Abstract
Vaccines are very cost-effective tools in combating infectious disease mortality and morbidity. Unfortunately, vaccines efficiently protecting against infection with malaria parasites are not available and are not likely to appear in the near future. An alternative strategy would be vaccines protecting against the disease and its consequences rather than against infection per se, by accelerating the development of the protective immunity that is normally acquired after years of exposure to malaria parasites in areas of stable transmission. This latter strategy is being energetically pursued to develop a vaccine protecting pregnant women and their offspring against mortality and morbidity caused by the accumulation of Plasmodium falciparum-infected erythrocytes in the placenta. It is based on a detailed understanding of the parasite antigen and the host receptor involved in this accumulation, as well as knowledge regarding the protective immune response that is acquired in response to placental P. falciparum infection. Nevertheless, it remains controversial in some quarters whether such a vaccine would have the desired impact, or indeed whether the strategy is meaningful. This article critically examines the relevance of several perceived obstacles to development of a vaccine against placental malaria.
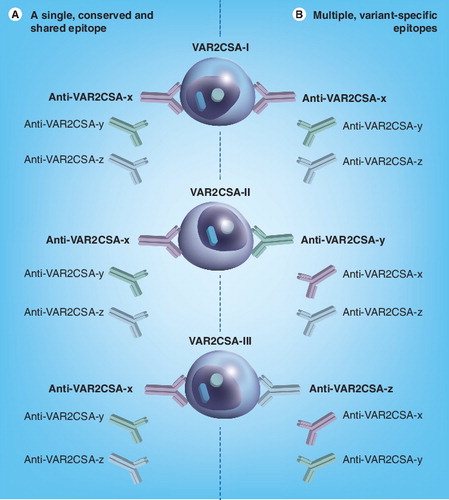
Assume a scenario, where three different clones of P. falciparum, each expressing a distinct variant of VAR2CSA (VAR2CSA-I, VAR2CSA-II, VAR2CSA-III) on the infected erythrocyte surface are exposed to a polyclonal anti-serum containing IgG with specificity for the three different epitopes in VAR2CSA (Anti-VAR2CSA-x, Anti-VAR2CSA-y, Anti-VAR2CSA-z). In the scenario represented in (A), the three clones are all recognized by one of the antibodies (anti-VAR2CSA-x that recognizes a single, conserved epitope shared by all three variants) but not by the two others. In the scenario shown in (B) the three clones are also recognized by only one antibody each, but a different one in each case (anti-VAR2CSA-x, anti-VAR2CSA-y and anti-VAR2CSA-z, respectively, each recognizing a distinct, variant-specific epitope). In both scenarios, each clone is recognized by one of the three VAR2CSA-specific antibodies present in the antiserum, leading to highly concordant antibody recognition of the three clones. However, only (A) involves an interclonally conserved epitope that is present in all three VAR2CSA variants (and recognized by cross-reactive antibody Anti-VAR2CSA-x).
Malaria in pregnancy & VAR2CSA-specific vaccination to prevent it
Each year approximately 55 million women living in areas with stable transmission of Plasmodium falciparum parasites become pregnant Citation[1]. Most of these women live in Africa, and it is among them that the large majority of episodes of malaria in pregnancy occur – episodes that every year cost aproximately 100,000 infants their lives in Africa alone Citation[2]. Although an additional 70 million pregnancies occur in areas with unstable transmission of P. falciparum parasites or with transmission of Plasmodium vivax only, these pregnancies are only at very limited risk of malaria-related complications Citation[1]. Not very much is known about the clinical impact of P. vivax infection during pregnancy, but it appears to be less striking Citation[3] and due to infection-related fever and anemia rather than to parasitemia per seCitation[4]. Thus, in terms of numbers and severity, by far the major part of the problem of malaria in pregnancy is caused by P. falciparum infections in women living in areas of stable transmission in Sub-Saharan Africa. It is these women a VAR2CSA-based vaccine is aimed at protecting.
The particular virulence of P. falciparum is closely related to the ability of P. falciparum-infected erythrocytes (IEs) to adhere to various vascular beds. This tissue-specific IE adhesion, or sequestration, is mediated by parasite-encoded proteins expressed on the IE surface, primarily members of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family (reviewed in Citation[5]). The other malaria parasites regularly infecting humans – Plasmodium vivax, Plasmodium malariae and Plasmodium ovale – do not possess antigens corresponding to PfEMP1, and erythrocytes infected by these species do not or only to a limited extent sequester. The schizont-infected cell agglutination (SICA) antigen family of Plasmodium knowlesi, which was recently shown to also naturally infect humans Citation[6,7], appears orthologous to PfEMP1 Citation[8], but little is known about the role of SICA proteins in the pathogenesis of human P. knowlesi infections.
The basis for development of VAR2CSA-based vaccines against placental malaria
Adults living in areas of stable P. falciparum transmission usually benefit from substantial clinical protection from malaria because of immunity acquired in response to P. falciparum infections during childhood (for a review see Citation[9]). Pregnant women, and in particular primigravidae, constitute an important exception to this general rule (for a review see Citation[10]). The infections in these pregnant women are often inconspicuous with limited overt symptoms and scant peripheral parasitemia. Nevertheless, placental parasitemia can be high, because IEs selectively accumulate in this organ Citation[11]. This has been known for a very long time, but it was only 15 years ago that it was discovered that the IEs accumulating in the placenta specifically adhere to the glycosaminoglycan chondroitin-4-sulfate (usually referred to as chondroitin sulfate A [CSA]) in the intervillous space Citation[12]. This pivotal finding was matched 7 years later, when the parasite ligand of the CSA receptor was identified as a particular PfEMP1 variant called VAR2CSA Citation[13,14].
