Abstract
Hyporesponsiveness (immune tolerance) follows vaccination with meningococcal polysaccharide and many pneumococcal polysaccharide serotypes. Hyporesponsiveness after Haemophilus influenzae type b polysaccharide vaccination has not been directly observed, but may follow exposure during disease in some individuals. Use of currently licensed conjugate vaccines has not been associated with hyporesponsiveness to date, with the possible exception of pneumococcal serotype 3. Introduction of polysaccharide vaccines anywhere into a conjugate vaccination schedule may result in reduced immune responses on subsequent exposure. This review of vaccine-induced hyporesponsiveness and its potential clinical implications considers recent evidence suggesting that hyporesponsiveness may occur for specific components of combined conjugate vaccines, such as pneumococcal serotype 3. These data have implications for the development of new multivalent vaccines.
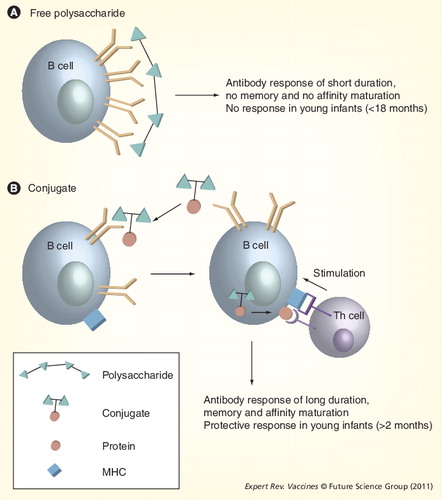
(A) B cells directly recognize vaccine polysaccharides. No interaction occurs between Th and B cells (B) B cells recognize the polysaccharide–protein conjugate of the vaccine. The protein carrier is processed and presented to Th cells in the context of the MHC, which induces T-cell activation. In turn, T cells stimulate the specific B cells to produce antibodies against the conjugate.
Th: T helper.
SBA (rabbit complement) GMTs against serogroup A (A) and C (B) in Gambian infants post-vaccination with MenAC-PS vaccine at 19.7 months or 5 years of age Citation[55,62]. Group MenAC-PS had received prior MenAC-PS at 3 and 6 months; group MenAC-CV+PS had received 1, 2 or 3 prior MenAC-CV doses and MenAC-PS at age 2 years; group MenAC-CV had received 1, 2 or 3 prior MenAC-CV doses and MenAC-PS at year 5. (C) SBA (rabbit complement) GMTs against serogroups A and C in Saudi Arabian 10–29-year olds post MenAC-PS in naive versus previously MenAC-PS-immunized subjects Citation[54]. (D) Percentage of toddlers (from a study conducted in Canada) with serogroup C SBAs ≥1:8 (human complement) after MenACWY-PS Citation[24]. Group MenACWY-PS received MenACWY-PS at study months 0, 2 and 14. Vaccine-naive subjects received MenACWY-PS at month 14. (E) SBA (human complement) GMTs against serogroup C after MenC-PS challenge in naive, MenC-PS or MenC-CV immunized adults Citation[53].
CV: Conjugate vaccine; GMT: Geometric mean antibody titer; MenAC: Meningococcal serogroups A and C; MenACYW: Meningococcal serogroup A, C, W-135 and Y; MenC: Meningococcal serogroup C; PS: Polysaccharide; SBA: Serum bactericidal antibodies.
Data taken from Citation[24,53–55,62].
![Figure 2. Contrasting responses following repeated meningococcal C or A polysaccharide vaccine.SBA (rabbit complement) GMTs against serogroup A (A) and C (B) in Gambian infants post-vaccination with MenAC-PS vaccine at 19.7 months or 5 years of age Citation[55,62]. Group MenAC-PS had received prior MenAC-PS at 3 and 6 months; group MenAC-CV+PS had received 1, 2 or 3 prior MenAC-CV doses and MenAC-PS at age 2 years; group MenAC-CV had received 1, 2 or 3 prior MenAC-CV doses and MenAC-PS at year 5. (C) SBA (rabbit complement) GMTs against serogroups A and C in Saudi Arabian 10–29-year olds post MenAC-PS in naive versus previously MenAC-PS-immunized subjects Citation[54]. (D) Percentage of toddlers (from a study conducted in Canada) with serogroup C SBAs ≥1:8 (human complement) after MenACWY-PS Citation[24]. Group MenACWY-PS received MenACWY-PS at study months 0, 2 and 14. Vaccine-naive subjects received MenACWY-PS at month 14. (E) SBA (human complement) GMTs against serogroup C after MenC-PS challenge in naive, MenC-PS or MenC-CV immunized adults Citation[53].CV: Conjugate vaccine; GMT: Geometric mean antibody titer; MenAC: Meningococcal serogroups A and C; MenACYW: Meningococcal serogroup A, C, W-135 and Y; MenC: Meningococcal serogroup C; PS: Polysaccharide; SBA: Serum bactericidal antibodies.Data taken from Citation[24,53–55,62].](/cms/asset/7b7fd299-c9c6-4cb3-b951-9682e292b787/ierv_a_11217495_f0002_b.jpg)
SBA (rabbit complement) GMTs before and after initial meningococcal serogroup A, C, W-135 and Y (MenACWY)-PS vaccination at 14 years of age, and before and after MenACWY-CV booster 3 years later (squares); SBA (rabbit complement) GMTs in vaccine naive subjects after MenACWY-CV at 17 years of age (circles).
Error bars represent the 95% CIs.
GMT: Geometric mean antibody titer; SBA: Serum bactericidal antibody.
Data taken from Citation[56].
![Figure 3. Effect of prior meningococcal polysaccharide vaccination on responses to meningococcal conjugate vaccine in adolescents.SBA (rabbit complement) GMTs before and after initial meningococcal serogroup A, C, W-135 and Y (MenACWY)-PS vaccination at 14 years of age, and before and after MenACWY-CV booster 3 years later (squares); SBA (rabbit complement) GMTs in vaccine naive subjects after MenACWY-CV at 17 years of age (circles).Error bars represent the 95% CIs.GMT: Geometric mean antibody titer; SBA: Serum bactericidal antibody.Data taken from Citation[56].](/cms/asset/90315e05-3931-4de6-84de-b96aa0c7707c/ierv_a_11217495_f0003_b.jpg)
Error bars represent the 95% CIs. Differences between the groups were statistically significant (p < 0.01) for all serotypes except OPA titers for serotype 19F.
GMC: Geometric mean antibody concentration; GMT: Geometric mean antibody titer; OPA: Opsonophagocytic assay: Pn: Pneumococcal; PS: Polysaccharide.
Data taken from Citation[86].
![Figure 4. Antipneumococcal antibody GMCs (A) and opsonophagocytic activity GMTs (B) in adults ≥70 years of age administered PCV-7 (n = 110) or 23vPn-PS followed 1 year later by PCV-7 (n = 78).Error bars represent the 95% CIs. Differences between the groups were statistically significant (p < 0.01) for all serotypes except OPA titers for serotype 19F.GMC: Geometric mean antibody concentration; GMT: Geometric mean antibody titer; OPA: Opsonophagocytic assay: Pn: Pneumococcal; PS: Polysaccharide.Data taken from Citation[86].](/cms/asset/62b9a0b1-820d-498b-9fe4-e00b144b54ba/ierv_a_11217495_f0004_b.jpg)
Triangles represent vaccine-naive subjects vaccinated at month 12–15 with 23vPn-PS; Subjects were vaccinated at 2, 4 and 6 months with 11Pn-PD and then boosted at 12–15 months with either 11Pn-PD (represented by circles) or with 23vPn-PS (represented by squares).
GMC: Geometric mean antibody concentration.
Data taken from Citation[95].
