and adults throughout the world. This threat has increased tremendously with the rapid and continuing development of international travel and mass population movements. In 2009, 19 countries previously considered polio-free reported cases and outbreaks caused by imported viruses emerging from Nigeria Citation[9].
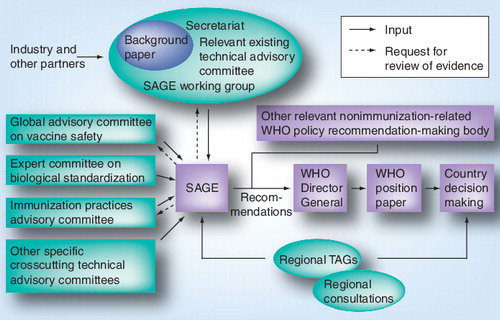
Procedure for the formulation of global recommendations: the immunization policy advisory framework
Formulating recommendations involves a systematic effort to gather scientific evidence, which is then considered carefully by the best experts. WHO uses its convening power to receive recommendations from independent external advisory committees comprising experts from various geographical and institutional backgrounds. The experts act in their own capacity, not on behalf of the countries or organizations they come from. They are not paid for this work and receive no personal benefit. The committees’ deliberations are issued in the form of advice to the WHO Director-General or her representatives. WHO then uses this advice to promulgate WHO immunization policy recommendations.
Since 2005, WHO has aimed to strengthen its normative and policy-setting functions for immunization and increase the acceptance of WHO policy recommendations on vaccines and immunization. It therefore made adjustments to its immunization-related advisory committees and their processes. This entailed amending the number and terms of reference of the committees, optimizing their coordination, and improving the mode of operating of the committees with particular emphasis on evidence-based decision making and transparency to enhance credibility and impact.
The main group involved with the development of global policy recommendations and strategic advice related to vaccines and immunization to WHO is the Strategic Advisory Group of Experts (SAGE). SAGE also provides support for regional and national programs through its development of immunization norms and good practices. Established in 1999 through the merging of two previous committees, notably the Scientific Advisory Group of Experts (which served the Program for Vaccine Development) and the Global Advisory Group (which served the EPI program), SAGE was restructured in 2005. Its activities and modes of operating were then adjusted to suit the requirements of WHO’s GIVS Citation[6]. The mandate of SAGE now extends to all vaccine-preventable diseases throughout all age groups Citation[10]. SAGE provides recommendations on issues ranging from research and development to vaccine administration and linkage with other health interventions.
Specifically, SAGE advises on:
• Major issues and challenges to be addressed with respect to achieving the goals of GIVS;
• The adequacy of progress towards the achievement of the goals of the GIVS;
• Immunization program response to current public health priorities;
• Policies, goals and targets including those related to vaccine research and development;
• Adequacy of WHO’s strategic plan and priority activities to achieve the GIVS goals considering the comparative advantages and respective roles of partner organizations;
• Cross-departmental activities and initiatives related to vaccine and immunization technologies, strategies and linkages with other health interventions;
• Engagement of WHO in partnerships that will enhance achievement of global immunization goals.
WHO immunization-related policy recommendations, including those in the WHO position papers on vaccines, follow the advisory processes established through/for SAGE. These position papers are summaries of information about licensed vaccines of public health interest, which are based on an extensive review and ranking of evidence by experts, and include inputs from interested stakeholders including industry. They are designed to be used by immunization and public health staff to make decisions about the public health value and use of specific vaccines. summarizes the pathways for the issuance of WHO recommendations. Over the past 5 years, SAGE has provided recommendations to WHO on the use of tetanus, Haemophilus influenza type b, rotavirus, mumps, Japanese encephalitis, pneumococcal conjugate and polysaccharide, BCG, rabies, human papillomavirus, typhoid, hepatitis B, measles, poliomyelitis, cholera and pertussis vaccines. These were used to develop new, or update previous, WHO position papers Citation[11–27]. Guidance was also provided on the use of H5N1 Citation[28] and H1N1 pandemic influenza vaccines Citation[8].
A number of technical advisory committees complement and support the work of SAGE. They cover a wide range of issues including technical analysis and guidance, development of norms and standards, vaccine safety, global research and vaccine design. The main groups are the Global Advisory Committee on Vaccine Safety (GACVS), the Expert Committee on Biological Standardization (ECBS), the Immunization Practice Advisory committee (IPAC), and the Quantitative Immunization and Vaccine Research Advisory Committee.
The ECBS was established in 1947 to set norms and standards for the manufacturing, licensing and control of biological products in order to guarantee the quality of vaccines and other biological products. ECBS is commissioned by the WHO to establish detailed recommendations and guidelines for the manufacturing, licensing and control of blood products, cell regulators, vaccines and related in vitro diagnostic tests. The committee also develops and disseminates reference preparations (i.e., international standard materials that are used as reference materials by manufacturers and regulatory authorities to calibrate regional, national or in-house working standards and which often form the basis for licensing and batch release Citation[29]). Historically, standards were established after a new vaccine had been licensed, but ECBS is now more proactive and steps in at the beginning of the production cycle. ECBS recommendations are published in the WHO Technical Report Series (more information on the committee is available at Citation[101]).