With this knowledge, scientists have since been working to develop a VAR2CSA-based vaccine specifically aimed at protecting women against placental P. falciparum infection Citation[15]. By immunization, the goal is to achieve before the first pregnancy a level of immunological protection corresponding to the level otherwise achieved only over several pregnancies. The aim is to induce VAR2CSA-specific antibodies that can inhibit adhesion of IEs to CSA in the placenta and opsonize VAR2CSA-positive IEs for phagocytosis. To the author’s knowledge, there is currently no credible alternative vaccination strategy to specifically reduce the problem of malaria in pregnancy.
Reasons why VAR2CSA-based vaccination against malaria in pregnancy might not work
It is generally accepted that CSA-specific IE adhesion and VAR2CSA expression are centrally involved in the pathogenesis of placental P. falciparum infection, and that VAR2CSA is an important target of acquired immunity to the syndrome. Nevertheless, the utility of the PfEMP1-based intervention against malaria in pregnancy is regularly challenged with reference to a series of obstacles (Box 1). To the author’s knowledge, the significance of these hindrances and knowledge gaps has not previously been systematically reviewed and will be attempted here.
CSA might not be the only host receptor for placenta-sequestering IEs
The identification of CSA as the placental adhesion receptor for IEs Citation[12] was a momentous follow-up on reports that P. falciparum IEs can adhere to CSA in vitroCitation[16,17]. IEs from nonpregnant hosts do not have this phenotype. The discovery made it possible to develop a coherent theory accounting for previously unexplained epidemiological observations. Thus, the accumulation of IEs in the placenta might be explained by the abundance of CSA on the syncytiotrophoblast surface and in particular in the intervillous space. The high susceptibility of pregnant women to P. falciparum infection despite having acquired substantial protective immunity during childhood might be explained by the unavailability of CSA for IE sequestration in other tissues, and the consequent lack of VAR2CSA-specific immunity to CSA-adhering parasites in primigravidae. This supposition would also explain the marked concentration of susceptibility to malaria in pregnancy among primigravidae (because susceptibility to placental infection decreases as protection is acquired when the immune system is exposed to the parasite ligand binding to CSA). The alternative theory favored by most at the time (and still by some) was that malaria in pregnancy is an unfortunate but unavoidable consequence of immunosuppression required to protect the fetus from rejection. However, this general immunosuppression hypothesis cannot adequately explain the parity-dependent susceptibility to malaria in pregnancy.
On the other hand, the observation by Fried and Duffy did not rule out the existence of additional placental IE receptors, although those authors did not find any among the many they tested Citation[12]. For this reason, it has been repeatedly proposed since that CSA is just one among several receptors Citation[18]. The non-sulfated glycosaminoglycan hyaluronic acid and the neonatal Fcγ receptor have received particular attention in this respect due to high-profile reports of their involvement Citation[18,19], but present evidence weighs heavily against significant roles for either of these receptors in placental IE sequestration Citation[20–22]. Although adhesion of placental IEs to CD36 (a common phenotype among isolates from nonpregnant donors) was tested and excluded (significant adhesion in zero out of 11 isolates) as a placental receptor in the original report on CSA adhesion Citation[12], it has been reported in some studies as a minor phenotype (three out of 17 isolates Citation[23] and one out of seven islotates Citation[24]) among placental isolates. However, all the placental isolates with CD36-adhering IEs also contained CSA-adhering IEs, and the most parsimonious explanation is that these isolates included both IEs adhering to CSA in the placenta and IEs adhering to CD36 elsewhere Citation[23–26]. Even if some placenta-sequestering IEs were able to bind to CD36 in vitro, such adhesion is probably irrelevant in terms of placental sequestration, as CD36 does not appear to be expressed by the syncytiotrophoblast Citation[27].
The theory that acquired protective immunity to malaria in pregnancy is mediated by antibodies interfering with CSA-specific IE adhesion was first substantiated shortly after the identification of CSA as a placental receptor for adhesion of P. falciparum IEs Citation[28]. Primigravidae were found not to possess IgG inhibiting CSA-specific IE adhesion, whereas IgG from multigravidae had high levels of such antibodies. Follow-up studies have confirmed and extended the theory that the parasite ligand mediating IE adhesion to CSA in the placenta is antigenically distinct from parasite molecules responsible for IE sequestration in other tissues. Thus, levels of IgG with specificity for CSA-adhering IEs were found to be much lower in P. falciparum-exposed men and children than in sympatric women Citation[23,29], and not significantly different from levels in nonexposed control donors Citation[29,30]. Furthermore, levels of CSA-adhering IE-specific IgG among P. falciparum-exposed women were found to correlate with the parity of the donors Citation[23,28–30]. The clinical importance of these antibodies is supported by studies demonstrating correlations between their levels and protection from maternal anemia, low infant birth weight and premature delivery Citation[31,32]. The absence of similar associations for IgG with specificity for IE surface antigens expressed by clonally identical P. falciparum not adhering to CSA strengthens the likelihood of a causal relationship between levels of CSA-adhering IE-specific IgG and clinical protection from adverse pregnancy outcome Citation[32].
Taken together, these observations strongly suggest that CSA is the major, and very likely the only, placental receptor for clinically significant adhesion of IEs in the placenta. In any case, no other receptor has been identified to which only placental IEs adhere, and that has a tissue distribution making its involvement in the pathogenesis of placental malaria plausible.