![Figure 5. Anti-pneumococcal antibody concentrations (non-22F ELISA) against serotype 3 following 11Pn-PD vaccination in infants.Triangles represent vaccine-naive subjects vaccinated at month 12–15 with 23vPn-PS; Subjects were vaccinated at 2, 4 and 6 months with 11Pn-PD and then boosted at 12–15 months with either 11Pn-PD (represented by circles) or with 23vPn-PS (represented by squares).GMC: Geometric mean antibody concentration.Data taken from Citation[95].](/cms/asset/a1e2862f-186b-47b7-9bfe-2dbe1bf2ed71/ierv_a_11217495_f0005_b.jpg)
Background
Polysaccharide vaccines
Haemophilus influenzae type b (Hib), Neisseria meningitidis and Streptococcus pneumoniae are encapsulated bacteria that together have been the most common causes of childhood meningitis in industrialized and developing countries Citation[1,2]. Hib and pneumococcus are also important causes of pneumonia and otitis media in children Citation[3,4]. All three of these bacteria are encased by a polysaccharide (PS) capsule that is the most important virulence determinant owing to its properties as a physical barrier, and its interaction with complement that allows it to circumvent the host antibacterial defense system Citation[5–9]. Thus, the ability of the host to produce specific antibodies against capsular PS plays a pivotal role in the defense against most encapsulated bacteria Citation[10,11].
The ability of the immune system to respond to PS develops late in ontogeny, and most PSs (known as TI-2 antigens) are poorly immunogenic in infants until the age of 18–21 months. Recent studies have shown that infants 12 months of age can respond to all 23 vaccine serotypes Citation[12], although exposure to PS in children under 2 years of age is not without risk Citation[13]. The marginal zone of the spleen, which is lacking in human neonates, plays an important role in the initiation and development of a TI-2 antibody response Citation[14,15]. Exposure to PS in older children and adults induces an antibody response, but no immune memory and no affinity maturation occurs. There are some notable exceptions to this rule: for example, meningococcal serogroup A PS vaccines (MenA-PS) induce an immune response in infants less than 1 year of age. However, this is short lived Citation[16] and long-term effects on booster responses are not known Citation[17,18]. Pneumococcal serotype 3 also induces an immune response at early age Citation[19].
Glycoconjugate vaccines
The coupling of a PS to a protein carrier allows B cells stimulated by PS to induce immunological memory . Thus, a key feature of conjugate vaccines is their ability to induce immunological priming in infants, with robust immune memory responses on subsequent booster vaccination with plain or conjugate PS Citation[20–25]. Compared with vaccination with PS, vaccination of infants with conjugate vaccines increases the amount of IgG antibodies produced and increases the IgG:IgM ratio on repeated vaccination. The predominance of the IgG1 subclass increases further upon booster immunization Citation[26]. Avidity maturation of antibodies is also apparent following primary and booster vaccination with conjugate vaccines Citation[27–34]. Importantly, infants lacking antibody responses to native PSs (i.e., infants with immunoglobulin subclass deficiencies) are able to mount antibody responses to conjugate vaccines Citation[35,36].
Hyporesponsiveness
Hyporesponsiveness (immune tolerance) refers to the inability of the individual to mount an immune response after booster vaccination of at least the same, or of a greater magnitude, than the response that was induced after primary vaccination. Hyporesponsiveness may occur when vaccination begins very early in life, and has been observed, for example, following administration of diphtheria–tetanus–whole-cell pertussis vaccine Citation[37,38] or a combined diphtheria–tetanus–acellular pertussis vaccine Citation[39] or diphtheria–tetanus vaccine Citation[40] to newborns. Repeated doses of some PS vaccines, notably meningococcal serogroup C (MenC) vaccines, also results in hyporesponsiveness, regardless of the age at which vaccination occurs. To date, hyporesponsiveness was thought not to be a feature of repeated vaccination with conjugate vaccines. Hyporesponsiveness is easily demonstrated both by ELISA Citation[41] and functional assays Citation[42,43].
The immune mechanisms responsible for the development of immune hyporesponsiveness are not known but several mechanisms acting alone or in concert have been proposed. Both natural immunity to specific PSs arising following exposure to bacteria or to cross-reacting bacteria, and immunity induced by conjugate vaccination will result in T-cell-dependent responses and the development of memory B cells. Subsequent exposure to PS induces a T-cell-independent immune response whereby immune memory cells are stimulated but not replenished, resulting in overall depletion of the memory-cell pool and attenuated responses on re-exposure to the same PS Citation[42]. Studies conducted in Gambian infants and in adolescents in Saudi Arabia, shown in , illustrate the development of hyporesponsiveness after repeated meningococcal PS (Men-PS) vaccination.
The response to exposure to PSs through infection or carriage is age dependent. Both infection and nasopharyngeal carriage of pneumococci in young infants result in serotype-specific hyporesponsiveness to subsequent vaccination Citation[41,44,45]. By contrast, antibody responses of adults to a first injection of a PS vaccine have features of a memory antibody response, with evidence of somatic hypermutation of the genes encoding the variable regions, and a predominance of IgG antibody Citation[46–48], indicating that infection or carriage is sufficient to induce immune memory responses by adulthood. Hyporesponsiveness may persist for years, possibly because of persistence of the PS within immune cells with ongoing neutralization of specific antibody, or by ongoing binding to new B cells Citation[49].
Another potential mechanism could include a role for dendritic cells. An in vitro study showed that in dendritic cells previously exposed to pneumococcal PS, the response to subsequent stimulation with lipopolysaccharide induced production of IL-10, an immunosuppressive cytokine, rather than IL-12, an immunostimulatory cytokine, with potentially important downstream effects on Tregs and antigen-specific antibody responses Citation[50]. However, since hyporesponsiveness is specific for a given PS antigen Citation[41], nonspecific regulatory mechanisms can be excluded. This leaves depletion of the B-memory pool (in older children and adults) Citation[42] and B-cell fatigue/unresponsiveness due to early binding/de-activation of B cells via unconjugated PS (young children) Citation[41] as the most likely explanations.
Hib vaccines
Hyporesponsiveness following repeated exposure to the Hib capsular PS polyribosyl-ribitol-phosphate (PRP) has not been observed. However, children who had recovered from Hib disease and who were subsequently immunized with Hib PS vaccine showed evidence of hyporesponsiveness Citation[51].
The immune response and antibody characteristics following repeated PRP-PS vaccination in children of various ages was evaluated in a key study conducted in Finland Citation[52]. Children between 3 and 17 months of age received two PRP-PS doses 2–6 months apart; children between 18 and 35 months, and 36 and 47 months of age received a single PRP dose. All children in each age bracket were re-vaccinated 3.5 years later. An unimmunized group acted as control. Immune responses to the initial vaccination were age dependent, with the highest responses observed in children over the age of 18 months. On re-vaccination after 3.5 years, antibody increases matched the responses in children of the same age receiving their first dose. No immune tolerance and no immune memory were demonstrated.
Meningococcal vaccines
Immune hyporesponsiveness to N. meningitidis serogroup C PS (MenC-PS) has been demonstrated in adults Citation[53], adolescents Citation[54], children Citation[24] and infants Citation[55]. Primary vaccination with MenC conjugate vaccine (MenC-CV) does not prevent the development of hyporesponsiveness to MenC on subsequent PS exposure. Furthermore, hyporesponsiveness to meningococcal serogroup C after repeated PS vaccination is unable to be completely reversed by subsequent conjugate vaccination Citation[32,56–60]. This suggests that production of immune memory cells by MenC-CV is impaired by exposure to MenC-PS, either before or after MenC-CV vaccination, and has implications for scheduling of meningococcal vaccines in older populations. Hyporesponsiveness to MenC-PS appears dose related, with more pronounced effects when doses over 10 µg are administered Citation[61].
Some of the striking features of the immune response to repeated MenC-PS are illustrated by a study conducted in The Gambia, in which infants vaccinated with two doses of meningococcal serogroups A and C (MenAC)-PS failed to develop serum bactericidal antibodies (SBAs) against serogroup C on re-vaccination with MenAC-PS during their second year of life Citation[55]. The SBA-MenC titer in MenAC-PS vaccinees was almost ten-times lower than the titer observed in vaccine-naive children of the same age receiving their first MenAC-PS dose.
At 2 years of age, a subset of MenAC-CV-primed children in the same study received a booster dose of MenC-PS during the second year of life. All MenAC-CV-primed children received a booster dose of MenAC-PS at 5 years of age. SBAs for serogroup C were sixfold lower after the year 5 MenAC-PS booster in those children who had received a MenAC-PS booster during the second year of life, than in children who had received no additional vaccination in their second year Citation[62].