The GACVS was established in 1999 to respond promptly to vaccine safety issues of potential global importance. The committee does not directly determine immunization policies, but it does express its scientific opinion on vaccine safety, which could result in policy changes Citation[30]. The committee evaluates vaccine safety by thoroughly reviewing the latest developments in basic science, epidemiology and clinical practice. The committee works in close cooperation with relevant stakeholders, including experts from national authorities, academic institutions and the pharmaceutical sector. The committee is at liberty to request, monitor and evaluate specific studies that seek to explore a possible link between vaccines or their components and adverse effects. The impartiality of the committee is essential. While GACVS focuses on risk assessment, SAGE deals with risk management. GACVS has, on occasion, found that the alleged harmfulness of certain vaccines to be unsubstantiated, yet has also promptly recognized, when the evidence was clear, the link between a given vaccine and particular adverse effects Citation[31]. In addition to the reports published in the Weekly Epidemiological Record, emphasis is placed on making information available promptly via the website where all the committee’s findings can be consulted Citation[102].
The IPAC was established in June 2010 and represents an expansion of the mandate for the earlier Technologies and Logistics Advisory Committee Citation[32,103]. IPAC’s mandate is to advise WHO on the formulation of immunization strategies and operational standards, the tools and technologies necessary to reach and sustain high levels of immunization coverage as required in GIVS, and to promote immunization services of high quality. IPAC’s main focus is on practices at an operational and procedural level. The recommendations of IPAC will need to be endorsed by SAGE.
The Quantitative Immunization and Vaccine Research Advisory Committee advises WHO on the estimations of the burden of vaccine-preventable diseases, modeling of vaccine interventions, economic evaluations of vaccines, immunizations, related technologies and interventions, and analytical components of operational and implementation research Citation[104].
Technical advisory groups on immunization have also been established in each of the six WHO regions (Africa, the Americas, the Eastern Mediterranean, Europe, Southeast Asia and the Western Pacific). While names differ between the Regions (Task Force on Immunization, Technical Consultative Group, and European Technical Advisory Group of Experts, Technical Advisory Group), the functions of these groups are essentially similar. They provide WHO Regional Directors and countries in the respective regions with recommendations on regional immunization priorities and strategies in light of particular regional epidemiological and social issues. Recommendations from these groups are also brought to the attention of Regional Committees, the regional equivalents to the global World Health Assembly. These groups make regional recommendations or recommendations at a national or local level that countries should follow.
Countries have autonomy for decision making regarding their national policies and strategies in the light of existing problems and allowing for optimal solutions to be specifically adapted. Countries are responsible for implementing their own national programs and monitoring the resulting impact. Key to improving routine immunization programs and introducing new vaccines and immunization technologies is for countries to ensure that they have the necessary evidence and clear processes to enable informed decision making. Similarly, such evidence and processes are needed to justify the continuation of, or any necessary adjustments to, existing immunization programs and policies. At the global level, the goal is therefore not to prescribe rigid recommendations or immunization schedules that all programs must follow, but rather to offer a framework that countries can adapt to existing schedules and local epidemiological, economical and other circumstances in the context of other health priorities Citation[29].
The majority of industrialized and an increasing number of developing countries have established national technical advisory bodies to guide their immunization policies; other countries are working towards or contemplating the establishment of such bodies. These advisory bodies are often referred to as NITAGs. NITAGs are committees involving national experts supplying guidance to policy makers and program managers to enable them to make evidence-based immunization-related policy and program decisions. One of WHO’s priorities is the supporting of the establishment/strengthening of NITAGs that can convert global or regional policy recommendations into national policy. This is part of the process to ensure evidence-based decision making at country level, which is particularly needed in view of the complexity of the immunization programs and the cost of new vaccines Citation[33].
Strategic Advisory Group of Experts
Composition & membership selection process
SAGE has 15 members, who are renowned immunization, vaccine and public health experts from around the world. Members serve in their personal capacity and represent a broad range of disciplines encompassing many aspects of immunization and vaccines, for example, epidemiology, public health, vaccinology, pediatrics, internal medicine, infectious diseases, immunology, drug regulation, program management, immunization delivery, healthcare administration, health economics and vaccine safety Citation[105].
The membership of SAGE also reflects a spectrum of professional affiliation (e.g., academia, clinical practice, research institutes and governmental agencies including national immunization programs, public health departments and regulatory authorities), the three strategic areas of WHO’s work relating to immunization (accelerating innovation, ensuring quality and safety, and maximizing access and links with other health interventions), and geographical and diversity balance.
SAGE undergoes a regular rotation of membership. Members are appointed to serve an initial term of 3 years, which can only be renewed once. Periodic public calls for nominations are issued. After determination of eligibility, nominations are submitted to an independent selection panel including representatives of key partner organizations. From the pool of nominees, the panel identifies the most suitable members on the basis of their qualifications, ability to contribute to the accomplishment of SAGE’s objectives and consideration of the expertise already available in the group. Those members are then proposed for appointment by the WHO Director-General. Preference is given to experts with a wider scope of expertise.