VAR2CSA might not be the only parasite ligand involved in placental IE sequestration
Obviously, the identification of CSA as the placental IE adhesion receptor spurred a hunt for the corresponding parasite ligand. The focus was on the PfEMP1 proteins expressed on the IE surface Citation[33]. These proteins had just been shown to be encoded by a multigene family involved in antigenic variation and switches in IE adhesion phenotype Citation[34–36]. A clonally variant – and immunologically distinct – antigen selectively expressed during infection of pregnant women seemed probable, as it would explain why protective immunity does not develop prior to the first pregnancy. After some initial false leads, the search resulted in the identification of a PfEMP1-encoding var gene (var2csa) that is selectively transcribed by all placental parasites as well as by parasites selected in vitro for IE adhesion to CSA Citation[13,14,37–39]. Like all other var genes, var2csa encodes a modular protein composed of a series of so-called Duffy-binding-like (DBL) and cysteine-rich inter-domain region (CIDR) domains Citation[36]. However, the domains encoded by var2csa are structurally distinct from those of other PfEMP1 proteins Citation[40], as would be expected from the functional specialization of VAR2CSA Citation[13]. Several studies have documented that transcription of var2csa by placental and CSA-adhering parasites results in IE surface expression of the corresponding approximately 350-kDa PfEMP1 protein (VAR2CSA) Citation[14,25,41].
A number of DBL and CIDR domains from PfEMP1 proteins other than VAR2CSA have been reported to have some affinity for CSA Citation[42–45]. However, these are probably irrelevant in terms of placental malaria Citation[46], and the central importance of VAR2CSA was further strengthened when it was recently shown that its affinity for CSA is several orders of magnitude higher than described for any other PfEMP1 domain Citation[47,48]. Furthermore, selective knockout of var2csa abrogates or greatly diminishes the ability to select for IE adhesion to CSA in vitroCitation[49–51]. Finally, clinical protection from placental malaria is associated with VAR2CSA-specific IgG levels Citation[14], IE adhesion to CSA is efficiently inhibited by recombinant VAR2CSA and by VAR2CSA-specific antibodies Citation[25,47,48], and the naturally acquired IgG response to CSA-adhering IEs is completely focused on VAR2CSA Citation[52].
Several recent studies have mapped the CSA-binding region of VAR2CSA to the DBL2X-CIDRPAM region Citation[53–55], with only minimal involvement of the previously implicated DBL3-X domain Citation[56–58]. The involvement of a low molecular weight (22 kDa) unidentified protein in binding of VAR2CSA-positive IEs to CSA Citation[59] has not been substantiated.
Direct identification by mass spectrometry of PfEMP1 on the surface of placental and CSA-adhering IEs has proved difficult Citation[60,61], probably due to the low abundance of PfEMP1 in the IE membrane, complicated by technological difficulties Citation[62]. This approach nevertheless yielded data pointing to a non-PfEMP1 hypothetical conserved protein (PFI1785w), as peptides from this protein were exclusively identified in placental IE samples Citation[61]. Selective transcription of this and four additional conserved proteins (including PFD1140w) among placental parasites has since been reported in whole-genome transcriptional profiling studies Citation[39,63]. Since surface expression of VAR2CSA is restricted to CSA-adhering IEs, serum IgG with specificity for CSA-adhering IEs and VAR2CSA is generally absent from men (sex specificity), whereas the levels of these antibodies among women increase with the number of pregnancies experienced (parity dependency) Citation[14,29,30,64]. The higher serum levels of IgG with specificity for PFD1140w Citation[39] and PFI1785w Citation[63] among P. falciparum-exposed women than men were therefore taken as evidence that these antigens are also of relevance to malaria in pregnancy. However, truly sex-specific antibody recognition of either PFD1140w or PFI1785w was not apparent (but difficult to evaluate because of the lack of control samples from nonexposed donors), and parity dependency was not demonstrated in either study Citation[39,63]. Furthermore, it is not yet known whether one or both these proteins are present on the IE surface, let alone involved in IE sequestration in the placenta.
Gysin et al. have described adhesion of ring-stage-infected erythrocytes (8 h postinvasion) to placenta tissue in vitroCitation[65]. Ring-stage IE adhesion would exclude a role for PfEMP1, as these proteins (including VAR2CSA) are not present on the IE surface until about 16 h after parasite invasion, and the responsible parasite ligand was identified as RAP2 in a subsequent study by the same authors Citation[66]. However, these findings are difficult to reconcile with the heavily documented stage-specific accumulation of mature IEs in the placenta in vivoCitation[11], and to the author’s knowledge, evidence for the involvement of RAP2 in the pathogenesis of placental malaria has not been forthcoming.
Taken together, these observations strongly point to VAR2CSA as the major, and very likely only, P. falciparum ligand mediating clinically important adhesion of IEs in the placenta. In any case, substantial evidence of significant alternative candidates is not yet available.
P. falciparum parasites sequestering in tissues other than the placenta might be involved in the pathogenesis of malaria in pregnancy
Pregnant women are unquestionably at increased risk of malaria compared with their nonpregnant peers, and the consequences of infection tend to be more severe Citation[67,68]. Pregnant women appear to be particularly attractive to the mosquitoes that transmit the disease Citation[69,70], and perhaps pregnancy-induced immune modulation temporarily reduces their ability to control a malaria parasite infection – whether acquired during pregnancy or before conception Citation[71]. Unfortunately, there is very little data available regarding the role in the pathogenesis of malaria in pregnancy of parasites not binding to CSA, not expressing VAR2CSA and sequestering in tissues other than the placenta. Such parasites, if present in a pregnant woman before the placenta has developed sufficiently to support VAR2CSA-mediated intervillous IE adhesion to CSA, could potentially be a Trojan horse source of placental infection due to their potential for switching to expression of VAR2CSA. That way, placental parasitemia might not necessarily require infection during the last two thirds of pregnancy when the placenta is fully formed, but could possibly originate from a clinically inconspicuous or completely asymptomatic infection acquired earlier in pregnancy – or even long before conception. This might explain the borderline association between first-trimester infections and low infant birth weight Citation[72,73], and the less than expected seasonality of malaria in pregnancy observed in several studies (reviewed in Citation[74]). Finally, it is the most reasonable explanation for cases of placental malaria in women without exposure to parasite transmission for several years prior to conception Citation[75,76]. But it is speculation, and as far as the author is aware, studies specifically designed to study the Trojan horse hypothesis have not been done, and such studies may not in fact be practical.