Studies in children, adolescents and adults show that post-vaccination SBA titers against serogroup C after MenC-CV were lower when subjects had previously received PS vaccine, than in vaccine-naive subjects Citation[32,56–60].
Serogroup A PS appears to stimulate the immune system in a different way to serogroup MenC-PS. MenA-PS vaccination induces short-lived immune responses in children under 18 months of age Citation[63,64]; however, it remains uncertain whether MenA-PS is capable of inducing immune memory when administered to children of this age Citation[55,62,64]. In the Gambian study, serogroup A responses were generally of a greater magnitude, and an effect of previous MenA-PS exposure on responses to subsequent MenA-PS was not demonstrated . However, at least one study showed an apparent immune memory response to serogroup A, with higher SBA titers against serogroup A in MenAC-PS-primed and boosted subjects Citation[54,61].
A second dose of MenAC-PS in adults, administered 6 months after a first dose, resulted in SBA titers against serogroup A that were approximately a third of the magnitude of titers observed after the first dose Citation[65]. In MenA-PS primed adolescents, subsequent vaccination with tetravalent meningococcal serogroup A, C, W-135 and Y (MenACWY)-CV induced bactericidal titers against serogroup A that were lower than those observed in vaccine-naive adolescents that had received their first dose of MenACWY-CV Citation[56].
Responses to repeated exposure to serogroup W-135 and Y PSs have been less well studied. Like MenC-PS, meningococcal serogroup W-135 (MenW-135)-PS and meningococcal serogroup Y (MenY)-PS vaccines are poorly immunogenic in young children Citation[63]. However, clinical trial data in an adolescent population vaccinated with two doses of tetravalent MenACWY-PS vaccine showed that, similarly to MenA, post-dose two SBA geometric mean titers (GMT)s against MenW-135 and MenY approximated or exceeded post-primary titers Citation[56]. MenACWY-CV administered to adolescents previously vaccinated with tetravalent MenACWY-PS induced lower responses against all serogroups, including W-135 and Y, than vaccine-naive adolescents given their first MenACWY-CV dose . The impact on the MenC responses was higher as compared with serogroups A, W-135 and Y. A total of 1 year after vaccination with a full (50 µg) or fractional (either 10 or 5 µg) dose of MenACWY-PS, in a clinical trial in Uganda, 120 individuals (aged 2–19 years) were all re-vaccinated with a full dose of MenACWY-PS Citation[66]. After prior immunization with a full dose, SBA titers following re-vaccination were significantly lower as compared with those following primary vaccination for serogroups A, C and W-135. For serogroups C, W-135 and Y, hyporesponsiveness was not observed when using fractional doses as primary immunization, but for serogroup A, some degree of hyporesponsiveness was observed for the 10-µg dose but not the 5-µg dose.
Following a single dose of MenACWY-CV in Saudi children aged 5–8 years, serogroup C SBA titers but not serogroups A, Y and W-135 SBA titers, were shown to be significantly lower in those who had previously received two doses of MenACWY-PS before 2 years of age, as opposed to those naive to MenACWY-PS Citation[67,68].
Pneumococcal vaccines
Licensed pneumococcal PS (Pn-PS) vaccines contain 23 serotypes that differ in their capacity to induce an immune response. In a study in Finland using a 14-valent (14v) Pn-PS vaccine, infants were vaccinated at 7 months of age and re-vaccinated 6 months later at 13 months of age Citation[19]. Responses in terms of anticapsular antibodies and class-specific antibodies fell into three broad categories: poor immunogens (serotypes 1, 6A, 12 and 23F), characterized by weak initial responses and weaker booster responses, with predominantly IgM elicited by the booster dose; intermediate immunogens (serotypes 7F, 9N, 14, 19F and 25), characterized by moderate responses after the primary and booster doses with mixed IgG, IgM and IgA responses after vaccination; and stronger immunogens (serotypes 3, 4, 8 and 18C) with good initial responses but weaker booster responses with mixed class-specific antibodies after booster. In this age group, only serotype 3 and 18C PSs induced responses that provided any advantage over unvaccinated controls.
The immune response to pneumococcal PSs is age dependent, being higher in adults Citation[12,69]. In children 12–18-months of age vaccinated with two 23-valent (23v)Pn-PS vaccine doses 1 year apart, hyporesponsiveness was observed for serotypes 4, 6B, 9V, 18C and 23F, whereas modest booster responses were observed for serotypes 14 and 19F Citation[70]. Additionally, in a Finnish study, serotype 3 also induced hyporesponsiveness Citation[19]. Because the serotypes tested in individual studies vary, it is difficult to identify which serotypes are most prone to induce hyporesponsiveness on repeated PS vaccination. However, the majority of studies in healthy adults and children show reduced responses to many or most serotypes following repeated Pn-PS vaccine, consistent with hyporesponsiveness Citation[69,71–78]. It has to be noted that early generation pneumococcal anti-PS antibody detection assays were not fully specific Citation[79]. Later in the era of conjugate vaccines, such assays have been improved Citation[79].
The immune response to pneumococcal conjugate vaccines (PCVs) is also highly age dependent. PCVs containing seven (PCV-7), 10 (PCV-10; also referred to as PHiD-CV) or 13 (PCV-13) serotypes are currently licensed for use in infants and children. For the licensed vaccines, the characteristics of a T-cell-dependent immune response typical of conjugate vaccines have been demonstrated for all of the serotypes contained in the vaccines. Robust immune responses are induced in infants, with good booster responses on re-vaccination and evidence of immunological priming Citation[80,81], although responses to serogroup 6B and 23F tended to be lower than other serotypes, reflecting poorer immunogenicity of these serotypes after PS vaccination Citation[19,82]. It has been observed that fewer doses or lower conjugate dosage given early in life may favor the development of immune memory B cells as a consequence of the lower induction of antibody-producing plasma B cells Citation[83].
In adults, the immune response to PCV at a 2-µg PS dosage appears similar to PS at 25 µg, therefore whether conjugate vaccines offer any advantage over the PS vaccine in this age group is uncertain. Some studies show post-PCV antibody responses and booster responses on re-exposure to PS that are not different to consecutive 23vPn-PS vaccine doses Citation[84,85]. In a dose-range study in elderly adults with a history of vaccination with 23v-PS, antibody concentrations after vaccination with PCV-7 (pediatric dosage) were in the same range as antibody concentrations following a further 23vPn-PS vaccine dose for most serotypes Citation[85]. However, a dose response was observed, with a trend toward higher antibody concentrations following higher PCV-7 dosages (4 µg) compared with the 23vPn-PS vaccine group. A challenge dose of 23vPn-PS vaccine administered 1 year later failed to induce booster responses to any serotype in PCV-7-vaccinated or 23vPn-PS-vaccinated adults, regardless of the PCV-7 dose initially administered Citation[85].
It remains controversial whether Pn-PS vaccine-induced hyporesponsiveness can be ‘corrected’ by subsequent vaccination with PCV. In elderly adults given PCV-7, antibody concentrations and opsonophagocytic responses were significantly lower if they had received a previous 23vPn-PS vaccine dose than in vaccine-naive adults given their first PCV-7 vaccination Citation[86]. In 24–30-month-old children administered PCV-7, only anti-6B and anti-23F antibody concentrations were lower in subjects who had received 23vPn-PS vaccine 12 months earlier Citation[70].
Only one study has investigated the immune response to consecutive 23vPn-PS vaccine doses in PCV-primed individuals. 23vPn-PS challenge administered to children primed with PCV-7 in infancy, and who did or did not receive 23vPn-PS at 12 months of age resulted in hyporesponsiveness in the 23vPn-PS-boosted children Citation[78].
Can glycoconjugate vaccines induce immune hyporesponsiveness?