SAGE uses a rigorous process to manage potential conflicts of interest and regularly looks for ways to improve its procedures. Prior to being appointed as SAGE members and prior to renewal of a term, nominees and current SAGE members are required to complete a declaration of interest using a standard form. Individuals with a potential conflict of interest that could affect the impartiality and independence of their advice will not be retained for membership. Members of SAGE update their declarations of interest regularly (i.e., ahead of each 6-monthly meeting). The WHO Secretariat consults with the SAGE Chairperson to discuss any interests that are disclosed and a decision is taken on appropriate measures. If members have interests that are relevant to a meeting, the interests are disclosed to the group, and members may be excluded from discussions or decision making on those topics. Potential conflicts of interests, however, are rare as early screening of personal and professional interests prevent conflicts from arising. A register of members’ interests is maintained by WHO and summaries of members’ interests relevant to the meeting’s topics are published on the website. Although serving on SAGE represents a significant time commitment, SAGE members are not remunerated for their participation on SAGE. Only meeting-related travel expenses are covered by WHO in accordance with the organization’s rules.
Functioning of SAGE & conduct of meetings
SAGE normally meets twice annually in April and November. The frequency of meetings may, however, be adjusted as necessary. For example, an extraordinary meeting occurred in July 2009 to deal with urgently needed advice on vaccination against the influenza A (H1N1) 2009 pandemic Citation[8]. Regular meetings run normally over 2–3 days.
SAGE deliberations are undertaken in an open forum with a view to ensure transparency of the decision-making process. Decisions and recommendations are, as a rule, taken by consensus. UNICEF, the Secretariat of the GAVI Alliance, and WHO Headquarters and Regional Offices, Chairs of WHO regional technical advisory groups and of other important WHO headquarters’ technical advisory groups participate as observers in SAGE meetings.
The WHO invites other observers to SAGE meetings, including representatives from international professional organizations (such as the International Pediatric Society, the World Medical Association and the International Council of Nurses), other nongovernmental organizations (such as Médecins Sans Frontières and OXFAM International), technical agencies (such as the US CDC, the UK Health Protection Agency and the European CDC), donor organizations, country representatives, vaccine manufacturers’ associations, immunization technologies and other industry experts.
Additional and specific contributions may be elicited, as appropriate, to contribute expert information on agenda items for which the appropriate expertise is not held by SAGE.
The participation of the many organizations mentioned above and involved in immunization is important. There is full transparency to all of the available evidence and scope of discussion. This helps build credibility and facilitates the ‘buy in’ by organizations and countries. Representatives from the various institutions may also bring valuable contributions to the discussion including submitting the views from their respective organizations. The Chair invites participants to make comments to ensure that there is no undue influence nor imbalance in contributions during the meeting. The decisions on any recommendations rest with SAGE members. At the end of each session, the Chair summarizes the key points made by SAGE members, proposes conclusions, and calls for any objection or suggestions for modifications from members to the proposed summaries/conclusions. The conclusions and recommendations are adapted until there is consensus among members.
The SAGE Chair briefs the WHO Director-General after the meeting and within 2 months of the meetings, the conclusions and recommendations are published in WHO’s Weekly Epidemiological Record. Initially published in English and French, reports are also translated in the additional four official WHO languages, that is Arabic, Chinese, Russian and Spanish and are posted on the WHO website. WHO recommendations are also actively disseminated to the intended target audiences and particularly to country-level officials. The SAGE recommendations are shared promptly with national immunization managers and regional technical advisory groups.
Development of recommendations & the basis for decision making
In advance of its deliberations, SAGE is provided with reviews of the evidence and background documentation. Some topics do require a preceding review of the evidence by some of the technical advisory committees, such as a review of vaccine safety issues by the GACVS. A comprehensive background paper may be prepared as was the case for discussions on the use of new vaccines against the human papillomavirus Citation[106]. When questions for SAGE are particularly focused, such as the updating of a recommendation on the specific route of administration for rabies vaccine or for deciding on the need for a second routine dose of measles vaccine, then SAGE is presented with the specific relevant evidence. SAGE is provided with both published and as yet unpublished evidence.
There are three models for the preparation of background information and evidence review by SAGE, specifically through work done by the WHO Secretariat, the work of an existing relevant technical advisory committee, or through a SAGE working group. The latter has become a more common route for consideration of more complex issues. As of June 2010, there were seven SAGE working groups: H5N1 influenza, pertussis, meningococcal, rubella, hepatitis A, measles and seasonal influenza vaccines.
Working groups are established on a time-limited basis. They review and provide evidence-based information and options for recommendations together with implications of the various options that will then be discussed openly by SAGE Citation[107]. The need and charge for a working group are discussed and agreed upon during SAGE meetings or at the preparatory teleconferences. Each working group operates under specific terms of reference developed jointly by SAGE and the Secretariat. Each group is composed of two or more SAGE members (one of whom functions as the working group Chair), and additional appropriate experts. Representatives of partners’ organizations and members of regional technical consultative groups may be included. SAGE members and experts who have topic-specific conflicts of interest cannot serve on the working groups. Public calls are made for the identification of experts to serve on the working groups. WHO staff serve as Secretariat for the working groups.
The SAGE working groups do not submit consensus advice or recommendations directly to WHO but are accountable to SAGE. Working group Chairs, other working group members, and working groups per se are not empowered to speak on behalf of SAGE. Rather, they are utilized by SAGE to gather and organize information upon which SAGE can deliberate and act. Thus, while working groups should examine an area in detail and define the issues, including the development of options for recommendations, the actual processes culminating in development of recommendations must occur in the open public forum of SAGE meetings.