Apart from this putative indirect role, it is repeatedly suggested that parasites not expressing VAR2CSA and sequestering in organs other than the placenta also play a direct and important role in malaria in pregnancy Citation[26,77]. Although peripheral blood P. falciparum parasitemia in pregnant women living in stable parasite transmission areas often seems to be derived from a parasite population with a placental sequestration focus Citation[23,78–80], such a population may also be just one of several populations present in the peripheral blood Citation[12,23,79]. These populations can be clonally different from each other Citation[81] or genotypically identical subpopulations expressing different PfEMP1 proteins and with different tissue tropisms.
In summary, there is currently no substantial evidence that nonplacental (sub-)populations play a significant direct role in the pathogenesis of malaria in pregnancy. By contrast, there is a very substantial body of evidence that placenta-sequestering IEs adhering to CSA through VAR2CSA are an unequivocal and substantial cause of adverse pregnancy outcome. Determination of the relative importance of placental and nonplacental infections for adverse pregnancy outcome may have to await results of clinical trials of vaccination against placental parasitemia.
Antibodies with specificity for CSA-adhering IEs might not be sufficient to control placental parasitemia
There is substantial evidence linking IgG specific for CSA-adhering and VAR2CSA-positive IEs to protection from adverse pregnancy outcome Citation[14,28,30–32]. However, there is also evidence that placental parasitemia can persist in the presence of this type of immunity Citation[28,82–85], and this is often cited to question the importance of IgG-mediated immunity to CSA-adhering IEs. It is usually overlooked that the statistical power to detect a relationship between IgG to CSA-adhering IEs and pregnancy outcome was compromised in several studies by the overall low levels of potentially inhibitory antibodies due to concomitant use of insecticide-treated bed nets Citation[85], inclusion of primigravidae only Citation[84,86], or by the small sample size Citation[82]. Nevertheless, these studies raise the important issue of the quantity (levels) and quality (function) of IgG to IE surface antigens needed to substantially impact placental parasitemia.
Let us look at the role of quantity first. In one study, where both IgG levels to CSA-adhering IEs and placental parasite loads were quantified, a clear inverse correlation between the two was observed in multigravidae but not among primigravidae Citation[30]. In another study, placental parasite density was inversely correlated with infant birth weight Citation[82]. In combination, these data suggest that it is high placental parasite densities that really matter in terms of adverse pregnancy outcome, and that high IgG levels to CSA-adhering IEs are required to (and can) reduce them sufficiently. If this indeed is the case, it follows that simple recording of the absence or presence of specific IgG and placental parasitemia is insufficient when relating acquired immunity to protection. Further quantitative data are clearly needed.
The aforementioned data also indicate that qualitative differences in CSA-adhering IE-specific IgG may be important, a notion supported by the finding that levels of IgG to CSA-adhering IEs and levels of IgG capable of inhibiting IE adhesion to CSA do not always correlate well Citation[82]. Recent data clearly support that IgG recognizing epitopes near the CSA-binding region of VAR2CSA can be potent inhibitors of IE adhesion to CSA, whereas antibodies binding to more distant epitopes are ineffective Citation[25,53–55,87–89]. Additional qualitative data are therefore needed.
Finally, the timing and rate of acquisition of the relevant antibodies are also likely to play a role. The primary IgG response to CSA-adhering IEs among primigravidae occurs later and develops slower than the secondary response typically seen among multigravidae Citation[90]. In fact, these differences, rather than qualitative differences in the IgG acquired, were suspected to be contributing to the absent correlation between levels of IgG to CSA-adhering IEs and placental parasite load among primigravidae Citation[30]. More longitudinal studies are clearly needed.
The interclonal diversity of critical VAR2CSA epitopes might prohibit development of an effective vaccine
VAR2CSA is a member of the PfEMP1 family of proteins. Each P. falciparum genome contains approximately 60 genes called var that encode different PfEMP1 variants with high sequence diversity and affinity for many different host receptors Citation[34–36]. The intraclonal variability among PfEMP1 proteins in general is replayed at a more limited scale within VAR2CSA itself, since many P. falciparum clones harbor more than one var2csa gene in a way that appears to be biologically significant Citation[91–93]. This intraclonal diversity is further compounded by substantial interclonal polymorphism, yielding a potentially very large number of antigenically distinct variants.
VAR2CSA is not only antigenically distinct from all other PfEMP1 variants Citation[14]; it is also characterized by a higher degree of interclonal conservation Citation[13,94]. Nevertheless, the extracellular domains of VAR2CSA are composed of highly conserved segments interspersed by stretches with very substantial amino acid diversity Citation[94,95]. There has therefore been concern that antibody epitopes of importance to acquired protective immunity might be preferentially located in variable regions of VAR2CSA as a consequence of diversifying selection pressure Citation[52,96], which would constitute a serious obstacle to the development of a VAR2CSA-based vaccine.
Immuno-epidemiological evidence uniformly shows a high concordance of serum antibody reactivity to genotypically distinct CSA-adhering and VAR2CSA-expressing IEs Citation[28,82,97], suggesting either the presence of interclonally conserved antibody epitopes or the presence of a broad repertoire of antibody specificities . Fortunately for vaccine development, several independent lines of research support the former possibility Citation[52,53,98–103]. Although one study has cast doubt on the functional importance of such interclonally conserved epitopes Citation[104], it is offset by several reports providing evidence of substantial interclonal conservation of epitopes recognized by IgG that can block CSA-specific adhesion Citation[25,47,87,88].
Clearly, much work remains, and studies of less heterogeneous antigens, such as AMA-1, indicate that unanticipated problems could lie ahead. Despite this caveat, the bulk of presently available evidence points to the presence of interclonally conserved and functionally important antibody epitopes in VAR2CSA that can be exploited in vaccine development.