Neither Hib nor MenC-CV induce hyporesponsiveness after repeated vaccination in infants or adults. A combined Hib–meningococcal serogroup CY-conjugate vaccine (Hib-MenCY) showed immune memory against Hib, MenC and MenY, with robust booster responses after primary vaccination in infancy Citation[87]. Tetravalent MenACWY-CVs differ in their immunogenicity in infants and adolescents. These differences are probably primarily due to differences in the protein conjugate used, but other differences between vaccines, including the size of the saccharide, conjugation chemistry and amount of free PS, may play a role. In infants, low responses against all serogroups were observed when diphtheria toxoid was used as the protein conjugate Citation[88], whereas higher responses against serogroups C, W-135 and Y, but not A, occurred when CRM197 (a mutated nontoxic diphtheria toxin) was used Citation[89]. In a head-to-head study in adolescents, serum bactericidal titers (human complement source) were statistically significantly higher following administration of MenACWY-CV conjugated to CRM197 compared with MenACWY-CV conjugated to diphtheria toxoid for all serogroups except MenC Citation[90]. Despite the reduced immunogenicity in infants, immune memory and strong booster responses have been demonstrated for both vaccines Citation[88,89]. MenACWY-CV conjugated to tetanus toxoid (TT) is under development and has demonstrated a good immunogenicity profile Citation[91].
Age may also be a factor in the risk of developing hyporesponsiveness to PCVs. In a study of vaccine-naive adults at least 75 years of age who received two doses of PCV-7 1 year apart, the post-booster antibody concentrations were lower than after the first PCV-7 dose for serotypes 9V, 14, 18C and 19F (significant only for 9V; 5.7 vs 9.8; p = 0.02 Citation[86]). In HIV-infected adults given two PCV-7 doses 8 weeks apart, post-dose two responses were similar to, or lower than, post-dose one responses for all of the serotypes tested (4, 6B, 9V, 14 and 23F) Citation[92].
Serotype 3
Serotype 3 was included in a prototype 11-valent PCV, in which all serotypes were conjugated to protein D (a recombinant nonlipidated form of a highly conserved, 42-kDa cell surface lipoprotein of nontypeable H. influenzae; vaccine referred to as 11Pn-PD), but was subsequently removed from the final formulation, resulting in the 10-valent marketed product Citation[93]. The removal of serotype 3 was related to the observation that immune responses after the booster dose to serotype 3 differed from those to other serotypes, and to its failure to demonstrate efficacy against acute otitis media (AOM) Citation[93,94]. Serotype 3 was the only serotype out of the 11 for which post-booster antipneumococcal antibody GMCs were lower than post-primary GMCs after three doses of 11Pn-PD in infancy Citation[95]. Although 11Pn-PD-primed infants did appear to mount a response to a toddler dose of 23v-PS, the absence of a control group that received only the toddler 23v-PS dose means that it is not possible to conclude if the post-23v-PS response to serotype 3 was also blunted in 11Pn-PD-primed infants. Serotype 3 is included in PCV-13, but studies to date also show low or no booster responses against this serotype . Although it cannot be excluded that some priming has occurred, a first dose of 11Pn-PD or PCV-13 administered to toddlers in the second year of life induced higher antibody concentrations than those in subjects of the same age receiving their fourth dose Citation[95,96].
Clinical aspects of hyporesponsiveness
The clinical implications of the development of hyporesponsiveness are largely unknown. Theoretically, a reduced immune response following a booster dose of a PS vaccine could lead to increased susceptibility to disease on exposure; however, clinical experience is lacking.
It has been clearly demonstrated that some individuals are unable to respond to particular serotypes following invasive pneumococcal disease (IPD) and repeated vaccination with PCV-7 in children Citation[44], or 23vPn-PS in adults Citation[97]. The exact mechanisms for this are not known, but could reflect either immune paralysis due to large pneumococcal PS antigen loads during IPD, and/or a potential genetic basis for nonresponse to individual pneumococcal serotypes. Recently, it has been demonstrated in two separate studies Citation[41,45] that nasopharyngeal carriage by a pneumococcus results in a significantly impaired antibody response to the specific serotype after vaccination with PCV.
The importance of immune memory & kinetics of the immune response in protection against encapsulated bacteria
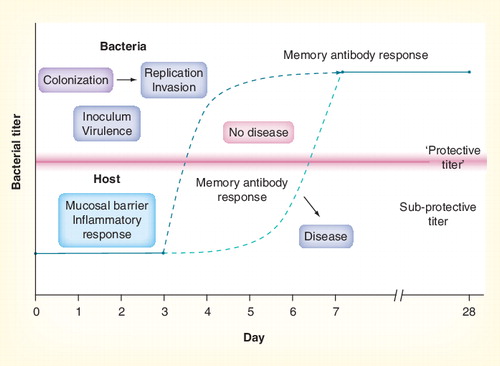
Immune memory responses may be unable to protect against disease if the time from exposure to achieving protective antibody levels is greater than the time between exposure and development of invasive disease .
It is generally accepted that invasive meningococcal disease usually occurs shortly (often 1–7 days) after acquisition of a new strain Citation[98,99]. By contrast, in primed subjects, anti-MenC-PS antibodies rise above baseline some time after day 3 following MenC-PS challenge Citation[53]. The observation that children with MenC-CV vaccine failure showed a normal anamnestic response to infection suggests that, for MenC, circulating antibody is more important than booster responses in preventing invasive disease Citation[100].
The situation is probably the same for Hib. Negative efficacy (not statistically significant from 0% efficacy) against invasive Hib disease was shown in Minnesota, USA, when the Hib-PS vaccine was widely implemented in 24–34-month-old children Citation[101]. Hib-PS was associated with increased disease susceptibility for the first week after vaccination, possibly because of saturation of circulating antibodies by antigen binding Citation[102], highlighting the importance of circulating antibody in disease protection. Examination of Hib vaccine failures in the UK also suggests that normal immune memory responses may be too slow to be effective in preventing invasive Hib disease Citation[103].
A study in adults primed and boosted with PCV-7 showed that circulating IgG antibody-forming cells (plasma cells) rose above baseline levels by day 4–5 and peaked between day 6 and 7 after booster Citation[104]. Booster was also accompanied by a rise in circulating memory B cells. Antibody levels rose above baseline by day 6 and peaked at day 15 after the booster dose. Antibody responses following vaccination appeared after day 3 and peak by 7–10 days after vaccination. This relatively slow response provides several (3–6) days for which an invasive bacteria can replicate unchecked and cause systemic disease if protective antibody concentrations are not already present. Thus, maintenance of protective antibody levels in serum may be critical in preventing invasive disease.
The mechanisms responsible for maintenance of antibodies in the long term are not known. Studies with MenC-CVs suggest that the degree of antibody persistence over time is age dependent Citation[105]. One explanation is that fewer memory B cells, required for ongoing renewal of a plasma cell population that continues to secrete specific antibody, are produced by infants, leading to accelerated antibody loss over time compared with older age groups Citation[106,107]. Thus, in infants, a booster dose of conjugate vaccine resulting in memory B-cell proliferation may be important in maintaining adequate levels of circulating antibody Citation[108]. This appears the case for Hib and MenC, but the kinetics for pneumococcal colonization and invasion could be different.
In the case of IPD, immune memory may also be a correlate of protection. One can hypothesize that heavily capsulated pneumococci can adsorb the existing antibodies, leaving the immune memory response as the critical protective component. Adsorption of existing antibodies has also been demonstrated between 1–3 days post-booster immunization of toddlers and adults with 25 µg plain Hib-PS Citation[109].
A case report of serotype 14 breakthrough disease in a subject with circulating anti-serotype 14 antibodies but an impaired immune memory response supports the hypothesis of immune memory as a correlate of protection Citation[110]. This may be particularly true for serotype 3 pneumococci that are heavily encapsulated (adsorption of pre-existing antibodies upon infection as well as hyporesponsiveness induced by immunization) and would explain the absence of protection against serotype 3 AOM after pediatric conjugate immunization and against IPD after adult PS immunization Citation[93,111].
Conclusion
Hyporesponsiveness occurs following repeated PS vaccination with meningococcal vaccines and many pneumococcal vaccine serotypes. Although hyporesponsiveness has not been observed in clinical studies of Hib-PS, hyporesponsiveness may follow clinical exposure to Hib in some individuals. In infants, use of currently licensed conjugate vaccines has not been associated with hyporesponsiveness, with the possible exception of pneumococcal serotype 3. It appears that introduction of PS vaccines anywhere into the vaccination schedule may result in reduced immune responses on subsequent exposure, even in conjugate-vaccinated subjects. Using PSs to demonstrate immune memory has been a basic requirement for licensure of new conjugate vaccines. Given the potential consequence of hyporesponsiveness in individual subjects, this practice should be discouraged Citation[42], as reflected in recent WHO recommendations Citation[112].