In making its recommendations, SAGE takes into consideration issues such as disease epidemiology (disease burden including age specific mortality, morbidity and societal impact; projections for future disease burden; specific risk groups; epidemic potential; disease occurrence over time; serogroup or serotype distribution for serogroup or serotype-specific vaccines; and changes in epidemiology over time), clinical characteristics (clinical management of disease; disease severity; primary/secondary/tertiary care implications; long-term complications of disease; and medical requirements), vaccine and immunization characteristics (efficacy; effectiveness and population impact of vaccine; indirect effects; vaccine safety; cold chain and logistics concerns; vaccine availability and supply; vaccine markets and demands; vaccine schedules; schedule acceptability; and ability to deliver), economic considerations (disease; vaccine and vaccine delivery costs; perspective for vaccine price reduction; vaccine cost and cost–effectiveness of immunization programs; and affordability of immunization), health system opportunities and the existence of, and interaction with, other existing intervention and control strategies.
A careful and critical appraisal of the scientific evidence is a necessary step in recommendation and guidance development. A strong evidence base, when available, is critical to ensure the most appropriate recommendations. While the evidence reviewed is the result of scientific endeavors, evaluating the quality of the evidence and making recommendations are activities that require expert interpretation and judgement. In addition to the results of data themselves, consideration should be given to the methodology and study design used to conduct such studies. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach is one of many frameworks developed over the years to assess the quality of evidence Citation[34]. In April 2007, SAGE adopted the use of the GRADE methodology to score the quality of evidence in support of key recommendations included in the WHO vaccine position papers.
When information is lacking, SAGE may make provisional recommendations and request the WHO and international community at large to initiate specific research projects. In the absence of specific evidence, in urgent situations, SAGE may also have to make recommendations relying mostly on expert judgement.
In an attempt to minimize delays between vaccine availability and issuance of recommendations on vaccine use, it is important that SAGE anticipates the availability of new vaccines and identifies any gaps in knowledge that may prevent timely recommendations being made.
SAGE’s work needs to be coordinated with other possible preventative interventions and policies to control diseases, for example, immunization against human papillomavirus as part of cervical cancer prevention and future immunization against malaria as part of malaria control programs. Careful consideration was given in 2009 to integration of Intermittent Preventative Treatment of malaria in infants when given at the routine EPI-scheduled immunizations Citation[35].
After each meeting, presentations delivered at the SAGE meeting are made available on the SAGE website together with relevant background documents.
Scope of SAGE’s work & agenda setting
Agenda items include topics presented for information purposes, for discussion or for decision. Each meeting’s agenda is composed of both recurrent items that are mostly for information and a series of six to ten specific items for decision. For the latter, a SAGE output and recommendation is normally expected but such items can also be for information, such as vaccine horizon scanning to help the committee keep abreast of new developments.
Recurrent agenda items include reports from: WHO headquarters, key advisory committees, the GAVI Alliance and from the WHO regions. The WHO report highlights progress in the implementation of previous recommendations. The Secretariat keeps a tracking sheet of all SAGE’s recommendations that apply to the Secretariat Citation[108]. This tracking sheet is regularly updated and highlights the key actions taken in response to the recommendations and their progress. At each meeting, three of the six WHO regional offices deliver reports on their situation, their challenges and their progress; in this way, each region is reviewed annually. SAGE is also informed of policies and recommendations set by the WHO Regional technical advisory groups. These reports are essential to keep SAGE abreast of key local issues, priorities, progress and challenges in the implementation of its recommendations.
Specific topics reviewed by SAGE over each previous year fall under two broad categories: recommendations on vaccine use, and strategic issues that can relate to new or existing vaccines, vaccine delivery/operational issues, financial sustainability and surveillance.
SAGE works with WHO to develop its priorities of work and agendas for forthcoming meetings. The views of countries, regions and partners are solicited. Requests for advice from countries are generally channelled through the regional technical advisory groups and regional offices. A 2–3-year ‘horizon list’ of items for SAGE discussion is maintained by the Secretariat.
In view of the limited number of topics that can be discussed at any given meeting, the final list of agenda items requires both consideration of the importance and urgency of the expected output from SAGE and the level of readiness that would lead to a fruitful session. If critical pieces of evidence are lacking or the necessary compilation and review of evidence cannot be achieved in good time, ahead of the meeting, the related session will be postponed. Preparation for a session may require anywhere from 2 months to several years.
The final list of agenda items is normally settled 2 months ahead of each regular biennial SAGE meeting during the first of two preparatory teleconferences. These teleconferences take place 2 months and 1 month prior to dates set for the meetings.
A list of topics discussed by SAGE is available on the web through the agenda search tool Citation[109].
Vaccine position papers
Since 1998, WHO regularly produces and updates evidence-based vaccine position papers that summarize information on available licensed vaccines against infectious diseases of public health interest. These papers are concerned primarily with vaccines used in large-scale immunization programs. The format of these papers has been adjusted over time and they now contain four sections: an introduction, a section providing information on the respective disease (disease epidemiology, the pathogen, the disease), a section providing information on the available vaccines (composition, safety, immune response, efficacy and effectiveness, cost–effectiveness and any other relevant issue), and the WHO position on the optimal vaccine use.
The position papers are produced for use mainly by national public health officials and immunization program managers. However, they may also be of interest to international funding agencies, the vaccine manufacturing industry, the medical community and the scientific media.