Expert commentary
A vaccine specifically against placenta-sequestering IEs is not the only – or even the best – solution to malaria in pregnancy. Obviously, a P. falciparum vaccine inducing efficient (preferably sterile), long-lasting (life- or at least decades long) immunity to all parasites regardless of their tissue tropism would be a superior solution to the problem. Such a vaccine would be expected to protect against all P. falciparum malaria syndromes, including placental malaria. The problem, however, is that such a vaccine does not exist – despite many attempts to create it, encouraging results and high hopes. It remains an open question whether it ever will. By contrast, a vaccine based on conserved parasite antigens and inducing relatively short-lasting, partial immunity may well be around the corner. Given to small children, such a vaccine is likely to have a substantial effect on malaria-related morbidity and mortality early in life, but unlikely to prevent placental malaria – and therefore unlikely to have a major impact on malaria in pregnancy. A vaccine specifically aimed at protecting pregnant women – and particularly their unborn babies – from the adverse consequences of placental P. falciparum infection would be an extremely useful supplement to a partially efficacious pediatric vaccine against malaria. Admittedly, such a vaccine does not exist either, but if the resources for its development can be found, it is a goal that is potentially reachable in the not too distant future. It is a goal that appears much more realistic than an ideal vaccine inducing life-long, sterile immunity to all P. falciparum parasites. The available evidence indicates that a vaccine based on VAR2CSA that can inhibit IE adhesion to CSA in the placental intervillous space and opsonize VAR2CSA-positive IEs for phagocytosis would markedly reduce mortality and morbidity from malaria in pregnancy. It would not prevent all forms or types of malaria in pregnant women, but it would probably remove a very substantial proportion of the problems suffered by pregnant women living in areas of stable P. falciparum transmission. It follows that even a highly efficacious VAR2CSA-based vaccine should not be relied upon as the only measure to protect pregnant women in low-endemicity areas against malaria, because they are likely to be susceptible to P. falciparum parasites other than those sequestering in the placenta. What transmission patterns and intensities would render vaccination with a VAR2CSA-based vaccine cost–beneficial is beyond the scope of this article, but clearly something that must be considered carefully.
Overall, there are still lacunae in our knowledge, and we should strive to fill these. Some knowledge gaps have been mentioned earlier; others have been described elsewhere Citation[2,105]. Our recent finding that VAR2CSA-positive IEs appear to be able to shield themselves from immune recognition illustrates that further complications might well lie ahead Citation[89]. However, none of them cast serious doubt that a vaccine capable of markedly reducing placental P. falciparum parasite loads would be highly likely to have a major impact on maternal and infant morbidity and mortality caused by malaria in pregnancy Citation[2,74]. It is the author’s opinion that using weakly supported obstacles such as those discussed earlier and listed in Box 1 (except for the last bullet point) as arguments that may delay or jeopardize the development of such a vaccine could be irresponsible Citation[77].
Five-year view
Progress in the next 5 years regarding development of a vaccine to protect pregnant women against placental P. falciparum infection will depend mainly on the availability of funding. In the last decade, there has been tremendous progress in the understanding of the pathogenesis and immunology of placental malaria, which constitutes an exceptionally solid evidence-based foundation for clinical vaccine development. Despite this, the recent decline in funding for the preclinical research required to define optimal vaccine constructs and formulations constitutes a very serious and immediate threat to continued progress. Similar difficulties can be envisioned regarding funding for the clinical trials themselves. The reasons for this precarious situation are many; the international economic setback, lack of political awareness, and the change in the malaria research agenda from control to elimination and eradication are important examples. Finally, a solution must be found to the practical problem of being able to formally document in a clinical trial the protective effect of VAR2CSA-based vaccination. Pregnant trial participants are likely to receive standard preventive treatment against placental malaria and to be issued with insecticide-treated bed nets. These interventions are known to markedly reduce the incidence of placental malaria, and on top of this, transmission of P. falciparum parasites at many potential trial sites has been declining in recent years. Under such circumstances it may well prove to be very difficult to demonstrate convincing vaccine efficacy, even when testing an efficacious vaccine.
If these obstacles can be overcome, it is reasonable to speculate that an experimental, VAR2CSA-based vaccine could be in clinical trials within the next 5 years. Hopefully, consensus among sponsors, policy-makers and researchers regarding the appropriate target product profiles and clinical development plans for such a vaccine can be achieved. This will require broad discussions involving all stakeholders and considering all available evidence.
In all likelihood, the eventual target population of a placental malaria vaccine is girls at puberty. A vaccine inducing sufficiently long-lasting immunological memory to allow vaccination against placental malaria as part of routine childhood vaccination programs might not be achievable, and a vaccine with duration of protection so short that it must be given during pregnancy is probably not worth pursuing. On this premise, a vaccine against placental malaria combined with the already available vaccine against cervical carcinoma appears an attractive option.
Box 1. Potential obstacles to VAR2CSA-based vaccination against malaria in pregnancy.
VAR2CSA-based vaccine development will be compromised if:
• CSA is not the only clinically significant host receptor for placental sequestration of Plasmodium falciparum IEs
• VAR2CSA is not the only clinically significant P. falciparum ligand mediating CSA-specific placental sequestration of IEs
• The pathogenesis of malaria in pregnancy depends critically and directly on parasite populations sequestering outside the placenta
• Antibodies to CSA-adhering IEs cannot adequately control placental parasitemia
• Interclonal diversity of VAR2CSA prevents its use in vaccination
• Sufficient funding for preclinical research and clinical trials is not available
Key issues
• The major part of the problem of maternal and infant morbidity and mortality related to malaria in pregnancy is the result of placental Plasmodium falciparum infections in women living in areas of stable P. falciparum transmission.