The notion that hyporesponsiveness may occur for specific components of combined conjugate vaccines, such as pneumococcal serotype 3, is new and poses challenges for the development of vaccines with increased valency.
Expert commentary
Polysaccharide vaccines have been instrumental in preventing disease due to pneumococcus and meningococcus for decades. Immune hyporesponsiveness following repeated exposure to PS has long been recognized. Although the clinical implications have not been extensively studied, there is nevertheless a theoretical risk for increased disease susceptibility for a prolonged period when hyporesponsiveness occurs. The clinical risk could conceivably be greater in developing countries where recurrent exposure to pathogens is more likely.
Conjugate vaccines were developed primarily for use in infants, in whom most PS vaccines are ineffective. For meningococcal conjugate vaccines where the problem of hyporesponsiveness appears to have been overcome, ongoing use of Men-PS vaccines should be avoided as far as possible, particularly in children less than 2 years of age Citation[113] and when sustained protection necessitating re-vaccination is likely to be required. This is the case for travelers from industrialized to nonindustrialized countries, and in countries where meningococcal epidemics regularly occur, for example, during the Hajj pilgrimage and in countries within the African meningitis belt. At present, however, Men-PS vaccines continue to play an important role in preventing deaths due to invasive disease in developing countries. Mass vaccination with a trivalent ACW-135 PS vaccine in Burkino Faso has proved effective in outbreak management Citation[114]. In settings where conjugate vaccines are not available and/or unaffordable, the benefits of Men-PS vaccination currently outweigh the theoretical risks of induction of hyporesponsiveness Citation[113]. However, by providing boostable immunity, widespread meningococcal conjugate vaccination promises enormous benefits over PS vaccination for these populations.
Caution should also be used in administering Pn-PS vaccines to children less than 2 years of age, as suggested by a recent study showing increased susceptibility to acute lower respiratory tract infections Citation[13].
Diversity among pneumococcal serotypes has made development of multivalent PCVs complex. Protein conjugation of pneumococcal PS has not overcome development of hyporesponsiveness in adults with subsequent plain PS immunization.
Available evidence suggests that serotype 3 conjugate vaccination in infants induces a degree of hyporesponsiveness, but further investigation is needed to understand infant responses to this serotype. Serotype-specific post-marketing surveillance will be needed after introduction of PCV-13 containing serotype 3 to evaluate protective efficacy of this vaccine antigen against IPD.
Serotype replacement of vaccine serotypes with nonvaccine serotypes in highly vaccinated populations means that PCVs containing new serotypes, or more serotypes, will be called for in years to come. It is likely that new conjugation methods, including new carrier proteins, will be needed to successfully incorporate these additional serotypes with potentially diverse capsular phenotypes and immunological characteristics, and to avoid immune interferences Citation[115]. Adequate testing in prelicensure clinical trials will be needed to evaluate safety and immunogenicity of novel vaccines. However, the recent licensure of PHiD-CV, the first vaccine to employ protein D as a carrier protein, demonstrates the feasibility of this approach.
The search for extended and more immunogenic PCVs for use in older adults is ongoing, although pediatric use of multivalent conjugates may lead to the disappearance of pediatric vaccine types in adults via indirect herd immunity effects related to reduced transmission, and may therefore lead to the emergence of nonvaccine types in adults. Therefore, there is also active research ongoing to discover pneumococcal protein antigens that may prevent serotype replacement and broaden vaccine coverage for pediatric and adult use.
Five-year view
New PCVs containing more than 13 serotypes are unlikely to become available in the next 5 years. The question of whether the introduction of pneumococcal serotype 3 into PCV-13 will result in protection against serotype 3 disease will be resolved through post-marketing surveillance of IPD-causing serotypes. Wider availability of effective conjugate vaccines such as PHiD-CV and PCV-13, as well as MenA-TT, MenACWY-CRM197 and MenACWY-TT, will see the phasing out of PS vaccines for disease prevention, particularly in developing countries where more effective vaccines providing long-lasting immunity are needed. Development of broadly protective meningococcal serogroup B vaccines, potentially based on nonconjugate technologies, will move ahead. Development of PCVs for use in adults and the elderly will continue, but refinements in conjugation immunology and technology will see development of a new generation of PCVs for use in these age groups. The outcome of a PCV-13 adult community-acquired pneumonia efficacy trial will become known and this will impact the views on how to best prevent adult community-acquired pneumococcal pneumonia Citation[201]. In addition to the aspect of possible enhanced protection with conjugates versus existing 23vPn-PS vaccines, the aspect of serotype coverage in an era when pediatric conjugate immunization may lead to dominance of nonvaccine types in adult pneumococcal disease will impact vaccine development. A higher valency PCV (up to 23 or more) may then be required to cope with adult nonvaccine types. Alternatively, pneumococcal protein antigens will be developed to extend vaccine coverage beyond 10–13-valent PCVs.
Table 1. Immune responses to pneumococcal serotypes in different populations illustrating hyporesponsiveness.
Table 2. Immune responses to pneumococcal serotype 3.
Key issues
• Hyporesponsiveness (or immune tolerance) refers to the inability of the individual to mount an immune response after repeated vaccination or colonization that is less than the same magnitude of immune response induced after primary vaccination and in the absence of colonization.
• Hyporesponsiveness may occur after colonization with encapsulated bacteria or after repeated exposure to polysaccharide vaccines used in humans and is antigen dependent. It may also be dose- and age dependent, and may be long lasting.
• Immune hyporesponsiveness to repeated Neisseria meningitidis serogroup C polysaccharide exposure has been demonstrated in all age groups. Limited available data suggests that all four meningococcal serogroup polysaccharides (A, C, W-135 and Y) have the potential to induce immune hyporesponsiveness at high doses, including in older age groups.
• Most studies in healthy adults and children show evidence of hyporesponsiveness to many or most pneumococcal serotypes following repeated exposure via vaccination.
• Repeat doses of neither Haemophilus influenzae type b (Hib) nor meningococcal serogroup A, C, W-135 or Y conjugate vaccines induce hyporesponsiveness in infants or adults. However, hyporesponsiveness occurs in children and adults who receive pneumococcal polysaccharide after pneumococcal conjugate vaccine (PCV), and hyporesponsiveness to pneumococcal serotype 3 in infants has been observed after booster doses of PCV.
• The notion that hyporesponsiveness may occur for specific components of combined conjugate vaccines, such as pneumococcal serotype 3, is new and poses challenges for the development of larger valency conjugate vaccines.
• The clinical implications of the development of hyporesponsiveness are largely unknown but could theoretically lead to increased susceptibility to disease on exposure, particularly in the absence of residual antibodies. Furthermore, the capacity to respond to bacterial exposure may come too late for meningococcal and Hib-invasive disease due to the fast kinetics of exposure to invasion overtaking immune memory recall. In the case of pneumococcal disease, there is some evidence that a recall response correlates with protection, suggesting that the kinetics of pneumococcal colonization to invasion is somewhat slower compared with meningococcal and Hib kinetics.
• In the case of pneumococcal serotype 3 disease, the combination of a large quantity of capsule upon exposure leading to adsorption of residual antibodies, and the absence of induced immune memory by conjugate or polysaccharide immunization, may lead to disease susceptibility and the absence of vaccine efficacy.
• Multivalent PCVs have demonstrated induction of antibodies and immune memory, and have been shown to be efficacious (except for serotype 3) in young children. PCVs are being studied for efficacy in older adults. Even if efficacious in older adults, the clinical impact of these vaccines may be limited due to the reduced prevalence of pediatric serotypes in older adults, linked to herd immunity effects from pediatric immunization and reduced transmission of pediatric serotypes to adults.
• Pneumococcal protein antigens are being investigated as an alternative/additive option to broaden the coverage of pneumococcal immunization in children and adults and to avoid serotype replacement.