The papers are drafted or updated based on an extensive literature review and are the result of a wide-ranging consultative process by various experts and interest groups both inside and outside the WHO. Initial drafts are sent for review by regional advisers, interested parties, world experts in the specific area covered by the vaccine, industry and SAGE members. Since April 2006, the drive for new or updated position papers has followed the discussions and recommendations of SAGE Citation[29].
Grading tables that assess the quality of the evidence are also developed and are posted on the website. These tables are referenced in the position papers and follow the GRADE approach Citation[34].
The position papers are prepared in English, published in English and French in the Weekly Epidemiological Record of the WHO and are made available on the web (together with a list of key relevant references that have been used for the development or updating of the position papers). The position papers, like SAGE meeting reports, are subsequently translated into the other four WHO headquarters’ official languages. One page summaries and PowerPoint presentations summarizing the main content and recommendations from the vaccine position papers are also prepared and are posted on the website.
Contribution to achieving the global goals & impact of recommendations
Relevance of SAGE discussions to the achievement of global goals
The following are examples of agenda items discussed by SAGE that are of great importance in achieving the GIVS goals.
It has been estimated that 24 million children annually are not immunized or their immunizations are delayed and innovative ways are needed to reach them. Following a request from SAGE in November 2007, the results of detailed analyses of such children were discussed at the October 2009 meeting Citation[34]. The analyses by The Swiss Tropical Institute considered children who had received no vaccinations and those who had received one dose or more of any of the following vaccines (BCG, diphtheria–tetanus–pertussis, oral polio vaccine, and measles-containing vaccines) but were not fully immunized. The CDC performed a systematic review of peer-reviewed literature. IMMUNIZATIONbasics reviewed the gray literature (studies, reviews or reports written after 1980 that had not been published or were published in publications that are not peer reviewed) from studies in low-income and middle-income countries.
SAGE concluded that factors such as the distance from vaccination sites, the motivation of healthcare staff, lack of resources and false contraindications were key determinants for children remaining unvaccinated or undervaccinated. Demand-side factors, including family characteristics, parental attitudes and knowledge, the caregiver’s educational level and religious beliefs, also affected whether a child was immunized. The importance of understanding local determinants was emphasized. Operational research at the local level is important for understanding and addressing these gaps.
There have been several discussions on mortality reduction from measles with adjustment of immunization strategies based on the analysis of country experiences combined with mathematical modeling Citation[36,37]. SAGE has provided criteria that can be used by countries and regions to make rational decisions on: first, when to start a second dose of measles-containing vaccine delivered through routine services (routine MCV2); second, the optimal age of administration of routine MCV2; and third, when regular vaccination campaigns can be suspended in place of routine MCV2. SAGE has approved a comprehensive program of work to assess the feasibility of measles eradication and has also highlighted the need for resources both from WHO and from donors prior to setting a measles eradication goal Citation[34].
SAGE also issued recommendations on the use of new vaccines such as those against rotavirus infection and pneumococcal disease (discussed previously). Successful implementation of these vaccines has a major potential to contribute to the mortality reduction goals Citation[37,38].
In setting the future agenda, developing integrated strategies will be of increasing importance: examples are comprehensive approaches to disease control, be it for meningitis, pneumonia, diarrheal diseases, cancer or epidemic/pandemic prevention. In 2009, SAGE endorsed the co-administration of intermittent preventive treatment in infants for malaria at the same time as routine immunization visits, concluding that using immunization contacts to assist another child health program was a positive contribution to the well-being of children that would help develop and strengthen sustainable health services Citation[34].
SAGE has been involved in repeated discussions on the direct or indirect impact of the financing of immunization. One financing instrument for new vaccines is the Advanced Market Commitment (AMC). This involves a financial commitment being made by donors to subsidize vaccine demand by GAVI-eligible countries at a set purchase price as long as the vaccine in question meets a specific Target Product Profile (TPP). The goal of an AMC is to motivate suppliers and accelerate vaccine introduction Citation[39]. The TPP sets the minimal technical requirements for efficacy and safety that a candidate product must meet. SAGE endorsement of the TPP for pneumococcal conjugate vaccines was an essential step in the AMC process for that particular product. SAGE has also been concerned about the financing of vaccines for low–middle-income countries that are not eligible for GAVI support Citation[40].
Impact of WHO recommendations
Since the impact of WHO recommendations depend on so many external factors, determining those that are based on SAGE’s input and evaluating precisely their specific contribution to achieving the GIVS goals is not easy.
In industrialized countries, the introduction of Haemophilus influenza type b (Hib) vaccine more than 15 years ago has almost eliminated Hib-related disease. Despite a position paper in 1998 recommending its use Citation[41], by the end of 2005 only 65 of the world’s 156 nonindustrialized countries (42%) had introduced this vaccine Citation[42]. However, by the end of 2009, 154 states (80%) had introduced Hib vaccination. Multiple factors contributed to the accelerated introduction of new vaccines in the last 4 years Citation[43]. This included the reinforcement of SAGE’s recommendation on the use of the Hib vaccine in light of recent data, which led to the publication by WHO of a revised position statement recommending the global use of Hib vaccine even in the absence of local surveillance data Citation[12]. In low-income countries, the uptake of these new vaccines has been greatly facilitated by the recent assistance from the GAVI Alliance and the GAVI Fund and the advance of the GAVI Alliance-supported Hib Initiative Citation[43]. By the end of 2009, only 32 (16%) and 22 (11.5%) of 193 WHO Member States had introduced pneumococcal conjugate and rotavirus vaccines, respectively, in their routine immunization programs. SAGE has recommended the worldwide use of the pneumococcal conjugate vaccine Citation[38] and recommended the use of two recently licensed rotavirus vaccines Citation[37]. These recommendations helped to secure a commitment to support the introduction of these vaccines by the GAVI Alliance, which will enable them to be used in some of the world’s poorest countries. The impact of the SAGE recommendations will hopefully contribute to the wider utilization of these vaccines.