• The particular virulence of P. falciparum malaria is related to the expression of proteins (in particular PfEMP1) on the infected erythrocyte surface, where they mediate adhesion to a range of vascular host receptors.
• Placental sequestration of infected erythrocytes is mediated by the PfEMP1 protein VAR2CSA. This parasite ligand has nanomolar affinity for the placental host receptor chondroitin sulfate A (CSA).
• There is no firm evidence supporting a direct role of P. falciparum parasites not expressing VAR2CSA in the pathogenesis of malaria in pregnancy.
• Clinical protection against adverse pregnancy outcome due to placental malaria is mediated by IgG with specificity for CSA-adhering, VAR2CSA-expressing infected erythrocytes.
• Interclonally conserved and functionally important epitopes that could be exploited in the development of a vaccine against placental malaria are present in VAR2CSA.
• Inadequate funding appears to be the most serious obstacle to the successful development of a VAR2CSA-based vaccine to protect against maternal and neonatal morbidity and mortality in areas of stable transmission of P. falciparum parasites.
Financial & competing interests disclosure
The author is listed as a co-inventor of patent P33116PC01-WO2004067559 A, (30 December 2003): ‘Compounds useful in the diagnosis and treatment of pregnancy-associated malaria’. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notes
CSA: Chondroitin sulfate A; IE: Infected erythrocytes.
References
- Dellicour S, Tatem AJ, Guerra CA, Snow RW, Ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med.7, e1000221 (2010).
- Desai M, Ter Kuile FO, Nosten F et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis.7, 93–104 (2007).
- Nosten F, McGready R, Simpson JA et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet354, 546–549 (1999).
- Poespoprodjo JR, Fobia W, Kenangalem E et al. Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin. Infect. Dis.46, 1374–1381 (2008).
- Tembo D, Montgomery J. Var gene expression and human Plasmodium pathogenesis. Future Microbiol.203(5), 1185–1196 (2010).
- Singh B, Sung LK, Matusop A et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet363, 1017–1024 (2004).
- Cox-Singh J, Davis TM, Lee KS et al.Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis.46, 165–171 (2008).
- Korir CC, Galinski MR. Proteomic studies of Plasmodium knowlesi SICA variant antigens demonstrate their relationship with P. falciparum EMP1. Infect. Genet. Evol.6, 75–79 (2006).
- Hviid L. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop.95, 270–275 (2005).
- Hviid L. The immuno-epidemiology of pregnancy-associated malaria: a variant surface antigen-specific perspective. Parasite Immunol.26, 477–486 (2004).
- Beeson JG, Amin N, Kanjala M, Rogerson SJ. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect. Immun.70, 5412–5415 (2002).
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulphate A in the human placenta. Science272, 1502–1504 (1996).
- Salanti A, Staalsoe T, Lavstsen T et al. Selective upregulation of a single distinctly structured var gene in CSA-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol.49, 179–191 (2003).
- Salanti A, Dahlbäck M, Turner L et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med.200, 1197–1203 (2004).
- Smith JD, Deitsch KW. Pregnancy-associated malaria and the prospects for syndrome-specific antimalaria vaccines. J. Exp. Med.200, 1093–1097 (2004).
- Robert C, Pouvelle B, Meyer P et al. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res. Immunol.146, 383–393 (1995).
- Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med.182, 15–20 (1995).
- Flick K, Scholander C, Chen Q et al. Role of non-immune IgG bound to PfEMP1 in placental malaria. Science293, 2098–2100 (2001).
- Beeson JG, Rogerson SJ, Cooke BM et al. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med.6, 86–90 (2000).
- Rowe JA, Shafi J, Kai OK, Marsh K, Raza A. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am. J. Trop. Med. Hyg.66, 692–699 (2002).
- Creasey A, Staalsoe T, Raza A, Arnot D, Rowe JA. Nonspecific Immunoglobulin M binding and chondroitin sulfate A binding are linked phenotypes of Plasmodium falciparum isolates implicated in malaria during pregnancy. Infect. Immun.71, 4767–4771 (2003).
- Muthusamy A, Achur RN, Valiyaveettil M et al. Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta and IRBC adherence upregulates the receptor expression. Am. J. Pathol.170, 1989–2000 (2007).
- Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis.180, 464–472 (1999).
- Rogerson SJ, Beeson JG, Mhango CG, Dzinjalamala FK, Molyneux ME. Plasmodium falciparum rosette formation is uncommon in isolates from pregnant women. Infect. Immun.68, 391–393 (2000).
- Barfod L, Dobrilovic T, Magistrado P et al. CSA-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J. Immunol.185, 7553–7561 (2010).
- Rovira-Vallbona E, Dobano C, Bardaji A et al. Transcription of var genes other than var2csa in Plasmodium falciparum parasites infecting Mozambican pregnant women. J. Infect. Dis.204, 27–35 (2011).
- Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect. Immun.65, 1251–1257 (1997).
- Fried M, Nosten F, Brockman A, Brabin BT, Duffy PE. Maternal antibodies block malaria. Nature395, 851–852 (1998).
- Ricke CH, Staalsoe T, Koram K et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol.165, 3309–3316 (2000).
- Staalsoe T, Megnekou R, Fievet N et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis.184, 618–626 (2001).
- Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun.71, 6620–6623 (2003).
- Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against the clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet363, 283–289 (2004).
- Leech JH, Barnwell JW, Miller LH, Howard RJ. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med.159, 1567–1575 (1984).
- Baruch DI, Pasloske BL, Singh HB et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell82, 77–87 (1995).
- Smith JD, Chitnis CE, Craig AG et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell82, 101–110 (1995).
- Su X, Heatwole VM, Wertheimer SP et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell82, 89–100 (1995).