Acknowledgements
The authors are indebted to Dan Granoff for donating Figure 6. The authors thank Joanne Wolter and Ulrike Krause for assistance in preparation of the manuscript.
Financial & competing interests disclosure
Jan Poolman is an employee of GlaxoSmithKline Biologicals. Ray Borrow has received assistance to attend scientific meetings from Pfizer, Novartis, Sanofi Pasteur and Baxter Bioscience and has served as an ad hoc consultant for Pfizer, GlaxoSmithKline Biologicals, Novartis, Sanofi Pasteur and Baxter Bioscience. Industry honoraria received for consulting, lecturing and writing are paid directly into Central Manchester and Manchester Children’s University Hospitals NHS Trust endowment fund. Ray Borrow has performed contract research on behalf of the Health Protection Agency (funded by Pfizer, Novartis, Baxter Bioscience, GlaxoSmithKline Biologicals and Sanofi Pasteur). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was utilized in the production of this manuscript. Joanne Wolter assisted in writing the first draft of the manuscript. Funding for this assistance was provided by GlaxoSmithKline Biologicals.
References
- Schuchat A, Robinson K, Wenger JD et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N. Engl. J. Med.337(14), 970–976 (1997).
- Peltola H. Burden of meningitis and other severe bacterial infections of children in Africa: implications for prevention. Clin. Infect. Dis.32(1), 64–75 (2001).
- Cripps AW, Otczyk DC. Prospects for a vaccine against otitis media. Expert Rev. Vaccines5(4), 517–534 (2006).
- Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull. World Health. Organ.86(5), 408–416 (2008).
- Weller PF, Smith AL, Anderson P, Smith DH. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J. Infect. Dis.135(1), 34–41 (1997).
- Winkelstein JA, Bocchini JA Jr, Schiffman G. The role of the capsular polysaccharide in the activation of the alternative pathway by the pneumococcus. J. Immunol.116(2), 367–370 (1976).
- Kugelberg E, Gollan B, Tang CM. Mechanisms in Neisseria meningitidis for resistance against complement-mediated killing. Vaccine26(Suppl. 8), 134–139 (2008).
- Anderson P, Johnston RB Jr, Smith DH. Human serum activities against Haemophilus influenzae type b. J. Clin. Invest.51(1), 31–38 (1972).
- Weller PF, Smith AL, Smith DH, Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J. Infect. Dis.138(4), 427–436 (1978).
- Santosham M, Reid R, Ambrosino DM et al. Prevention of Haemophilus influenzae type b infections in high-risk infants treated with bacterial polysaccharide immune globulin. N. Engl. J. Med.317(15), 923–929 (1987).
- Ambrosino DM, Barrus VA, DeLange GG, Siber GR. Correlation of the Km(1) immunoglobulin allotype with anti-polysaccharide antibodies in Caucasian adults. J. Clin. Invest.78(2), 361–365 (1986).
- Balloch A, Licciardi PV, Russell FM, Mulholland EK, Tang ML. Infants aged 12 months can mount adequate serotype-specific IgG responses to pneumococcal polysaccharide vaccine. J. Allergy Clin. Immunol.126(2), 395–397 (2010).
- O’Grady KA, Lee KJ, Carlin JB et al. Increased risk of hospitalization for acute lower respiratory tract infection among Australian indigenous infants 5–23 months of age following pneumococcal vaccination: a cohort study. Clin. Infect. Dis.50(7), 970–978 (2010).
- Humphrey JH. Splenic macrophages: antigen presenting cells for T1–2 antigens. Immunol. Lett.11(3–4), 149–152 (1985).
- Timens WA. The Human Spleen. Development and Role in the Immune System. University of Groningen, The Netherlands (1985) (Thesis).
- Reingold AL, Broome CV, Hightower AW et al. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide A vaccine. Lancet326(8447), 114–118 (1985).
- Vergnano S, Heath P. Neisseria meningitides serogroup A vaccines: an overview. Expert Rev. Vaccines2(4), 571–582 (2003).
- Ismail AA. Harris SL, Granoff DM. Serum group A anticapsular antibodies in a Sudanese population immunized with meningococcal polysaccharide vaccine during a group A epidemic. Pediatr. Infect. Dis. J.23(8), 748–755 (2004).
- Koskela M, Leinonen M, Häivä VM, Timonen M, Mäkelä PH. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr. Infect. Dis.5(1), 45–50 (1986).
- Käyhty H. Difficulties in establishing a serological correlate of protection after immunization with Haemophilus influenzae conjugate vaccines. Biologicals22(4), 397–402 (1994).
- Lepow ML, Barkin RM, Berkowitz CD et al. Safety and immunogenicity of Haemophilus influenzae type b polysaccharide–diphtheria toxoid conjugate vaccine (PRP-D) in infants. J. Infect. Dis.156(4), 591–596 (1987).
- Weinberg GA, Einhorn MS, Lenoir AA, Granoff PD, Granoff DM. Immunologic priming to capsular polysaccharide in infants immunized with Haemophilus influenzae type b polysaccharide–Neisseria meningitidis outer membrane protein conjugate vaccine. J. Pediatr.111(1), 22–27 (1987).
- Granoff DM, Sheetz KE, Nahm MH, Madassery JV, Shackelford PG. Further immunologic evaluation of children who develop Haemophilus disease despite previous vaccination with type b polysaccharide vaccine. Monogr. Allergy.23, 256–268 (1988).
- MacDonald NE, Halperin SA, Law BJ, Forrest B, Danzig LE, Granoff DM. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA280(19), 1685–1689 (1998).
- Anderson PW, Pichichero ME, Stein EC et al. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J. Immunol.142(7), 2464–2468 (1989).
- Ambrosino DM, Sood SK, Lee MC et al. IgG1, IgG2 and IgM responses to two Haemophilus influenzae type b conjugate vaccines in young infants. Pediatr. Infect. Dis. J.11(10), 855–859 (1992).
- Anttila M, Eskola J, Ahman H, Käyhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis.177(6), 1614–1621 (1998).
- Harris SL, Finn A, Granoff DM. Disparity in functional activity between serum anticapsular antibodies induced in adults by immunization with an investigational group A and C Neisseria meningitidis–diphtheria toxoid conjugate vaccine and by a polysaccharide vaccine. Infect. Immun.71(6), 3402–3408 (2003).
- Lucas AH, Granoff DM. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide–protein conjugates. J. Immunol.154(8), 4195–4202 (1995).
- Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis.177(4), 1112–1115 (1998).
- Joseph H, Ryall R, Bybel M et al. Immunogenicity and immunological priming of the serogroup a portion of a bivalent meningococcal A/C conjugate vaccine in 2-year-old children. J. Infect. Dis.187(7), 1142–1146 (2003).
- Borrow R, Southern J, Andrews N et al. Comparison of antibody kinetics following meningococcal serogroup C conjugate vaccine between healthy adults previously vaccinated with meningococcal A/C polysaccharide vaccine and vaccine-naive controls. Vaccine19(23–24), 3043–3050 (2001).
- Ekström N, Ahman H, Verho J et al. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun.73(1), 369–377 (2005).
- Poolman J, Kaufhold A, De Grave D, Goldblatt D. Clinical relevance of lower Hib response in DTPa-based combination vaccines. Vaccine19(17–19), 2280–2285 (2001).
- Lenoir AA, Pandey JP, Granoff DM. Antibody responses of black children to Haemophilus influenzae type b polysaccharide–Neisseria meningitidis outer-membrane protein conjugate vaccine in relation to the Km(1) allotype. J. Infect. Dis.157(6), 1242–1245 (1988).
- Gross S, Blaiss MS, Herrod HG. Role of immunoglobulin subclasses and specific antibody determinations in the evaluation of recurrent infection in children. J. Pediatr.121(4), 516–522 (1992).
- Provenzano RW, Wetterlow LH, Sullivan CL. Immunization and antibody response in the newborn infant: I. Pertussis inoculation within twenty-four hours of birth. N. Engl. J. Med.273(18), 959–965 (1965).