In 2008, an independent Stakeholder’s Panel was asked by the WHO to investigate the impact of policy recommendations and norms and standards on immunization set by the WHO. The panel’s mandate also included the effects of recommendations formulated by WHO key advisory committees, especially those of SAGE. The panel’s review was informed by a country survey aimed at understanding the impact of WHO guidance on vaccines and immunization on key national level decision makers and eliciting suggestions for improvement in content, communication and access Citation[110]. The panel concluded that “WHO vaccine advisory committees play an increasingly central role in determining global and national vaccine policy. In particular, SAGE recommendations have become a necessary step in the pathway to the introduction and use of vaccines, especially in developing countries and, as a consequence, have clear and significant impact.” The panel further commented that, “because policy recommendations are only part of an integrated process leading to successful immunization, it is not possible to enumerate specific children who have been successfully immunized because of the resulting improved vaccine advisory committees procedures and policies. The GAVI Alliance now predicates its actions on SAGE recommendations and WHO vaccine position papers. Countries, particularly developing countries, reported that WHO recommendations are central to their policy-making process. Evidence of SAGE recommendations driving new vaccine introduction and immunization practice includes the rapidly expanding use of Hib and pneumococcal vaccines. Committee meetings are highly visible and well attended, and reviews by these committees are viewed as critical to the policy pathway for adoption of new vaccines. WHO should be proud of its accomplishments to date to increase the qualifications and credibility of members, transparency of process, effective use of evidence, and quality of resulting reports and recommendations” Citation[111]. The stakeholder’s panel recommended that the WHO take immediate steps to consolidate and build on the successes of its vaccine advisory committees reformation. The panel concluded that the WHO needs to better engage the country offices in the dissemination of information at a country level. As a result, the WHO is ensuring the translation of policy recommendations in all WHO headquarters’ official languages and is taking a more proactive approach to the dissemination of related information through country offices. Summaries of position papers are posted on the website together with PowerPoint presentations highlighting the key points of each position paper. In addition, the WHO recommendations contained in the position papers are being published in the journal Vaccine.
The credibility of SAGE processes including its culture of evaluation and communication of decisions are likely to be drivers of considerable influence. Not only do SAGE recommendations have an impact on agencies investing in immunization, but they also have impact on accelerating the late-stage development of vaccines such as a malaria vaccine Citation[35].
SAGE recommendations are expected to lead to higher level policy whose purpose is to accelerate the achievement of current and future global goals. Topics discussed at the WHO Executive Board meeting in January 2010 included measles eradication; a draft resolution on the prevention and treatment of pneumonia; and the prevention and control of viral hepatitis. These topics were then presented to the World Health Assembly in May 2010 at which Bangladesh requested that cholera prevention and control in Asia and Africa be included on the Executive Board’s May 2010 agenda. All of these discussions have built on previous policy recommendations made by SAGE.
Expert commentary & five-year view
The last 5 years have seen a progressive improvement in the functioning of SAGE so that the committee works to the highest standards of quality and transparency with respect to the review of scientific evidence and has become increasingly relevant to countries and partners. SAGE’s relevance extends to all WHO Member States. One of the strengths of SAGE is its willingness and readiness to change. Within the next 5 years, the GIVS will come to an end. A process to review its impact and develop a new vision and new goals for the next 10 years is now starting. As the capacity for decision making at country level is strengthened in particular with the development of national technical advisory committees on immunization, there will be an increased requirement for effective dialog with and between countries and regions.
Box 1. Global goals from the Global Immunization Vision and Strategy 2006–2015.
By 2010
• Countries will reach at least 90% national vaccination coverage and at least 80% vaccination coverage in every district or equivalent administrative unit
• Globally, mortality due to measles will have been reduced by 90% compared with the 2000 level
By 2015 or earlier
• The vaccination coverage goal reached in 2010 will have been sustained
• Global childhood mortality due to vaccine-preventable diseases will have been reduced by at least two-thirds compared with 2000 levels
• Every person eligible for immunization included in national programs will have been offered vaccination with vaccines of assured quality according to established national schedules
• Immunization with newly introduced vaccines will have been offered to the entire eligible population within 5 years of these new vaccines in national immunization programs
• All countries will have developed the capacity at all levels to conduct case-based surveillance of vaccine-preventable diseases, supported by laboratory confirmation where necessary, in order to measure vaccine coverage accurately and use these data appropriately
• All national immunization plans will have been formulated as an integral component of sector-wide plans for human resources, financing and logistics
• All national immunization plans will have been formulated, costed and implemented so as to ensure that human resources, funding and supplies are adequate
Key issues
• A series of global goals embracing the guiding principles of the 2005 Global Immunization Vision and Strategy have been set for the period to 2015. The achievement of these goals will be critical to the attainment of the Millennium Development Goals and in particular those that relate to mortality reduction in children less than 5 years of age.