- Tuikue Ndam NG, Salanti A, Bertin G et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis.192, 331–335 (2005).
- Duffy MF, Caragounis A, Noviyanti R et al. Transcribed var genes associated with placental malaria in Malawian women. Infect. Immun.74, 4875–4883 (2006).
- Francis SE, Malkov VA, Oleinikov AV et al. Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect. Immun.75, 4838–4850 (2007).
- Gardner MJ, Hall N, Fung E et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature419, 498–511 (2002).
- Magistrado P, Salanti A, Tuikue Ndam NG et al. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J. Infect. Dis.198, 1071–1074 (2008).
- Buffet PA, Gamain B, Scheidig C et al.Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl Acad. Sci. USA96, 12743–12748 (1999).
- Reeder JC, Cowman AF, Davern KM et al. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl Acad. Sci. USA96, 5198–5202 (1999).
- Degen R, Weiss N, Beck HP. Plasmodium falciparum: cloned and expressed CIDR domains of PfEMP1 bind to chondroitin sulfate A. Exp. Parasitol.95, 113–121 (2000).
- Khattab A, Kun J, Deloron P, Kremsner PG, Klinkert M-Q. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J. Infect. Dis.183, 1165–1169 (2001).
- Resende M, Ditlev SB, Nielsen MA et al. Chondroitin sulphate A (CSA)-binding of single recombinant Duffy-binding-like domains is not restricted to Plasmodium falciparum erythrocyte membrane protein 1 expressed by CSA-binding parasites. Int. J. Parasitol.39, 1195–1204 (2009).
- Khunrae P, Dahlbäck M, Nielsen MA et al. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J. Mol. Biol.397, 826–834 (2010).
- Srivastava A, Gangnard S, Round A et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl Acad. Sci. USA107, 4884–4889 (2010).
- Viebig NK, Gamain B, Scheidig C et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Reports6, 775–781 (2005).
- Duffy MF, Maier AG, Byrne TJ et al. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum.Mol. Biochem. Parasitol.148, 117–124 (2006).
- Viebig NK, Levin E, Dechavanne S et al. Disruption of var2csa gene impairs placental malaria associated adhesion phenotype. PLoS ONE2, e910 (2007).
- Barfod L, Bernasconi N, Dahlbäck M et al. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol. Microbiol.63, 335–347 (2007).
- Bigey P, Gnidehou S, Doritchamou J et al. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J. Infect. Dis.204, 1125–1133 (2011).
- Dahlbäck M, Jørgensen LM, Nielsen MA et al. The chondroitin sulphate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J. Biol. Chem.286, 15908–15917 (2011).
- Srivastava A, Gangnard S, Dechavanne S et al. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS ONE6, e20270 (2011).
- Higgins MK. The structure of a chondroitin sulfate-binding domain important in placental malaria. J. Biol. Chem.283, 21842–21846 (2008).
- Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat. Struct. Mol. Biol.15, 932–938 (2008).
- Singh K, Gitti RK, Diouf A et al. Subdomain 3 of Plasmodium falciparum VAR2CSA DBL3X is identified as a minimal chondroitin sulfate A binding region. J. Biol. Chem.285, 24855–24862 (2010).
- Gowda AS, Madhunapantula SV, Achur RN, Valiyaveettil M, Bhavanandan VP, Gowda DC. Structural basis for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin 4-sulfate and design of novel photoactivable reagents for the identification of parasite adhesive proteins. J. Biol. Chem.282, 916–928 (2007).
- Fried M, Wendler JP, Mutabingwa TK, Duffy PE. Mass spectrometric analysis of Plasmodium falciparum erythrocyte membrane protein-1 variants expressed by placental malaria parasites. Proteomics4, 1086–1093 (2004).
- Fried M, Hixson KK, Anderson L, Ogata Y, Mutabingwa TK, Duffy PE. The distinct proteome of placental malaria parasites. Mol. Biochem. Parasitol.155, 57–65 (2007).
- Lasonder E, Ishihama Y, Andersen JS et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature419, 537–542 (2002).
- Tuikue Ndam N, Bischoff E, Proux C et al.Plasmodium falciparum transcriptome analysis reveals pregnancy malaria associated gene expression. PLoS ONE3, e1855 (2008).
- Tuikue Ndam NG, Salanti A, Le-Hesran J-Y et al. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of Senegalese pregnant women. J. Infect. Dis.193, 713–720 (2006).
- Pouvelle B, Buffet PA, Lepolard C, Scherf A, Gysin J. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat. Med.6, 1264–1268 (2000).
- Douki LJB, Sterkers Y, Lepolard C et al. Adhesion of normal and Plasmodium falciparum ring-infected erythrocytes to endothelial cells and the placenta involves the rhoptry-derived ring surface protein-2. Blood101, 5025–5032 (2003).
- Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull. World Health Organ.61, 1005–1016 (1983).
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg.64, 28–35 (2001).
- Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet355, 1972 (2000).
- Ansell J, Hamilton KA, Pinder M, Walraven GE, Lindsay SW. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans. R. Soc. Trop. Med. Hyg.96, 113–116 (2002).
- Guilbert LJ. There is a bias against type 1 (inflammatory) cytokine expression and function in pregnancy. J. Repr. Immunol.32, 105–110 (1996).
- Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am. J. Trop. Med. Hyg.76, 849–854 (2007).
- Huynh BT, Fievet N, Gbaguidi G et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am. J. Trop. Med. Hyg.85, 214–220 (2011).
- Brabin BJ, Romagosa C, Abdelgalil S et al. The sick placenta – the role of malaria. Placenta25, 359–378 (2004).
- Giobbia M, Tonon E, Zanatta A, Cesaris L, Vaglia A. Late recrudescence of Plasmodium falciparum malaria in a pregnant woman: a case report. Int. J. Infect. Dis.9, 234–235 (2005).