- Barrett CD, Timm EA, Molner JG, Wilner BI, Fahey MF, McLean IW. Multiple antigen for immunization against poliomyelitis, diphtheria, pertussis, and tetanus. II. Response of infants and young children to primary immunization and eighteen-month booster. Am. J. Public Health49(5), 644–655 (1959).
- Halasa NB, O’Shea A, Shi JR, LaFleur BJ, Edwards KM. Poor immune responses to a birth dose of diphtheria, tetanus, and acellular pertussis vaccine. J. Pediatr.153(3), 327–332 (2008).
- Lieberman JM, Greenberg DP, Wong VK et al. Effect of neonatal immunization with diphtheria and tetanus toxoids on antibody responses to Haemophilus influenzae type b conjugate vaccines. J. Pediatr.126(2), 198–205 (1995).
- Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J. Infect. Dis.201(10), 1570–1579 (2010).
- Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr. Infect. Dis. J.26(8), 716–722 (2007).
- Russell FM, Carapetis JR, Burton RL et al. Opsonophagocytic activity following a reduced dose 7-valent pneumococcal conjugate vaccine infant primary series and 23-valent pneumococcal polysaccharide vaccine at 12 months of age. Vaccine29(3), 535–544 (2011).
- Borrow R, Stanford E, Waight P et al. Serotype-specific immune unresponsiveness to pneumococcal conjugate vaccine following invasive pneumococcal disease. Infect. Immun.76(11), 5305–5309 (2008).
- Vakevainen M, Soininen A, Lucero M et al. Serotype-specific hyporesponsiveness to pneumococcal conjugate vaccine in infants carrying pneumococcus at the time of vaccination. J. Pediatr.157(5), 778–783 (2010).
- Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, Goldblatt D. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol.30(4), 1214–1223 (2000).
- Mäkelä O, Mattila P, Rautonen N, Seppälä I, Eskola J, Käyhty H. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). J. Immunol.139(6), 1999–2004 (1987).
- Shackelford PG, Granoff DM, Nelson SJ, Scott MG, Smith DS, Nahm MH. Subclass distribution of human antibodies to Haemophilus influenzae type b capsular polysaccharide. J. Immunol.138(2), 587–592 (1987).
- O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect. Dis.7(9), 597–606 (2007).
- Meltzer U, Goldblatt D. Pneumococcal polysaccharides interact with human dendritic cells. Infect. Immun.74(3), 1890–1895 (2006).
- Holmes SJ, Granoff DM. The biology of Haemophilus influenzae type b vaccination failure. J. Infect. Dis.165(Suppl. 1), 121–128 (1992).
- Käyhty H, Karanko V, Peltola H, Mäkelä PH. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics74(5), 857–865 (1984).
- Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J. Infect. Dis.178(3), 870–748 (1998).
- Jokhdar H, Borrow R, Sultan A et al. Immunologic hyporesponsiveness to serogroup C but not serogroup A following repeated meningococcal A/C polysaccharide vaccination in Saudi Arabia. Clin. Diagn. Lab. Immunol.11(1), 83–88 (2004).
- Leach A, Twumasi PA, Kumah S et al. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide–protein conjugate vaccine. J. Infect. Dis.175(1), 200–204 (1997).
- Keyserling H, Papa T, Koranyi K et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch. Pediatr. Adolesc. Med.159(10), 907–913 (2005).
- Vu DM, de Boer AW, Danzig L et al. Priming for immunologic memory in adults by meningococcal group C conjugate vaccination. Clin. Vaccine Immunol.13(6), 605–610 (2006).
- Richmond P, Kaczmarski E, Borrow R et al. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J. Infect. Dis.181(2), 761–764 (2000).
- MacDonald NE, Halperin SA, Law BJ, Danzig LE, Granoff DM. Can meningococcal C conjugate vaccine overcome immune hyporesponsiveness induced by previous administration of plain polysaccharide vaccine? JAMA283(14), 1826–1827 (2000).
- Southern J, Deane S, Ashton L et al. Effects of prior polysaccharide vaccination on magnitude, duration, and quality of immune responses to and safety profile of a meningococcal serogroup C tetanus toxoid conjugate vaccination in adults. Clin. Diagn. Lab. Immunol.11(6), 1100–1104 (2004).
- Gold R, Lepow ML, Goldschneider I, Draper TL, Gotschlich EC. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Invest.56(6), 1536–1547 (1975).
- MacLennan J, Obaro S, Deeks J et al. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J. Infect. Dis.183(1), 97–104 (2001).
- Al-Mazrou Y, Khalil M, Borrow R et al. Serologic responses to ACYW135 polysaccharide meningococcal vaccine in Saudi children under 5 years of age. Infect. Immun.73(5), 2932–2939 (2005).
- Käyhty H, Karanko V, Peltola H, Sarna S, Mäkelä PH. Serum antibodies to capsular polysaccharide vaccine of group A Neisseria meningitidis followed for three years in infants and children. J. Infect. Dis.142(6), 861–868 (1980).
- Borrow R, Joseph H, Andrews N et al. Reduced antibody response to revaccination with meningococcal serogroup A polysaccharide vaccine in adults. Vaccine19(9–10), 1129–1132 (2000).
- Næss LM, Guerin PJ, Rosenqvist E, Kristiansen LH, Caugant DA. Influence of prior vaccination with fractional doses of a tetravalent meningococcal polysaccharide vaccine on serum bactericidal antibody responses after revaccination. Presented at: 10th Meeting of the European Meningococcal Disease Society (EMGM). Manchester, UK, 17–19 June 2009.
- Borrow R. Meningococcal disease and prevention at the Hajj. Travel Med. Infect. Dis.7(4), 219–225 (2009).
- Khalil M, Al-Mazrou Y, Bravo C et al. Response to quadrivalent meningococcal conjugate vaccine in Saudi Arabian children who previously received 2 doses of quadrivalent meningococcal polysaccharide vaccine before 2 years of age. Presented at: 10th Meeting of the European Meningococcal Disease Society (EMGM). Manchester, UK, 17–19 June 2009.
- Borgoño JM, McLean AA, Vella PP et al. Vaccination and revaccination with polyvalent pneumococcal polysaccharide vaccines in adults and infants. Proc. Soc. Exp. Biol. Med.157(1), 148–154 (1978).
- Blum MD, Dagan R, Mendelman PM et al. A comparison of multiple regimens of pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine and pneumococcal polysaccharide vaccine in toddlers. Vaccine18(22), 2359–6723 (2000).
- Mufson MA, Hughey DF, Turner CE, Schiffman G. Revaccination with pneumococcal vaccine of elderly persons 6 years after primary vaccination. Vaccine9(6), 403–407 (1991).
- Törling J, Hedlund J, Konradsen HB, Örtqvist Å. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine22(1), 96–103 (2003).
- Heidelberger M, Dilapi MM, Siegel M, Walter AW. Persistence of antibodies in human subjects injected with pneumococcal polysaccharides. J. Immunol.65(5), 535–541 (1950).
- Musher DM, Groover JE, Rowland JM et al. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin. Infect. Dis.17(1), 66–73 (1993).
- Lawrence EM, Edwards KM, Schiffman G, Thompson JM, Vaughn WK, Wright PF. Pneumococcal vaccine in normal children. Primary and secondary vaccination. Am. J. Dis. Child.137(9), 846–850 (1983).
- Jackson LA, Benson P, Sneller VP et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA281(3), 243–248 (1999).
- Sell SH, Wright PF, Vaughn WK, Hompson J, Schiffman G. Clinical studies of pneumococcal vaccines in infants. I. Reactogenicity and immunogenicity of two polyvalent polysaccharide vaccines. Rev. Infect. Dis.3(Suppl.), S97–S107 (1981).
- Russell FM, Carapetis JR, Balloch A et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide at 12 months of age in a randomised controlled trial. Vaccine28(19), 3341–3349 (2010).
- Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin. Vaccine Immunol.17(1), 134–142 (2010).
- Eskola J, Kilpi T, Palmu A et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med.344(6), 403–409 (2001).
- Black S, Shinefield H, Fireman B et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J.19(3), 187–195 (2000).
- Lockhart SP, Hackell JG, Fritzell B. Pneumococcal conjugate vaccines: emerging clinical information and its implications. Expert Rev. Vaccines5(4), 553–564 (2006).