• The Strategic Advisory Group of Experts (SAGE) on immunization is the overarching WHO advisory committee providing advice on issues ranging from vaccine research and development to immunization delivery. Its remit extends to all vaccine-preventable diseases and focuses on the issuance of policy and strategy recommendations on the use of specific vaccines, which then form the basis for WHO vaccine position papers. SAGE therefore plays an essential role with respect to policy development, program implementation and progress monitoring.
• SAGE’s membership and processes are aimed at ensuring a balanced view that takes account of benefits and risks, cost and opportunities.
• SAGE considers its recommendations in the context of the wider health system and public health needs and it tries to keep advice on vaccines in the perspective of other health interventions.
• SAGE is composed of independent experts rather than by representatives of organizations. Processes are in place to prevent and manage conflicts of interest with detailed screening of declarations of interest prior to nomination for membership and prior to each meeting. Any relevant interests and subsequent action in terms of members’ participation in meetings or at specific discussions are disclosed publicly.
• SAGE deliberations occur in a transparent manner during plenary meetings that are open to members of the vaccine community. The transparency of the process extends to the public posting of information and evidence that served as the basis for SAGE’s decision making.
• SAGE’s recommendations are evidence based. In making its recommendations, in addition to vaccine effectiveness and safety issues, SAGE considers issues such as epidemiology, clinical characteristics, programmatic issues, vaccine availability, economic considerations, health system opportunities and the existence of, and interaction with, other established intervention and control strategies.
• The interaction between SAGE and the regional and country levels is bidirectional. Global recommendations are important to drive progress and offer a framework that countries can adapt to local epidemiological, economical and other circumstances in the context of their other health priorities. In turn, hearing from countries and regions on priorities, need for direction and feedback on their ability to implement recommendations and any challenges encountered is essential to give context and relevance to SAGE’s work.
• WHO recommendations, which derive from SAGE recommendations, are used by countries and other key immunization partners, such as the GAVI Alliance and industry, look at SAGE recommendations to guide their investment decisions.
Disclaimer
Philippe Duclos and Jean-Marie Okwo-Bele are World Health Organization staff members. The opinions expressed in this article are those of the authors and do not necessarily represent the decisions, official policy or opinions of the World Health Organization.
Financial & competing interests disclosure
Since 2005, Philippe Duclos has served as Executive Secretary of the Strategic Advisory Group of Experts and and David Salisbury has been a member of SAGE from 2003 through 2010 and Chaired the group from 2005 through August 2010. Jean-Marie Okwo-Bele is the Director of the Department of Immunization, Vaccines and Biologicals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Charter of the United Nations and Statute of the International Court of Justice, United Nations. Department of Public Information, United Nations, PA, USA (1985).
- World Health Organization. Basic Documents, 45th Edition. World Health Organization, Geneva, Switzerland (2005).
- Resolution WHA27.57. WHO expanded programme on immunization. In: Handbook of Resolutions and Decisions of the World Health Assembly and the Executive Board Volume II 1973–1984. World Health Organization, Geneva, Switzerland, 139 (1985).
- WHO, UNICEF, World Bank. State of the World’s Vaccines and Immunization. 3rd Edition. World Health Organization, Geneva, Switzerland (2009).
- Poland GA, Henderson D. Thirty years after smallpox: celebration and sobering thoughts. Vaccine28(24), 4013–4014 (2010).
- World Health Organization and UNICEF. GIVS Global Immunization Vision and Strategy 2006–2015. World Health Organization, Geneva, Switzerland, 80 (2005).
- United Nations Children’s Fund (UNICEF). The State of the World’s Children 2006. UNICEF, New York, USA (2005).
- Strategic Advisory Group of Experts on immunization – report of the extraordinary meeting on the influenza A (H1N1) 2009 pandemic, 7 July 2009. Wkly Epidemiol. Rec.84(30), 301–304 (2009).
- Conclusions and recommendations of the Advisory Committee on Poliomyelitis Eradication, November 2009. Wkly Epidemiol. Rec.85(1–2), 1–12 (2010).
- Conclusions and recommendations from the Immunization Strategic Advisory Group. Wkly Epidemiol. Rec.81(1), 2–11 (2006).
- Tetanus vaccine: WHO position paper. Wkly Epidemiol. Rec.81(20), 198–208 (2006).
- WHO Position Paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol. Rec.81(47), 445–452 (2006).
- Rotavirus vaccines: WHO Position Paper. Wkly Epidemiol. Rec.82(32), 285–295 (2007).
- Rotavirus vaccines: an update. Wkly Epidemiol. Rec.84(51–52), 533–537 (2009).
- Mumps virus vaccines: WHO position paper. Wkly Epidemiol. Rec.82(7), 50–60 (2007).
- Japanese encephalitis vaccines: WHO position paper. Wkly Epidemiol. Rec.81(34/35), 331–340 (2006).
- Pneumococcal conjugate vaccine for childhood – WHO position paper. Wkly Epidemiol. Rec.82(12), 93–104 (2007).
- 23-valent pneumococcal polysaccharide vaccine: WHO position paper. Wkly Epidemiol. Rec.83(42), 373–384 (2008).
- Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol. Rec.82(21), 193–196 (2007).
- Rabies vaccines – WHO position paper. Wkly Epidemiol. Rec.85(32), 309–320 (2010).
- Human papillomavirus vaccines: WHO position paper. Wkly Epidemiol. Rec.84(15), 117–132 (2009).
- Typhoid vaccines: WHO position paper. Wkly Epidemiol. Rec.83(6), 49–60 (2008).
- Hepatitis B vaccines: WHO position paper Wkly Epidemiol. Rec.84(40), 405–420 (2009).
- Measles vaccines: WHO position paper. Wkly Epidemiol. Rec.84(35), 349–360 (2009).
- Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Wkly Epidemiol. Rec.85(23), 213–228 (2010).
- Cholera vaccines: WHO position paper. Weekly Epidemio. Rec.85(13), 117–128 (2010).
- Pertussis: WHO position paper. Wkly Epidemiol. Rec.85(6), 385–396 (2010).
- Meeting of the Strategic Advisory Group of Experts on immunization: recommendations on the use of licensed human H5N1 influenza vaccines in the interpandemic period. Wkly Epidemiol. Rec.84(24), 244–248 (2009).
- Duclos P, Okwo-Bele JM. Recommendations et politiques vaccinales mondiales: Le rôle de l’OMS. Médecine Sci.23(4), 409–416 (2007).
- Folb PI, Bernatowska E, Chen R et al. A global perspective on vaccine safety and public health: the global advisory committee on vaccine safety. Am. J. Pub. Health94(11), 1926–1931 (2004).
- Global Advisory Committee on Vaccine Safety, 16–17 December 2002. Wkly Epidemiol. Rec.78(4), 17–20 (2002).
- Meeting of the immunization Strategic Advisory Group of Experts, November 2008 – conclusions and recommendations. Wkly Epidemiol. Rec.84(1–2), 1–16 (2009).
- Bryson M, Duclos P, Jolly A, Cakmak N. A global look at national immunization technical advisory groups. Vaccine28(Suppl. 1), A13–A17 (2010).
- Guyatt GH, Oxman AD, Vist GE et al. for the GRADE Working Group. GRADE: an emerging consensus on rating recommendations quality of evidence and strength of GRADE. Br. Med. J.336(7650), 924–926 (2008).
- Meeting of the Strategic Advisory Group of Experts on immunization, October 2009 – conclusions and recommendations. Wkly Epidemiol. Rec.84(50), 517–532 (2009).
- Meeting of the immunization Strategic Advisory Group of Experts, 10–11 April 2006: conclusions and recommendations. Wkly Epidemiol. Rec.81(21), 210–220 (2006).
- Meeting of the immunization Strategic Advisory Group of Experts, April 2009 – conclusions and recommendations. Wkly Epidemiol. Rec.84(23), 220–236 (2009).
- Meeting of the immunization Strategic Advisory Group of Experts, November 2006 – conclusions and recommendations. Wkly Epidemiol. Rec.82(1–2), 1–16 (2007).
- Meeting of the immunization Strategic Advisory Group of Experts, November 2007 – conclusions and recommendations. Wkly Epidemiol. Rec.83(1–2), 1–16 (2008).
- Meeting of the immunization Strategic Advisory Group of Experts, April 2008 – conclusions and recommendations. Wkly Epidemiol. Rec.83(22), 193–208 (2008).
- Global Programme for Vaccines and Immunization (GPV) WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol. Rec.73(10), 64–68 (1998).
- Duclos P, Okwo-Bele JM, Gacic-Dobo M, Cherian T. Global immunization: status, progress, challenges and future. BMC Int. Health Hum. Rights.9(Suppl. 1), S2 (2009).
- Ojo LR, O’Loughlin RE, Cohen AL et al. Global use of Haemophilus influenzae type b conjugate vaccine. Vaccine28(43), 7117–7122 (2010).
Websites
- WHO Expert Committee on Biological Standardization www.who.int/biologicals/expert_committee/en/
- Global Advisory Committee on Vaccine Safety (GACVS) www.who.int/vaccine_safety/en/index.html
- Immunization Practices Advisory Committee (IPAC) www.who.int/immunization_delivery/systems_policy/ipac/en/
- Report of October 2009 Quantitative Immunization and Vaccines Related Research Committee (QUIVER) meeting – A Hinman www.who.int/immunization/sage/QUIVER_Hinman102609.pdf
- SAGE members www.who.int/immunization/sage/members/en/index.html
- Human Papillomavirus (HPV) Vaccine Background Paper. September 2008 www.who.int/immunization/sage/hpvbgpaper_oct08.pdf
- SAGE terms of reference www.who.int/immunization/sage/SAGE_TORs_Full_21_11_08.pdf
- Tracking database of recommendations and action points www.who.int/immunization/sage/SAGE_issues_and_recs.pdf
- SAGE April 2010 http://apps.who.int/immunization/sage/search_topics/
- A stakeholders’ panel to evaluate the impact of strengthening WHO’s normative and policy setting functions for immunization, 2006–2010. Mid-term evaluation, report March 2009 www.who.int/immunization/sage/1_Stakeholders_panel_final_report_March_17.pdf
- Impact of WHO normative and policy guidance on vaccines and immunization – summary of survey report www.who.int/immunization/sage/2_McKinsey_Country_survey_summary.pdf