- Poilane I, Jeantils V, Carbillon L. Découverte fortuite de paludisme à Plasmodium falciparum au cours de la grossesse: à propos de deux cas. Gynecol. Obstet. Fertil.37, 824–826 (2009).
- Menéndez C, Moorthy VS, Reed Z, Bardají A, Alonso P, Brown GV. Development of vaccines to prevent malaria in pregnant women: WHO MALVAC meeting report. Expert Rev. Vaccines10(9), 1271–1280 (2011).
- Nguyen-Dinh P, Steketee RW, Greenberg AE, Wirima JJ, Mulenda O, Williams SB. Rapid spontaneous postpartum clearance of Plasmodium falciparum parasitaemia in African women. Lancet2, 751–752 (1988).
- Ofori MF, Staalsoe T, Bam V et al. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect. Immun.71, 1584–1586 (2003).
- Bottero J, Briand V, Agbowai C, Doritchamou J, Massougbodji A, Cot M. Spontaneous postpartum clearance of Plasmodium falciparum parasitemia in pregnant women, Benin. Am. J. Trop. Med. Hyg.84, 267–269 (2011).
- Kamwendo DD, Dzinjalamala FK, Snounou G et al.Plasmodium falciparum: PCR detection and genotyping of isolates from peripheral, placental, and cord blood of pregnant Malawian women and their infants. Trans. R. Soc. Trop. Med. Hyg.96, 145–149 (2002).
- Tuikue Ndam NG, Fievet N, Bertin G, Cottrell G, Gaye A, Deloron P. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J. Infect. Dis.190, 2001–2009 (2004).
- Cox SE, Staalsoe T, Arthur P et al. Maternal vitamin A supplementation and immunity to malaria in pregnancy in Ghanaian primigravids. Trop. Med Int. Health10, 1286–1297 (2005).
- Ataide R, Hasang W, Wilson DW et al. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS ONE5, e10807 (2010).
- Serra-Casas E, Menendez C, Bardaji A et al. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J. Infect. Dis.201, 123–131 (2010).
- Cox SE, Staalsoe T, Arthur P et al. Rapid acquisition of isolate-specific antibodies to chondroitin sulphate A-adherent Plasmodium falciparum isolates in Ghanaian primigravidae. Infect. Immun.73, 2841–2847 (2005).
- Nielsen MA, Pinto VV, Resende M et al. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect. Immun.77, 2482–2487 (2009).
- Magistrado PA, Minja D, Doritchamou J et al. High efficacy of anti DBL4ε-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine29, 437–443 (2010).
- Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc. Natl Acad. Sci. USA108, 12485–12490 (2011).
- O’Neill-Dunne I, Achur RN, Agbor-Enoh ST et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun.69, 7487–7492 (2001).
- Brolin KJ, Ribacke U, Nilsson S et al. Simultaneous transcription of duplicated var2csa gene copies in individual Plasmodium falciparum parasites. Genome Biol.10, R117 (2009).
- Sander AF, Salanti A, Lavstsen T et al. Multiple var2csa-type PfEMP1 genes located at different chromosomal loci occur in many Plasmodium falciparum isolates. PLoS ONE4, e6667 (2009).
- Sander AF, Salanti A, Lavstsen T et al. Positive selection of Plasmodium falciparum parasites with multiple var2csa-type PfEMP1 genes during the course of infection in pregnant women. J. Infect. Dis.203, 1679–1685 (2011).
- Rask TS, Hansen DA, Theander TG, Pedersen AG, Lavstsen T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes – divide and conquer. PLoS Comput. Biol.6, e1000983 (2010).
- Trimnell AR, Kraemer SM, Mukherjee S et al. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol. Biochem. Parasitol.148, 169–180 (2006).
- Dahlbäck M, Rask TS, Andersen PH et al. Epitope mapping and topographic analysis of VAR2CSA DBL3X involved in Plasmodium falciparum placental sequestration. PLoS Pathog.2, e124 (2006).
- Hommel M, Elliott SR, Soma V et al. Evaluating the antigenic diversity of placental binding Plasmodium falciparum variants and the antibody repertoire among pregnant women. Infect. Immun.78, 1963–1978 (2010).
- Elliott SR, Duffy MF, Byrne TJ et al. Cross-reactive surface epitopes on chondroitin sulfate A-adherent Plasmodium falciparum-infected erythrocytes are associated with transcription of var2csa.Infect. Immun.73, 2848–2856 (2005).
- Andersen P, Nielsen MA, Resende M et al. Structural insights into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog.4, e42 (2008).
- Soerli J, Barfod L, Lavstsen T, Bernasconi NL, Lanzavecchia A, Hviid L. Human monoclonal IgG selection of Plasmodium falciparum for the expression of placental malaria-specific variant surface antigens. Parasite Immunol.31, 341–346 (2009).
- Avril M, Cartwright MM, Hathaway MJ et al. Immunization with VAR2CSA-DBL5 recombinant protein elicits broadly cross-reactive antibodies to placental-type Plasmodium falciparum infected erythrocytes. Infect. Immun.78, 2248–2256 (2010).
- Fernandez P, Petres S, Mecheri S, Gysin J, Scherf A. Strain-transcendent immune response to recombinant Var2CSA DBL5-ε domain block P. falciparum adhesion to placenta-derived BeWo cells under flow conditions. PLoS ONE5(9), e12558 (2010).
- Gnidehou S, Jessen L, Gangnard S et al. Insight into antigenic diversity of VAR2CSA-DBL5e domain from multiple Plasmodium falciparum placental isolates. PLoS ONE5, e13105 (2010).
- Avril M, Hathaway MJ, Srivastava A et al. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLoS ONE6, e16622 (2011).
- Rogerson SJ, Hviid L, Duffy PE, Leke RFG, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis.7, 105–117 (2007).