- Russell FM, Licciardi PV, Balloch A et al. Safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine at 12 months of age, following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine in infancy. Vaccine28(18), 3086–3094 (2010).
- Shelly MA, Jacoby H, Riley GJ, Graves BT, Pichichero M, Treanor JJ. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun.65(1), 242–247 (1997).
- Jackson LA, Neuzil KM, Nahm MH et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide–protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine25(20), 4029–4037 (2007).
- de Roux A, Schmöle-Thoma B, Siber GR et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin. Infect. Dis.46(7), 1015–1023 (2008).
- Nolan T, Lambert S, Roberton D et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine25(51), 8487–8499 (2007).
- Rennels M, King J Jr, Ryall R, Papa T, Froeschle J. Dosage escalation, safety and immunogenicity study of four dosages of a tetravalent meninogococcal polysaccharide diphtheria toxoid conjugate vaccine in infants. Pediatr. Infect. Dis. J.23(5), 429–435 (2004).
- Pace D. Quadrivalent meningococcal ACYW-135 glycoconjugate vaccine for broader protection from infancy. Expert Rev. Vaccines8(5), 529–542 (2009).
- Jackson LA, Baxter R, Reisinger K et al. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin. Infect. Dis.49(1), e1–e10 (2009).
- Knuf M, Kieninger-Baum D, Habermehl P et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine28(3), 744–753 (2010).
- Feikin DR, Elie CM, Goetz MB et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine20(3–4), 545–553 (2001).
- Poolman J, Kriz P, Feron C et al. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine27(24), 3213–3222 (2009).
- Prymula R, Peeters P, Chrobok V et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet367(9512), 740–748 (2006).
- Nurkka A, Joensuu J, Henckaerts I et al. Immunogenicity and safety of the eleven valent pneumococcal polysaccharide-PD conjugate vaccine in infants. Pediatr. Infect. Dis. J23(11), 1008–1014 (2004).
- Grimprel E, Scott D, Laudat F, Baker S, Gruber W. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine given with routine pediatric vaccination to healthy infants in France. Presented at: 48th Annual ICAAC. Washington DC, USA, 25–28 October 2008.
- Örtqvist Å, Henckaerts I, Hedlund J, Poolman J. Non-response to specific serotypes likely cause for failure to 23-valent pneumococcal polysaccharide vaccine in the elderly. Vaccine25(13), 2445–2450 (2007).
- Edwards EA, Devine LF, Sengbusch GH, Ward HW. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand. J. Infect. Dis.9(2), 105–110 (1977).
- Andersen J, Berthelsen L, Jensen BB, Lind I. Surveillance of cases of meningococcal disease associated with military recruits studied for meningococcal carriage. Scand. J. Infect. Dis.32(5), 527–531 (2000).
- Auckland C, Gray S, Borrow R et al. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J. Infect. Dis.194(12), 1745–1752 (2006).
- Osterholm MT, Rambeck JH, White KE et al. Lack of efficacy of Haemophilus b polysaccharide vaccine in Minnesota. JAMA260(10), 1423–1428 (1988).
- Granoff DM, Osterholm MT. Safety and efficacy of Haemophilus influenzae type b polysaccharide vaccine. Pediatrics80(4), 590–592 (1987).
- McVernon J, Johnson PD, Pollard A, Slack MP, Moxon ER. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch. Dis. Child.88(5), 379–383 (2003).
- Clutterbuck EA, Salt P, Oh S, Marchant A, Beverley P, Pollard AJ. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology119(3), 328–337 (2006).
- Snape MD, Pollard AJ. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect. Dis.5(1), 21–30 (2005).
- Kelly DF, Snape MD, Perrett KP et al. Plasma and memory B-cell kinetics in infants following a primary schedule of CRM197-conjugated serogroup C meningococcal polysaccharide vaccine. Immunology127(1), 134–143 (2009).
- Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood114(24), 4998–5002 (2009).
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol.9(3), 213–220 (2009).
- Daum RS, Sood SK, Osterholm MT et al. Decline in serum antibody to the capsule of Haemophilus influenzae type b in the immediate postimmunization period. J. Pediatr.114(5), 742–747 (1989).
- O’Brien KL, Moïsi J, Romero-Steiner S et al. Pneumococcal antibodies in a child with type 14 pneumococcal conjugate vaccine failure. Vaccine27(12), 1863–1868 (2009).
- Joint Committee on Vaccination and Immunisation. Pneumococcal Sub-group. Minutes of the Pneumococcal subgroup on Tuesday 15th January 2009. Department of Health. Joint Committee on Vaccination and Immunisation. Pneumococcal Sub-group, London, UK (2009).
- World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Group A Meningococcal Conjugate Vaccines. WHO Press, Geneva, Switzerland (2006).
- De Wals P. Meningococcal C vaccines: the Canadian experience. Pediatr. Infect. Dis. J.23(12 Suppl.), S280–S284 (2004).
- Soriano-Gabarró M, Toé L, Tiendrebeogo SR et al. Effectiveness of a trivalent serogroup A/C/W135 meningococcal polysaccharide vaccine in Burkina Faso, 2003. Vaccine25(Suppl. 1), A92–A96 (2007).
- Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine28(34), 5513–5523 (2010).
- Schuerman L, Prymula R, Chrobok V, Dieussaert I, Poolman J. Kinetics of the immune response following pneumococcal PD conjugate vaccination. Vaccine25(11), 1953–1961 (2007).
- Kieninger DM, Kueper K, Steul K et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine28(25), 4192–4203 (2010).
- Bryant KA, Block SL, Baker SA et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics125(5), 866–875 (2010).
- Bryant KA, Block SL, Scott DA. Safety and immunogenicity of a 4th dose of 13-valent pneumococcal conjugate vaccine in healthy toddlers. Presented at: 26th Annual Meeting of the European Society for Paediatric Infectious Diseases. Graz, Austria, 13–17 May 2008.
- Gadzinowski J, Daniels ED, Giardina P et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine manufactured with and without polysorbate 80 in healthy infants given a 2, 3, 4 and 12 months of age. Presented at: 27th Annual Meeting of the European Society for Paediatric Infectious Diseases. Brussels, Belgium, 9–13 June 2009.
- Diez-Domingo J, Gurtman A, Bernaola E et al. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers receiving routine vaccinations in Spain. Presented at: 27th Annual Meeting of the European Society for Paediatric Infectious Diseases. Brussels, Belgium, 9–13 June 2009.
- Esposito S, Tansey S, Thompson A et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin. Vaccine Immunol.17(6), 1017–1026 (2010).
- Grimprel E, Laudat F, Baker SA et al. Safety and immunogenicity of a 13–valent pneumococcal conjugate vaccine given with routine pediatric vaccination to healthy infants in France. Presented at: 27th Annual Meeting of the European Society for Paediatric Infectious Diseases. Brussels, Belgium, 9–13 June 2009.
- Nurkka A, Ahman H, Yaich M, Eskola J, Käyhty H. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine20(1–2), 194–201 (2002).
- Puumalainen T, Dagan R, Wuorimaa T et al. Greater antibody responses to an eleven valent mixed carrier diphtheria- or tetanus-conjugated pneumococcal vaccine in Filipino than in Finnish or Israeli infants. Pediatr. Infect. Dis. J.22(2), 141–149 (2003).
- Dagan R, Käyhty H, Wuorimaa T et al. Tolerability and immunogenicity of an eleven valent mixed carrier Streptococcus pneumoniae capsular polysaccharide–diphtheria toxoid or tetanus protein conjugate vaccine in Finnish and Israeli infants. Pediatr. Infect. Dis. J.23(2), 91–98 (2004).
- Dagan R, Goldblatt D, Maleckar JR, Yaïch M, Eskola J. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect. Immun.72(9), 5383–5391 (2004).
Website
- Study Evaluating a 13-Valent Pneumococcal Conjugate Vaccine (13vPnC) in Adults (CAPITA) http://clinicaltrials.gov/ct2/show/NCT00744263?term=NCT00744263&rank=1